
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Margarida Quina | + 1357 word(s) | 1357 | 2020-04-13 12:06:27 | | | |
| 2 | Catherine Yang | Meta information modification | 1357 | 2020-04-20 07:51:59 | | | | |
| 3 | Catherine Yang | -2 word(s) | 1355 | 2020-10-29 05:18:16 | | |
Video Upload Options
Kraft pulp mills produce a fibrous material composed of wood cellulose fibers, constituting the main raw material for papermaking. The kraft process is the dominant technology in the world to produce pulp, allowing the recycling of most of the pulping chemical in two loops, commonly referred to as the sodium cycle and calcium cycle. A third cycle is also relevant to sodium sulphate recovery. Meanwhile, significant amounts of solid, liquid and gaseous emissions are generated, requiring treatment before release into the environment. Some inorganic solid wastes are of particular concern due to the high quantities generated, which are landfilled: green liquor dregs, slaker grits, lime mud, and boiler fly ash.
1. Introduction
Chemical processes are the most common for obtaining pulp of cellulose fibers, among which stands out the ‘sulfate process’, commonly known as ‘kraft process’ due to the high physical-mechanical resistance of the pulps produced (kraft means strength in German) and is currently the most widely used process in the world (80% of total chemical pulp) [[1][2]]. This process can be divided into four parts: raw material handling, chemical delignification of wood with efficient chemical and energy recovery systems, bleaching with high water consumption and the wastewater treatment system. In the cooking process, water-solubilized reagents (liquor) are added to the wood chips in a reaction vessel (digester) for 1 to 3 h at 150–170 °C [[3]]. Among the several advantages compared to other chemical processes, it can be highlighted the following [[4]]:
- Higher strength and flexibility of the produced pulps;
- Applicability to various wood species, regardless of their physico-chemical characteristics;
- The wide range of pulp applications;
- The efficient recovery of chemicals used in cooking, off-setting the high capital costs, which makes it economically more viable and competitive.
2. Kraft Pulp Mill Process: Chemical Recovery and the Generation of Inorganic Waste
The main active chemicals employed in the kraft process are sodium hydroxide (NaOH) and sodium sulfide (Na2S), commonly known as white liquor. Indeed, the designation sulfate process is due to the addition of sodium sulfate to replace lost chemical reagents. This mixture leads to lignin fragmentation and dissolution, while cellulose fibers are released [[5]]. The cooking reagents are not completely selective for lignin and there are also undesirable reactions of polysaccharides, mainly hemicelluloses, which due to their chemical structure are very susceptible to chemical attack. The cellulose fibers are recovered from the black liquor, which contains lignin and valuable chemicals. Figure 1 shows a simplified diagram of the phases to obtain pulp from wood, highlighting the cycles for recovering sodium, sulphur and calcium as well as for energy recovery. These cycles aim not only at the recovery of inorganic chemicals but also to obtain energy from the combustion of organic matter and prevent pollution to the environment. The main purpose of the sodium cycle (or causticizing process) is to convert the main components of green liquor (Na2CO3 and Na2S), in particular the inactive sodium carbonate (Na2CO3) from the burning process, into sodium hydroxide (NaOH), according to the Eq. (1), which is a central cooking chemical in white liquor:
Na2CO3 + Ca(OH)2 → 2 NaOH + CaCO3 (1)
During the causticizing process, the green liquor is clarified and transformed into white liquor, by removing insoluble compounds (green liquor dregs - GLD), which are then washed and removed out from the process. The CaCO3 from the causticizing reaction (lime mud – LM) is separated from the white liquor, washed and calcined at high temperature into CaO, according to Eq. (2) in the calcium cycle:
CaCO3 → CaO + CO2 (2)
This reaction is endothermic and releases CO2 to the atmosphere. For producing hydrated lime, CaO reacts with water through Eq. (3), which will be used as a reactant in Eq.(1)
CaO + H2O → Ca(OH)2 (3)
The insoluble fraction in the slaking operation is commonly named slaker grits (SG).
An important point related to the recovery of chemicals is a third cycle associated with the recovery boiler, which is commonly equipped with an electrostatic precipitator to capture the dust from the flue-gas. The particles composed mainly of Na2SO4 are returned to the furnace by mixing them with the black liquor.
