For the creation of tissue-engineered structures based on natural biopolymers with the necessary chemical, physical, adhesive, morphological, and regenerative properties, biocompatible materials based on polysaccharides and proteins are used. This entry is devoted to a problem of the technology of polymeric materials for biomedical purposes: the creation of biopolymer tissue engineering matrix and the development of a methodology for studying morphology and functional properties of their surface to establish the prospects for using the material for contact with living objects.
- biopolymers
- chitosan
- fibroin
1. Introduction
Modern technologies make it possible to develop composite materials based on biopolymers with unique desired properties obtained as a result of modification: bioreactivity and biodegradation in natural environmental conditions, bioinertness in the environment of a living organism, resistance to mechanical stress, etc. [1][2].
Biopolymers are actively used in scientific developments aimed at obtaining medical and biological products with specified and controlled properties [3][4][5]. One of the promising areas of such research is tissue engineering, the purpose of which is to develop methods for the regeneration of lost or damaged tissues, as well as entire organs, using new biomaterials and cellular technologies. The critical issue of tissue engineering is the creation of a suitable artificial biomimetic matrix—a scaffold for targeted tissue regeneration, providing conditions for cell growth and, ultimately, their integration with the surrounding tissue. [6]. Biomaterials can form matrices and scaffolds of various types (
) [7], which provide structural and functional support of cells on their surface, and their components regulate cell migration and proliferation [8], thereby affecting tissue formation, blood coagulation, adsorption of biomacromolecules and wound healing [9].
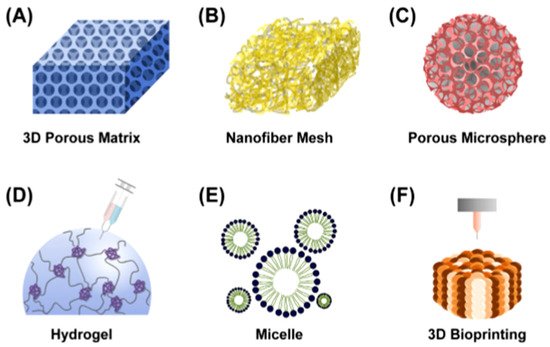
Different types of polymeric scaffolds for tissue engineering: (
) 3D porous matrix, (
) nanofibrous mesh, (
) porous microsphere, (
) hydrogel, (
) micelle and (
) 3D bioprinting [7].
The most common basis for creating materials capable of maintaining and regulating moisture, as well as adsorbing proteases from wound exudate, are proteins and polysaccharides (hyaluronic acid, alginates, starch, carrageenans, collagen, gelatin, dextran, polyhydroxyalkanoates, chitosan and fibroin) [10][11][12][13][14][15], many of which have antioxidant, antimicrobial and anti-inflammatory properties, reduce the risk of inflammation, and are highly effective biostimulants.
The surface activity of polysaccharides and proteins is able to regulate the enzymatic expression and optimal moisture content for wound healing while ensuring natural drainage [16][17]. Scaffolds based on these compounds provide the structural integrity of tissue structures, control the delivery of drugs and proteins to tissues, and serve as adhesives or barriers between tissue and various material surfaces [18]. In the process of biodegradation, they are split into simple compounds that can take an active part in metabolism at the cellular level or are excreted from the body [19].
Considering the unique properties—biocompatibility, gel-forming and mucoadhesive properties—and the ability to complex formation, and based on the quantitative analysis of publications devoted to the development of biopolymer matrices for tissue engineering, the most promising biopolymers for the development of a carrier support for attachment and growth of cells should include chitosan and silk fibroin [20][21][22][23][24]. Chitosan promotes tissue adhesion and regeneration due to its structural similarity with glycosaminoglycans, which are part of the extracellular matrix, and the structural protein silk fibroin is an excellent substrate for cell attachment and proliferation. Fibroin and chitosan, as well as composite materials based on them, provide the structural integrity of tissue structures, control the delivery of growth factors and enzymes to tissues and cell cultures, and support adhesion and proliferation and the functional activity of living cytoobjects.
To date, there are many methods for obtaining biopolymer matrices—casting films [25][26][27], spin-coating [27][28][29][30] or cryotropic gelation [31][32], electrospinning [33][34][35]. The method of electrospinning is widely known in tissue engineering, regenerative medicine, dentistry and matrices based on biopolymers, and compositions based on them have a number of unique properties that are distinguished by a number of authors, including Prof. Zafar [36][37].
