1000/1000
Hot
Most Recent

For the creation of tissue-engineered structures based on natural biopolymers with the necessary chemical, physical, adhesive, morphological, and regenerative properties, biocompatible materials based on polysaccharides and proteins are used. This entry is devoted to a problem of the technology of polymeric materials for biomedical purposes: the creation of biopolymer tissue engineering matrix and the development of a methodology for studying morphology and functional properties of their surface to establish the prospects for using the material for contact with living objects.
Modern technologies make it possible to develop composite materials based on biopolymers with unique desired properties obtained as a result of modification: bioreactivity and biodegradation in natural environmental conditions, bioinertness in the environment of a living organism, resistance to mechanical stress, etc. [1][2].
Biopolymers are actively used in scientific developments aimed at obtaining medical and biological products with specified and controlled properties [3][4][5]. One of the promising areas of such research is tissue engineering, the purpose of which is to develop methods for the regeneration of lost or damaged tissues, as well as entire organs, using new biomaterials and cellular technologies. The critical issue of tissue engineering is the creation of a suitable artificial biomimetic matrix—a scaffold for targeted tissue regeneration, providing conditions for cell growth and, ultimately, their integration with the surrounding tissue. [6]. Biomaterials can form matrices and scaffolds of various types (Figure 1) [7], which provide structural and functional support of cells on their surface, and their components regulate cell migration and proliferation [8], thereby affecting tissue formation, blood coagulation, adsorption of biomacromolecules and wound healing [9].
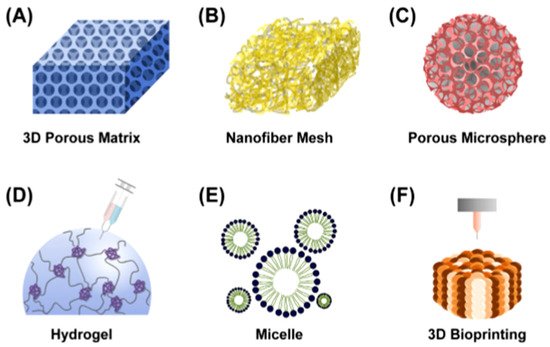
Figure 1. Different types of polymeric scaffolds for tissue engineering: (A) 3D porous matrix, (B) nanofibrous mesh, (C) porous microsphere, (D) hydrogel, (E) micelle and (F) 3D bioprinting [7].
The most common basis for creating materials capable of maintaining and regulating moisture, as well as adsorbing proteases from wound exudate, are proteins and polysaccharides (hyaluronic acid, alginates, starch, carrageenans, collagen, gelatin, dextran, polyhydroxyalkanoates, chitosan and fibroin) [10][11][12][13][14][15], many of which have antioxidant, antimicrobial and anti-inflammatory properties, reduce the risk of inflammation, and are highly effective biostimulants.
The surface activity of polysaccharides and proteins is able to regulate the enzymatic expression and optimal moisture content for wound healing while ensuring natural drainage [16][17]. Scaffolds based on these compounds provide the structural integrity of tissue structures, control the delivery of drugs and proteins to tissues, and serve as adhesives or barriers between tissue and various material surfaces [18]. In the process of biodegradation, they are split into simple compounds that can take an active part in metabolism at the cellular level or are excreted from the body [19].
Considering the unique properties—biocompatibility, gel-forming and mucoadhesive properties—and the ability to complex formation, and based on the quantitative analysis of publications devoted to the development of biopolymer matrices for tissue engineering, the most promising biopolymers for the development of a carrier support for attachment and growth of cells should include chitosan and silk fibroin [20][21][22][23][24]. Chitosan promotes tissue adhesion and regeneration due to its structural similarity with glycosaminoglycans, which are part of the extracellular matrix, and the structural protein silk fibroin is an excellent substrate for cell attachment and proliferation. Fibroin and chitosan, as well as composite materials based on them, provide the structural integrity of tissue structures, control the delivery of growth factors and enzymes to tissues and cell cultures, and support adhesion and proliferation and the functional activity of living cytoobjects.
To date, there are many methods for obtaining biopolymer matrices—casting films [25][26][27], spin-coating [27][28][29][30] or cryotropic gelation [31][32], electrospinning [33][34][35]. The method of electrospinning is widely known in tissue engineering, regenerative medicine, dentistry and matrices based on biopolymers, and compositions based on them have a number of unique properties that are distinguished by a number of authors, including Prof. Zafar [36][37].