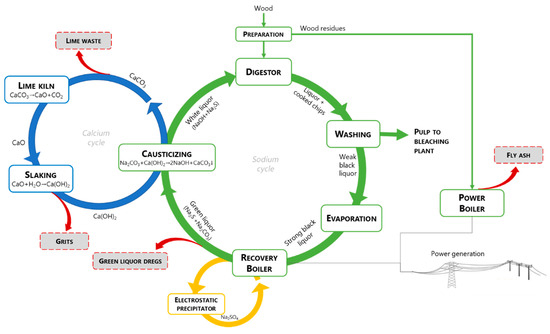
Figure 1. The chemical recovery loops and energy generation.
The generation points of the main four inorganic wastes produced in large quantities in the kraft pulp mill are shown in Figure 1, namely green liquor dregs (GLD), slaker grits (SG), lime mud (LM), and boiler fly ash (BFA). These wastes are of particular concern because despite several applications have been tested on a laboratory scale, few have reached the industrial scale, and thus the landfill is the main management method. GLD is generated during the clarification of green liquor and contain the insoluble material of the recovery boiler inorganic flux (smelt). The reported GLD suspended solids content is about 600–2000 mg/L and the pH is strongly alkaline [[6][7]]. Lime mud (LM) is a by-product formed during the causticizing reaction, which is separated from the white liquor, washed and calcined in the lime kiln, while a fraction is purged as waste and replaced by fresh CaCO3. Higher LM production occurs when there are differences between the production of white liquor and the production capacity of the lime kiln. The pH of LM can vary slightly but is often strongly alkaline [[8]]. Slaker grits (SG) are the coarse material removed from the discharge of the lime slaker to avoid build-up on causticizers and mechanical wear on filter components [[9]]. The solids content of SG is typically about 75% and the pH is usually higher than 12.5 [[9][10]]. The boiler fly ash (BFA) is generated in a biomass fluidized bed boiler, as a result of the combustion of wood bark and other wood residues for energy recovery. The fused particles are carried upwards along with the flue gas. As the flue gas approaches the low-temperature zones, the fused substances solidify to form fly ash, which is captured by cyclones, fabric filters and/or electrostatic precipitators (ESP) with cleaning efficiency above 99% [[11]]. The fly ash consists of fine particulates and precipitated volatiles, typically with a high specific surface area [[12]]. The pH of BFA is typically alkaline but lower than the observed for lime residues [[13]].
Closing the loops in kraft mills has environmental advantages but leads to the build-up in the liquor cycle of non-process elements (NPE), such as Ca, Mg, K, Mn, Ba, Fe, Al, Ni, Cu, Zn, etc., which may hinder the pulping, bleaching or chemicals recovery process. NPE enters the pulping process through the main raw materials, namely wood, make-up chemicals, water or may arise from the equipment corrosion [[14]]. In addition, the trend of closing the water cycle accumulates NPE such as Ca and K in the recovery cycle. Their accumulation may lead to filtration difficulties, precipitate formation, or even in undesirable catalytic effects. The purge of NPE from the recovery cycles is essential to maintain normal operating conditions. The process purges associated with GLD, SG, and LM are of great importance for the elimination of many NPE.
Data in Table 1 shows an overview of the specific production of GLD, SG, LM and BFA in kraft pulp mills, which is variable depending on the technology and other specific factors in each site.
Table 1. Specific waste generation in kraft pulp mills (kg/t AD).
| Wastes | Industrial Information (a) | [[15]] | [[16]] | [[17]] | [[18]] | [[19]] |
|---|---|---|---|---|---|---|
| GLD | 12 | 10–20 (b) | 12 | 15 | 4–20 | 12.8 |
| SG | 10 | 7 | 16 | |||
| LM | 25 | 10–20 | 15 | 13 | ||
| BFA | 30 | 9 (c) | 20 | 5 |
(a) Data provided by the Portuguese Industry (The Navigator Company); (b) includes green liquor dregs (GLD) and slaker grits (SG); (c) may be higher if biomass from external sources is also used; AD – air dried pulp.
Considering the pulp production worldwide, the wastes mentioned in Table 1 represent a huge amount. For example, according to the literature, in Finland (one of the main European pulp producers), about 100 kt of GLD were produced per year [[18]], and the world production can rise from 0.5 to 1.3 Mt [[19]]. Although kraft pulp mills have managed to close the chemicals loops, recover energy and reduce water consumption in the process, the current situation of inorganic waste can still be improved if industrial applications are developed to avoid landfill. In the future, the circular economy approaches will be fundamental to operate the transition from landfill to develop industrial applications for GLD, SG, LM and BFA.