Using the above methods, materials are obtained that differ in internal structure, pore size and mechanical properties of the surface [38], and morphology. The morphology of the surface of the biopolymer matrix, namely its roughness, is a very important parameter affecting the surface wettability, protein adsorption, cell attachment and further cell growth, as well as the course of the inflammatory reaction. Thus, the methodology for assessing the surface properties of biopolymer materials intended for use as matrices for tissue engineering is an important part of the research in the technology of biomedical materials.
2. Existing Directions and Methods of Obtaining Porous Biopolymer Matrix
Porous structures with a system of large interpenetrating pores can be obtained by freezing polysaccharide solutions containing a crosslinking reagent and lyophilizing a polymer hydrogel [39][40][41]. In this case, the characteristics of the initial system (molecular weight of the polymer, concentration, content of the cross-linking reagent) determine the structure of the resulting matrix (the nature and distribution of pores by size), as well as the degree of adhesion and spreading of cells, which, in turn, determine their subsequent growth and proliferation. A modification of this method is possible: a polymer solution is lyophilized, followed by the transformation of the material into an insoluble form by chemical crosslinking of macromolecules already in the solid phase. In this case, wide-porous spongy materials are obtained, which are called cryostructurates [42]. Currently, the electrospinning method is used to obtain fibrous mats consisting of sub-microfibers and nanofibers. Electrostatic forces are applied to the polymer solution from a jet, which, as the solvent evaporates, transforms into nanofibers and solidifies on the manifold. In recent years, the method of electrospinning nonwoven fibrous materials has been developed mainly for the processing of natural polymers [33][36][37], since materials based on them are characterized by biocompatibility and biodegradability in the body, and a fibrous surface with nano-sized structural elements promotes cell attachment and the formation of living tissues. Nanofibers derived from biopolymers activate fibroblasts, which migrate to the dermal layer, which releases the main components of the extracellular matrix to heal damaged tissue. The production of nanofibrous materials for various purposes, including scaffolds for tissue engineering, based on chitosan and, in particular, silk fibroin, by the method of electrospinning, was described in detail in the works of prof. Kaplan and other researchers, including prof. Zhang [43][44][45][46][47][48][49][50][51][52][53][54][55].
Another promising method for forming matrices from solutions of biopolymers for tissue engineering and regenerative medicine is the production of mono- and multilayer nano- and micro-polymer film coatings by the spin-coating method. A spinning coating allows one to obtain uniform thin films and coatings of varying thickness on a flat substrate using a special device—a spin-coater. The spin-coating process involves the application of the required volume of the polymer solution to the center of the substrate, and then acceleration and rotation of the substrate at high speed (3000–8000 rpm). The final coating thickness depends on the viscosity and nature of the initial solution and solvent, as well as on the parameters of centrifugation and temperature [56][57].
This method is used in developments related to tissue engineering due to the convenience of studying cell adhesion on a micro- and nanoscale surface (with known morphofunctional), proliferation, cytotoxicity when obtaining mono- and multilayer biopolymer coatings with desired sorption, hemostatic, antibacterial and hygienic properties [58][59][60][61].
Conventional films are produced by casting followed by solvent evaporation. These methods make it possible to include biologically active compounds, enzymes, and other protein compounds in their structure [62][63]. For improve the water-resistance and mechanical properties of proteins and polysaccharides, are used chemical crosslinking methods. Most of all, chitosan is suitable for preparing materials using cross-linking reagents, because of its amino groups, which are more reactive than the hydroxyl groups of other polysaccharides.
3. Preparation Biomaterials from the Chitosan Solutions
Chitosan is a polycationic biopolymer, a linear deacetylated polysaccharide derivative of natural-origin chitin. It is known that it consists of 2-acetamido-2-deoxy-β-D-glucose via a β (1 → 4) bond [64]. Chitosan can be characterized by the degree of deacetylation and molecular weight; these parameters can differ significantly, which affects the properties of biopolymer materials and their area of use. The influence of the different degrees of deacetylation and molecular weight of chitosan on the hydrophilicity, degradation, mechanical properties and biocompatibility of chitosan films was evaluated in [65]. The results showed that the degree of deacetylation affects the hydrophilicity and biocompatibility of the chitosan films. Reference [66] discusses the effects of molecular weight and degree of deacetylation cytotoxicity of chitosan molecules and nanoparticles. Chitosan molecules and nanoparticles exhibited comparable cytotoxicity against the A549 cells. Cytotoxicity of both chitosan entities was attenuated by decreasing polymer DD but was less affected by a decrease in Mw. The molecular weight, on the other hand, affected the rate of degradation and the mechanical properties. Chitosan, with a higher degree of deacetylation and molecular weight, was more suitable for tissue engineering applications [65]; these data are confirmed in Reference [67], which showed that activated fibroblasts appeared more in the higher deacetylation degree of chitosan.