Using the above methods, materials are obtained that differ in internal structure, pore size and mechanical properties of the surface [38], and morphology. The morphology of the surface of the biopolymer matrix, namely its roughness, is a very important parameter affecting the surface wettability, protein adsorption, cell attachment and further cell growth, as well as the course of the inflammatory reaction. Thus, the methodology for assessing the surface properties of biopolymer materials intended for use as matrices for tissue engineering is an important part of the research in the technology of biomedical materials.
Porous structures with a system of large interpenetrating pores can be obtained by freezing polysaccharide solutions containing a crosslinking reagent and lyophilizing a polymer hydrogel [39][40][41]. In this case, the characteristics of the initial system (molecular weight of the polymer, concentration, content of the cross-linking reagent) determine the structure of the resulting matrix (the nature and distribution of pores by size), as well as the degree of adhesion and spreading of cells, which, in turn, determine their subsequent growth and proliferation. A modification of this method is possible: a polymer solution is lyophilized, followed by the transformation of the material into an insoluble form by chemical crosslinking of macromolecules already in the solid phase. In this case, wide-porous spongy materials are obtained, which are called cryostructurates [42]. Currently, the electrospinning method is used to obtain fibrous mats consisting of sub-microfibers and nanofibers. Electrostatic forces are applied to the polymer solution from a jet, which, as the solvent evaporates, transforms into nanofibers and solidifies on the manifold. In recent years, the method of electrospinning nonwoven fibrous materials has been developed mainly for the processing of natural polymers [33][36][37], since materials based on them are characterized by biocompatibility and biodegradability in the body, and a fibrous surface with nano-sized structural elements promotes cell attachment and the formation of living tissues. Nanofibers derived from biopolymers activate fibroblasts, which migrate to the dermal layer, which releases the main components of the extracellular matrix to heal damaged tissue. The production of nanofibrous materials for various purposes, including scaffolds for tissue engineering, based on chitosan and, in particular, silk fibroin, by the method of electrospinning, was described in detail in the works of prof. Kaplan and other researchers, including prof. Zhang [43][44][45][46][47][48][49][50][51][52][53][54][55].
Another promising method for forming matrices from solutions of biopolymers for tissue engineering and regenerative medicine is the production of mono- and multilayer nano- and micro-polymer film coatings by the spin-coating method. A spinning coating allows one to obtain uniform thin films and coatings of varying thickness on a flat substrate using a special device—a spin-coater. The spin-coating process involves the application of the required volume of the polymer solution to the center of the substrate, and then acceleration and rotation of the substrate at high speed (3000–8000 rpm). The final coating thickness depends on the viscosity and nature of the initial solution and solvent, as well as on the parameters of centrifugation and temperature [56][57].
This method is used in developments related to tissue engineering due to the convenience of studying cell adhesion on a micro- and nanoscale surface (with known morphofunctional), proliferation, cytotoxicity when obtaining mono- and multilayer biopolymer coatings with desired sorption, hemostatic, antibacterial and hygienic properties [58][59][60][61].
Conventional films are produced by casting followed by solvent evaporation. These methods make it possible to include biologically active compounds, enzymes, and other protein compounds in their structure [62][63]. For improve the water-resistance and mechanical properties of proteins and polysaccharides, are used chemical crosslinking methods. Most of all, chitosan is suitable for preparing materials using cross-linking reagents, because of its amino groups, which are more reactive than the hydroxyl groups of other polysaccharides.
Chitosan is a polycationic biopolymer, a linear deacetylated polysaccharide derivative of natural-origin chitin. It is known that it consists of 2-acetamido-2-deoxy-β-D-glucose via a β (1 → 4) bond [64]. Chitosan can be characterized by the degree of deacetylation and molecular weight; these parameters can differ significantly, which affects the properties of biopolymer materials and their area of use. The influence of the different degrees of deacetylation and molecular weight of chitosan on the hydrophilicity, degradation, mechanical properties and biocompatibility of chitosan films was evaluated in [65]. The results showed that the degree of deacetylation affects the hydrophilicity and biocompatibility of the chitosan films. Reference [66] discusses the effects of molecular weight and degree of deacetylation cytotoxicity of chitosan molecules and nanoparticles. Chitosan molecules and nanoparticles exhibited comparable cytotoxicity against the A549 cells. Cytotoxicity of both chitosan entities was attenuated by decreasing polymer DD but was less affected by a decrease in Mw. The molecular weight, on the other hand, affected the rate of degradation and the mechanical properties. Chitosan, with a higher degree of deacetylation and molecular weight, was more suitable for tissue engineering applications [65]; these data are confirmed in Reference [67], which showed that activated fibroblasts appeared more in the higher deacetylation degree of chitosan.