References
- Bajpai, P. Environmentally Benign Approaches for Pulp Bleaching, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2012.
- Biermann, C.J. Handbook of Pulping and Papermaking, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 1996.
- Sjöström, E. Wood chemistry: Fundamentals and Applications, 2nd ed.; Academic Press: San Diego, CA, USA, 1993.
- Walker, J. Primary Wood Processing: Principles and Practice; Springer: Berlin/Heidelberg, Germany, 2006.
- Bajpai, P. Biotechnology for Pulp and Paper Processing, 2nd ed.; Springer: Singapore, 2018.
- Mohammad Golmaei; Teemu Kinnarinen; Eeva Jernström; Antti Häkkinen; Extraction of hazardous metals from green liquor dregs by ethylenediaminetetraacetic acid. Journal of Environmental Management 2018, 212, 219-227, 10.1016/j.jenvman.2018.01.078.
- Tikka, P. Papermaking Science and Technology, Book 6 (Part 2), 2nd ed.; Paper Engineers’ Association/Paperi ja Puu Oy: Helsinki, Finland, 2008.
- Jiajie He; Clifford R. Lange; Mark Dougherty; Laboratory study using paper mill lime mud for agronomic benefit. Process Safety and Environmental Protection 2009, 87, 401-405, 10.1016/j.psep.2009.08.001.
- Sanchez, D.; Tran, H. Treatment of Lime Slaker Grit and Green Liquor Dregs-Current Practice. In Proceedings of the TAPPI Engineering, Pulping & Environmental Conference, Philadelphia, Pennsylvania, 25–31 August 2005; pp. 1–9.
- R. Pöykiö; Hannu Nurmesniemi; Olli Dahl; G. Watkins; Kati Manskinen; Evaluation of the bio-accessible non-process element concentrations in slaker grits by synthetic sweat and gastric fluids extraction. Journal of Environmental and Occupational Science 2014, 3, 65, 10.5455/jeos.20140315081719.
- Mikkanen, P. Fly Ash Particle Formation in Kraft Recovery Boilers; Helsinki University of Technology: Espoo, Finland, 2000.
- L. Simão; Dachamir Hotza; F. Raupp-Pereira; Joao Labrincha; Oscar Rubem Klegues Montedo; Wastes from pulp and paper mills - a review of generation and recycling alternatives. Cerâmica 2018, 64, 371, 10.1590/0366-69132018643712414.
- Suthipong Sthiannopkao; Siranee Sreesai; Utilization of pulp and paper industrial wastes to remove heavy metals from metal finishing wastewater. Journal of Environmental Management 2009, 90, 3283-3289, 10.1016/j.jenvman.2009.05.006.
- Watkins, G.; Pöykiö, R.; Nurmesniemi, H.; Dahl, O. Earth construction and landfill disposal options for slaker grits. Res. J. Appl. Sci. Eng. Technol. 2010, 2, 757–764.
- European Commission. BREF Best Available Techniques (BAT) Reference Document for the Production of Pulp, Paper and Board; European Commission: Maastricht, The Netherlands, 2015.
- Modolo, R.C.E. Valorization of Solid Wastes from Cellulose and Paper Industry. PhD Thesis, University of Aveiro, Aveiro, Portugal, 2014.
- Kati Manskinen; Hannu Nurmesniemi; R. Pöykiö; Total and extractable non-process elements in green liquor dregs from the chemical recovery circuit of a semi-chemical pulp mill. Chemical Engineering Journal 2011, 166, 954-961, 10.1016/j.cej.2010.11.082.
- Hannu Nurmesniemi; Risto Poykio; Paavo Perämäki; Toivo Kuokkanen; The use of a sequential leaching procedure for heavy metal fractionation in green liquor dregs from a causticizing process at a pulp mill. Chemosphere 2005, 61, 1475-1484, 10.1016/j.chemosphere.2005.04.114.
- Isabel Martínez-Lage; Mirian Velay-Lizancos; Pablo Vázquez-Burgo; Marcos Rivas-Fernández; Cristina Vázquez-Herrero; Antonio Ramírez-Rodríguez; Isabel Martínez-Lage; Concretes and mortars with waste paper industry: Biomass ash and dregs. Journal of Environmental Management 2016, 181, 863-873, 10.1016/j.jenvman.2016.06.052.