The degree of deacetylation determines the fraction of free amino groups, which are much more reactive than the acetamide groups of chitin. The presence of an amino group in the anhydropyranose monomer unit ensures the solubility of chitosan in aqueous solutions of organic acids, which is possible when the degree of deacetylation is more than 60%.
shows the structure of the protonated form of chitosan.
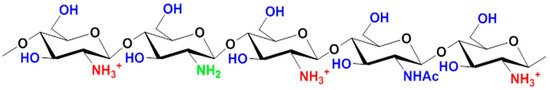
Chemical structure of the protonated form of chitosan.
Owing to its amino group, covalently cross-linked materials for various purposes with specified and controlled properties have been obtained [68]. Chemical crosslinking allows an irreversible transition from a chitosan solution to a hydrogel, and this technique is used in the preparation of various types of biomaterials [69][70][71]. The properties of chitosan hydrogels, the supramolecular and porous structure, the degree of swelling, and mechanical strength can vary due to changes in the crosslinking conditions [72][73].
Some cross-linking reagents have a certain toxicity and impart it to the resulting products, limiting their applications in medicine and tissue engineering. Therefore, recently, the use of an ecologically clean, bioactive and biocompatible covalent cross-linking agent of plant origin, genipin, has become widespread [74]. It is known as a hepatoprotective agent with pronounced anti-inflammatory, antioxidant and anti-diabetic properties, and is also actively used in the treatment of various inflammatory diseases and even cancer [75]. It was found that chitosan scaffolds, sponges, fibrous materials and products based on them spatially cross-linked by genipin not only are safe for the animal organism as a whole but also have no cytotoxic effect on various cell populations [76]. The obtained materials contribute to the proliferation of cytoorganisms; it was recorded that an increase in the amount of genipin directly affects improves cell adhesion. Depending on the degree of cross-linking, cells acquire different morphology when attached to the surface of the carrier material: single with minimal contact with the carrier, cell fusion, spreading, and local adhesion formations [31][32][77].
Chitosan is soluble in water only in an acidic media, when the primary amino groups of chitosan are protonated and the macromolecule acquires a positive charge; therefore, biopolymer matrices based on chitosan can be obtained only from its solution in an aqueous solution of an organic acid. Unlike chitosan, degummed silk—fibroin—is soluble in the entire pH range, and this, along with good fiber-forming ability, expands the possibilities of obtaining porous biopolymer matrices on its basis for growing cells.
4. Application Fibroin to the Preparation of Polymer Matrices
Silk fibroin, prepared from silkworms (mulberry silkworm of the
), has become a widely used biomaterial due to its unique physicomechanical and especially strength properties and high elasticity, biocompatibility, well-studied surface chemistry, controlled degradation, water and oxygen permeability, renewable reserves, low cost and ease of processing. The amino acid composition of
silk fibroin, purified from sericin, consists mainly of glycine (Gly) (43%), alanine (Ala) (30%) and serine (12%) and a small number of other amino acids [78]. A simplified fibroin formula is shown in
.
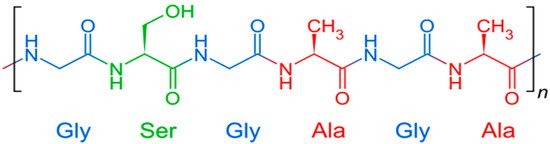
Chemical structure of fibroin.
Rather easy processing and processing of this raw material contribute to the production of biopolymer forms with different structures, including fibrous, film, and three-dimensional (3D) porous hydrogels, complex multi-level structures (micropatterned) [79].
Films of various sizes with a variable set of parameters and characteristics can be obtained from aqueous solutions of purified fibroin. For example, Wang et al. obtained nanoscale fibroin films from aqueous solutions using a layer-by-layer technique [53]. This method makes it possible to obtain biopolymer coatings of a given size and thickness. Such nanosized films excellently support the adhesion and proliferation of mesenchymal stem cells [53]. Composite coatings obtained by Li et al., using the layer-by-layer formation of a material based on fibroin, the addition of which significantly improves biocompatibility and hydrophilicity, in combination with the polysaccharide chitosan, and with the addition of heparin, increasing the antibacterial activity to 95%, the coatings have improved biological capacity [80]. It has been shown that the attachment of fibroblasts to silk films is the same as for films based on collagen [81]. Other mammalian and insect cells also showed good adhesion to fibroin coatings as compared to collagen films [82]. Chemically modified fibroin-based films are used to improve osteoblast cell attachment and bone formation [83]. Biocompatible fibrous materials that regulate the formation of vascularized reticular connective tissue are obtained by electrospinning from fibroin [84].