The degree of deacetylation determines the fraction of free amino groups, which are much more reactive than the acetamide groups of chitin. The presence of an amino group in the anhydropyranose monomer unit ensures the solubility of chitosan in aqueous solutions of organic acids, which is possible when the degree of deacetylation is more than 60%. Figure 2 shows the structure of the protonated form of chitosan.
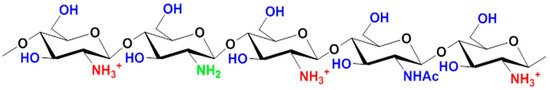
Figure 2. Chemical structure of the protonated form of chitosan.
Owing to its amino group, covalently cross-linked materials for various purposes with specified and controlled properties have been obtained [68]. Chemical crosslinking allows an irreversible transition from a chitosan solution to a hydrogel, and this technique is used in the preparation of various types of biomaterials [69][70][71]. The properties of chitosan hydrogels, the supramolecular and porous structure, the degree of swelling, and mechanical strength can vary due to changes in the crosslinking conditions [72][73].
Some cross-linking reagents have a certain toxicity and impart it to the resulting products, limiting their applications in medicine and tissue engineering. Therefore, recently, the use of an ecologically clean, bioactive and biocompatible covalent cross-linking agent of plant origin, genipin, has become widespread [74]. It is known as a hepatoprotective agent with pronounced anti-inflammatory, antioxidant and anti-diabetic properties, and is also actively used in the treatment of various inflammatory diseases and even cancer [75]. It was found that chitosan scaffolds, sponges, fibrous materials and products based on them spatially cross-linked by genipin not only are safe for the animal organism as a whole but also have no cytotoxic effect on various cell populations [76]. The obtained materials contribute to the proliferation of cytoorganisms; it was recorded that an increase in the amount of genipin directly affects improves cell adhesion. Depending on the degree of cross-linking, cells acquire different morphology when attached to the surface of the carrier material: single with minimal contact with the carrier, cell fusion, spreading, and local adhesion formations [31][32][77].
Chitosan is soluble in water only in an acidic media, when the primary amino groups of chitosan are protonated and the macromolecule acquires a positive charge; therefore, biopolymer matrices based on chitosan can be obtained only from its solution in an aqueous solution of an organic acid. Unlike chitosan, degummed silk—fibroin—is soluble in the entire pH range, and this, along with good fiber-forming ability, expands the possibilities of obtaining porous biopolymer matrices on its basis for growing cells.
Silk fibroin, prepared from silkworms (mulberry silkworm of the Bombyx mori), has become a widely used biomaterial due to its unique physicomechanical and especially strength properties and high elasticity, biocompatibility, well-studied surface chemistry, controlled degradation, water and oxygen permeability, renewable reserves, low cost and ease of processing. The amino acid composition of B. Mori silk fibroin, purified from sericin, consists mainly of glycine (Gly) (43%), alanine (Ala) (30%) and serine (12%) and a small number of other amino acids [78]. A simplified fibroin formula is shown in Figure 3.
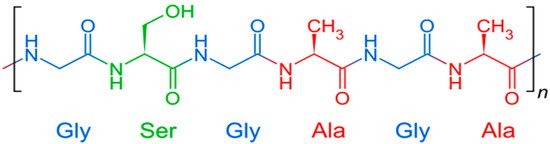
Figure 3. Chemical structure of fibroin.
Rather easy processing and processing of this raw material contribute to the production of biopolymer forms with different structures, including fibrous, film, and three-dimensional (3D) porous hydrogels, complex multi-level structures (micropatterned) [79].
Films of various sizes with a variable set of parameters and characteristics can be obtained from aqueous solutions of purified fibroin. For example, Wang et al. obtained nanoscale fibroin films from aqueous solutions using a layer-by-layer technique [53]. This method makes it possible to obtain biopolymer coatings of a given size and thickness. Such nanosized films excellently support the adhesion and proliferation of mesenchymal stem cells [53]. Composite coatings obtained by Li et al., using the layer-by-layer formation of a material based on fibroin, the addition of which significantly improves biocompatibility and hydrophilicity, in combination with the polysaccharide chitosan, and with the addition of heparin, increasing the antibacterial activity to 95%, the coatings have improved biological capacity [80]. It has been shown that the attachment of fibroblasts to silk films is the same as for films based on collagen [81]. Other mammalian and insect cells also showed good adhesion to fibroin coatings as compared to collagen films [82]. Chemically modified fibroin-based films are used to improve osteoblast cell attachment and bone formation [83]. Biocompatible fibrous materials that regulate the formation of vascularized reticular connective tissue are obtained by electrospinning from fibroin [84].