References
- Plank, J. Applications of Biopolymers and Other Biotechnological Products in Building Materials. Appl. Microbiol. Biotechnol. 2004, 66, 1–9.
- Rehm, B.H. Bacterial Polymers: Biosynthesis, Modifications and Applications. Nat. Rev. Microbiol. 2010, 8, 578–592.
- Ishihara, M.; Kishimoto, S.; Nakamura, S.; Sato, Y.; Hattori, H. Polyelectrolyte Complexes of Natural Polymers and Their Biomedical Applications. Polymers 2019, 11, 672.
- Samadian, H.; Maleki, H.; Allahyari, Z.; Jaymand, M. Natural Polymers-Based Light-Induced Hydrogels: Promising Biomaterials for Biomedical Applications. Coord. Chem. Rev. 2020, 420, 213432.
- George, A.; Sanjay, M.R.; Srisuk, R.; Parameswaranpillai, J.; Siengchin, S. A Comprehensive Review on Chemical Properties and Applications of Biopolymers and Their Composites. Int. J. Biol. Macromol. 2020, 154, 329–338.
- Ambekar, R.S.; Kandasubramanian, B. Progress in the Advancement of Porous Biopolymer Scaffold: Tissue Engineering Application. Ind. Eng. Chem. Res. 2019, 58, 6163–6194.
- Park, S.-B.; Lih, E.; Park, K.-S.; Joung, Y.K.; Han, D.K. Biopolymer-Based Functional Composites for Medical Applications. Prog. Polym. Sci. 2017, 68, 77–105.
- Greenwood, H.L.; Singer, P.A.; Downey, G.P.; Martin, D.K.; Thorsteinsdottir, H.; Daar, A.S. Regenerative Medicine and the Developing World. PLoS Med. 2006, 3, e381.
- Dieckmann, C.; Renner, R.; Milkova, L.; Simon, J.C. Regenerative Medicine in Dermatology: Biomaterials, Tissue Engineering, Stem Cells, Gene Transfer and Beyond. Exp. Dermatol. 2010, 19, 697–706.
- Francesko, A.; Blandón, L.; Vázquez, M.; Petkova, P.; Morató, J.; Pfeifer, A.; Heinze, T.; Mendoza, E.; Tzanov, T. Enzymatic Functionalization of Cork Surface with Antimicrobial Hybrid Biopolymer/Silver Nanoparticles. Acs Appl. Mater. Interfaces 2015, 7, 9792–9799.
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign Body Reaction to Biomaterials. In Seminars in Immunology; Elsevier: Amsterdam, The Netherlands, 2008; Volume 20, pp. 86–100.
- Baier, R.E.; Dutton, R.C. Initial Events in Interactions of Blood with a Foreign Surface. J. Biomed. Mater. Res. 1969, 3, 191–206.
- Shen, M.; Horbett, T.A. The Effects of Surface Chemistry and Adsorbed Proteins on Monocyte/Macrophage Adhesion to Chemically Modified Polystyrene Surfaces. J. Biomed. Mater. Res. 2001, 57, 336–345.
- Mrksich, M.; Whitesides, G.M. Using Self-Assembled Monolayers to Understand the Interactions of Man-Made Surfaces with Proteins and Cells. Annu. Rev. Biophys. Biomol. Struct. 1996, 25, 55–78.
- Klimek, K.; Ginalska, G. Proteins and Peptides as Important Modifiers of the Polymer Scaffolds for Tissue Engineering Applications—A Review. Polymers 2020, 12, 844.
- Chien, K.R. Regenerative Medicine and Human Models of Human Disease. Nature 2008, 453, 302–305.
- Legonkova, O.A.; Sukhareva, L.A. Thousand and One Polymer from Biostable to Biodegradable. In M. Radio-Soft; RadioSoft: Moscow, Russia, 2004; p. 272. ISBN 5-93274-008-6.
- Metcalfe, A.D.; Ferguson, M.W. Tissue Engineering of Replacement Skin: The Crossroads of Biomaterials, Wound Healing, Embryonic Development, Stem Cells and Regeneration. J. R. Soc. Interface 2007, 4, 413–437.
- Ikada, Y. Challenges in Tissue Engineering. J. R. Soc. Interface 2006, 3, 589–601.
- LogithKumar, R.; KeshavNarayan, A.; Dhivya, S.; Chawla, A.; Saravanan, S.; Selvamurugan, N. A Review of Chitosan and Its Derivatives in Bone Tissue Engineering. Carbohydr. Polym. 2016, 151, 172–188.
- Reza, E.-K.; Fateme, R.; Moghim, A.H.A.; Sima, S.; Behnam, T.; Maleki, A.; Hamid, M. Chitosan Hydrogel/Silk Fibroin/Mg (OH) 2 Nanobiocomposite as a Novel Scaffold with Antimicrobial Activity and Improved Mechanical Properties. Sci. Rep. Nat. Publ. Group 2021, 11, 1–13.
- Teimouri, A.; Ebrahimi, R.; Chermahini, A.N.; Emadi, R. Fabrication and Characterization of Silk Fibroin/Chitosan/Nano γ-Alumina Composite Scaffolds for Tissue Engineering Applications. RSC Adv. 2015, 5, 27558–27570.
- Characterization of Silk Fibroin/Chitosan 3D Porous Scaffold and In Vitro Cytology. Available online: (accessed on 2 February 2021).
- Pankongadisak, P.; Suwantong, O. Enhanced Properties of Injectable Chitosan-Based Thermogelling Hydrogels by Silk Fibroin and Longan Seed Extract for Bone Tissue Engineering. Int. J. Biol. Macromol. 2019, 138, 412–424.
- Wu, Y.-Y.; Jiao, Y.-P.; Xiao, L.-L.; Li, M.-M.; Liu, H.-W.; Li, S.-H.; Liao, X.; Chen, Y.-T.; Li, J.-X.; Zhang, Y. Experimental Study on Effects of Adipose-Derived Stem Cell–Seeded Silk Fibroin Chitosan Film on Wound Healing of a Diabetic Rat Model. Ann. Plast. Surg. 2018, 80, 572–580.
- Panjapheree, K.; Kamonmattayakul, S.; Meesane, J. Biphasic Scaffolds of Silk Fibroin Film Affixed to Silk Fibroin/Chitosan Sponge Based on Surgical Design for Cartilage Defect in Osteoarthritis. Mater. Des. 2018, 141, 323–332.
- Bhardwaj, N.; Kundu, S.C. Chondrogenic Differentiation of Rat MSCs on Porous Scaffolds of Silk Fibroin/Chitosan Blends. Biomaterials 2012, 33, 2848–2857.
- Ligler, F.S.; Lingerfelt, B.M.; Price, R.P.; Schoen, P.E. Development of Uniform Chitosan Thin-Film Layers on Silicon Chips. Langmuir 2001, 17, 5082–5084.
- Mironenko, A.; Modin, E.; Sergeev, A.; Voznesenskiy, S.; Bratskaya, S. Fabrication and Optical Properties of Chitosan/Ag Nanoparticles Thin Film Composites. Chem. Eng. J. 2014, 244, 457–463.
- Li, Q.; Qi, N.; Peng, Y.; Zhang, Y.; Shi, L.; Zhang, X.; Lai, Y.; Wei, K.; Soo Kim, I.; Zhang, K.-Q. Sub-Micron Silk Fibroin Film with High Humidity Sensibility through Color Changing. RSC Adv. 2017, 7, 17889–17897.
- Sazhnev, N.A.; Drozdova, M.G.; Rodionov, I.A.; Kil’deeva, N.R.; Balabanova, T.V.; Markvicheva, E.A.; Lozinsky, V.I. Preparation of Chitosan Cryostructurates with Controlled Porous Morphology and Their Use as 3D-Scaffolds for the Cultivation of Animal Cells. Appl. Biochem. Microbiol. 2018, 54, 459–467.
- Kil’deeva, N.R.; Kasatkina, M.A.; Drozdova, M.G.; Demina, T.S.; Uspenskii, S.A.; Mikhailov, S.N.; Markvicheva, E.A. Biodegradablescaffolds Based on Chitosan: Preparation, Properties, and Use for the Cultivation of Animal Cells. Appl. Biochem. Microbiol. 2016, 52, 515–524.
- Yu, P.; Guo, J.; Li, J.; Shi, X.; Wang, L.; Chen, W.; Mo, X. Repair of Skin Defects with Electrospun Collagen/Chitosan and Fibroin/Chitosan Compound Nanofiber Scaffolds Compared with Gauze Dressing. J. Biomater. Tissue Eng. 2017, 7, 386–392.
- Cai, Z.; Mo, X.; Zhang, K.; Fan, L.; Yin, A.; He, C.; Wang, H. Fabrication of Chitosan/Silk Fibroin Composite Nanofibers for Wound-Dressing Applications. Int. J. Mol. Sci. 2010, 11, 3529–3539.
- Aliramaji, S.; Zamanian, A.; Mozafari, M. Super-Paramagnetic Responsive Silk Fibroin/Chitosan/Magnetite Scaffolds with Tunable Pore Structures for Bone Tissue Engineering Applications. Mater. Sci. Eng. C 2017, 70, 736–744.
- Qasim, S.B.; Zafar, M.S.; Najeeb, S.; Khurshid, Z.; Shah, A.H.; Husain, S.; Rehman, I.U. Electrospinning of Chitosan-Based Solutions for Tissue Engineering and Regenerative Medicine. Int. J. Mol. Sci. 2018, 19, 407.
- Zafar, M.; Najeeb, S.; Khurshid, Z.; Vazirzadeh, M.; Zohaib, S.; Najeeb, B.; Sefat, F. Potential of Electrospun Nanofibers for Biomedical and Dental Applications. Materials 2016, 9, 73.
- Ali, S.; Sangi, L.; Kumar, N.; Kumar, B.; Khurshid, Z.; Zafar, M.S. Evaluating Antibacterial and Surface Mechanical Properties of Chitosan Modified Dental Resin Composites. Technol. Health Care 2020, 28, 165–173.
- Sampaio, G.Y.H.; Fook, A.C.B.M.; Fideles, T.B.; Cavalcanti, M.E.R.R.M.; Fook, M.V.L. Biodegradable Chitosan Scaffolds: Effect of Genipin Crosslinking. Available online: (accessed on 2 February 2021).
- Kumari, R.; Dutta, P.K. Physicochemical and Biological Activity Study of Genipin-Crosslinked Chitosan Scaffolds Prepared by Using Supercritical Carbon Dioxide for Tissue Engineering Applications. Int. J. Biol. Macromol. 2010, 46, 261–266.
- Nikonorov, V.V.; Ivanov, R.V.; Kil’deeva, N.R.; Bulatnikova, L.N.; Lozinskii, V.I. Synthesis and Characteristics of Cryogels of Chitosan Crosslinked by Glutaric Aldehyde. Polym. Sci. Ser. A 2010, 52, 828–834.
- Rodionov, I.A.; Grinberg, N.V.; Burova, T.V.; Grinberg, V.Y.; Shabatina, T.I.; Lozinsky, V.I. Cryostructuring of Polymer Systems. 44. Freeze-Dried and Then Chemically Cross-Linked Wide Porous Cryostructurates Based on Serum Albumin. e-Polymers 2017, 17, 263–274.
- Stoppel, W.L.; Ghezzi, C.E.; McNamara, S.L.; Iii, L.D.B.; Kaplan, D.L. Clinical Applications of Naturally Derived Biopolymer-Based Scaffolds for Regenerative Medicine. Ann. Biomed. Eng. 2015, 43, 657–680.
- Bhardwaj, N.; Nguyen, Q.T.; Chen, A.C.; Kaplan, D.L.; Sah, R.L.; Kundu, S.C. Potential of 3-D Tissue Constructs Engineered from Bovine Chondrocytes/Silk Fibroin-Chitosan for in Vitro Cartilage Tissue Engineering. Biomaterials 2011, 32, 5773–5781.
- Vepari, C.; Kaplan, D.L. Silk as a Biomaterial. Prog. Polym. Sci. 2007, 32, 991–1007.
- Bini, E.; Knight, D.P.; Kaplan, D.L. Mapping Domain Structures in Silks from Insects and Spiders Related to Protein Assembly. J. Mol. Biol. 2004, 335, 27–40.
- Cunniff, P.M.; Fossey, S.A.; Auerbach, M.A.; Song, J.W.; Kaplan, D.L.; Adams, W.W.; Eby, R.K.; Mahoney, D.; Vezie, D.L. Mechanical and Thermal Properties of Dragline Silk from the Spider Nephila Clavipes. Polym. Adv. Technol. 1994, 5, 401–410.
- Nazarov, R.; Jin, H.-J.; Kaplan, D.L. Porous 3-D Scaffolds from Regenerated Silk Fibroin. Biomacromolecules 2004, 5, 718–726.
- Wu, Y.-X.; Ma, H.; Wang, J.-L.; Qu, W. Production of Chitosan Scaffolds by Lyophilization or Electrospinning: Which Is Better for Peripheral Nerve Regeneration? Neural Regen. Res. 2021, 16, 1093.
- Kim, H.J.; Kim, H.S.; Matsumoto, A.; Chin, I.-J.; Jin, H.-J.; Kaplan, D.L. Processing Windows for Forming Silk Fibroin Biomaterials into a 3D Porous Matrix. Aust. J. Chem. 2005, 58, 716–720.
- Li, L.; Yang, H.; Li, X.; Yan, S.; Xu, A.; You, R.; Zhang, Q. Natural Silk Nanofibrils as Reinforcements for the Preparation of Chitosan-Based Bionanocomposites. Carbohydr. Polym. 2021, 253, 117214.
- Nazeer, M.A.; Yilgor, E.; Yilgor, I. Electrospun Polycaprolactone/Silk Fibroin Nanofibrous Bioactive Scaffolds for Tissue Engineering Applications. Polymer 2019, 168, 86–94.
- Wang, X.; Kim, H.J.; Xu, P.; Matsumoto, A.; Kaplan, D.L. Biomaterial Coatings by Stepwise Deposition of Silk Fibroin. Langmuir 2005, 21, 11335–11341.
- She, Z.; Jin, C.; Huang, Z.; Zhang, B.; Feng, Q.; Xu, Y. Silk Fibroin/Chitosan Scaffold: Preparation, Characterization, and Culture with HepG2 Cell. J. Mater. Sci. Mater. Med. 2008, 19, 3545–3553.
- Zhang, X.; Reagan, M.R.; Kaplan, D.L. Electrospun Silk Biomaterial Scaffolds for Regenerative Medicine. Adv. Drug Deliv. Rev. 2009, 61, 988–1006.
- Tyona, M.D. A Theoritical Study on Spin Coating Technique. Adv. Mater. Res. 2013, 2, 195–208.
- Norrman, K.; Ghanbari-Siahkali, A.; Larsen, N.B. 6 Studies of Spin-Coated Polymer Films. Annu. Rep. Sect. C Phys. Chem. 2005, 101, 174–201.
- Figallo, E.; Flaibani, M.; Zavan, B.; Abatangelo, G.; Elvassore, N. Micropatterned Biopolymer 3D Scaffold for Static and Dynamic Culture of Human Fibroblasts. Biotechnol. Prog. 2007, 23, 210–216.
- Shah, A.M.; Yu, M.; Nakamura, Z.; Ciciliano, J.; Ulman, M.; Kotz, K.; Stott, S.L.; Maheswaran, S.; Haber, D.A.; Toner, M. Biopolymer System for Cell Recovery from Microfluidic Cell Capture Devices. Anal. Chem. 2012, 84, 3682–3688.
- Kargl, R.; Mohan, T.; Köstler, S.; Spirk, S.; Doliška, A.; Stana-Kleinschek, K.; Ribitsch, V. Functional Patterning of Biopolymer Thin Films Using Enzymes and Lithographic Methods. Adv. Funct. Mater. 2013, 23, 308–315.
- Qiu, S.; Xu, X.; Zeng, L.; Wang, Z.; Chen, Y.; Zhang, C.; Li, C.; Hu, J.; Shi, T.; Mai, Y.; et al. Biopolymer Passivation for High-Performance Perovskite Solar Cells by Blade Coating. J. Energy Chem. 2021, 54, 45–52.
- Sampaio, S.; Taddei, P.; Monti, P.; Buchert, J.; Freddi, G. Enzymatic Grafting of Chitosan onto Bombyx Mori Silk Fibroin: Kinetic and IR Vibrational Studies. J. Biotechnol. 2005, 116, 21–33.
- Srbová, J.; Slováková, M.; Křípalová, Z.; Žárská, M.; Špačková, M.; Stránská, D.; Bílková, Z. Covalent Biofunctionalization of Chitosan Nanofibers with Trypsin for High Enzyme Stability. React. Funct. Polym. 2016, 104, 38–44.
- Ravi Kumar, M.N.V. A Review of Chitin and Chitosan Applications. React. Funct. Polym. 2000, 46, 1–27.
- Hsu, S.; Whu, S.W.; Tsai, C.-L.; Wu, Y.-H.; Chen, H.-W.; Hsieh, K.-H. Chitosan as Scaffold Materials: Effects of Molecular Weight and Degree of Deacetylation. J. Polym. Res. 2004, 11, 141–147.
- Huang, M.; Khor, E.; Lim, L.-Y. Uptake and Cytotoxicity of Chitosan Molecules and Nanoparticles: Effects of Molecular Weight and Degree of Deacetylation. Pharm. Res. 2004, 21, 344–353.
- Minagawa, T.; Okamura, Y.; Shigemasa, Y.; Minami, S.; Okamoto, Y. Effects of Molecular Weight and Deacetylation Degree of Chitin/Chitosan on Wound Healing. Carbohydr. Polym. 2007, 67, 640–644.
- Guibal, E. Interactions of Metal Ions with Chitosan-Based Sorbents: A Review. Sep. Purif. Technol. 2004, 38, 43–74.
- Pinto, R.V.; Gomes, P.S.; Fernandes, M.H.; Costa, M.E.V.; Almeida, M.M. Glutaraldehyde-Crosslinking Chitosan Scaffolds Reinforced with Calcium Phosphate Spray-Dried Granules for Bone Tissue Applications. Mater. Sci. Eng. C 2020, 109, 110557.
- Mikhailov, S.N.; Zakharova, A.N.; Drenichev, M.S.; Ershov, A.V.; Kasatkina, M.A.; Vladimirov, L.V.; Novikov, V.V.; Kildeeva, N.R. Crosslinking of Chitosan with Dialdehyde Derivatives of Nucleosides and Nucleotides. Mechanism and Comparison with Glutaraldehyde. Nucleosides Nucleotides Nucleic Acids 2016, 35, 114–129.
- Kasatkina, M.A.; Budantseva, N.A.; Kil’deeva, N.R. Preparation of Biologically Active Film-Forming Materials Based on Polyphosphate-Modified Chitosan. Pharm. Chem. J. 2016, 50, 250–257.
- Nettles, D.L.; Elder, S.H.; Gilbert, J.A. Potential Use of Chitosan as a Cell Scaffold Material for Cartilage Tissue Engineering. Tissue Eng. 2002, 8, 1009–1016.
- Kildeeva, N.; Chalykh, A.; Belokon, M.; Petrova, T.; Matveev, V.; Svidchenko, E.; Surin, N.; Sazhnev, N. Influence of Genipin Crosslinking on the Properties of Chitosan-Based Films. Polymers 2020, 12, 1086.
- Sung, H.-W.; Huang, R.-N.; Huang, L.L.H.; Tsai, C.-C. In Vitro Evaluation of Cytotoxicity of a Naturally Occurring Cross-Linking Reagent for Biological Tissue Fixation. J. Biomater. Sci. Polym. Ed. 1999, 10, 63–78.
- Shanmugam, M.K.; Shen, H.; Tang, F.R.; Arfuso, F.; Rajesh, M.; Wang, L.; Kumar, A.P.; Bian, J.; Goh, B.C.; Bishayee, A.; et al. Potential Role of Genipin in Cancer Therapy. Pharmacol. Res. 2018, 133, 195–200.
- Muzzarelli, R.A.A.; El Mehtedi, M.; Bottegoni, C.; Gigante, A. Physical Properties Imparted by Genipin to Chitosan for Tissue Regeneration with Human Stem Cells: A Review. Int. J. Biol. Macromol. 2016, 93, 1366–1381.
- Gao, L.; Gan, H.; Meng, Z.; Gu, R.; Wu, Z.; Zhang, L.; Zhu, X.; Sun, W.; Li, J.; Zheng, Y.; et al. Effects of Genipin Cross-Linking of Chitosan Hydrogels on Cellular Adhesion and Viability. Colloids Surf. B Biointerfaces 2014, 117, 398–405.
- McGrath, K.; Kaplan, D. Protein-Based Materials; Springer: Boston, MA, USA, 1997.
- Qi, Y.; Wang, H.; Wei, K.; Yang, Y.; Zheng, R.-Y.; Kim, I.S.; Zhang, K.-Q. A Review of Structure Construction of Silk Fibroin Biomaterials from Single Structures to Multi-Level Structures. Int. J. Mol. Sci. 2017, 18, 237.
- Li, L.; Wang, X.; Li, D.; Qin, J.; Zhang, M.; Wang, K.; Zhao, J.; Zhang, L. LBL Deposition of Chitosan/Heparin Bilayers for Improving Biological Ability and Reducing Infection of Nanofibers. Int. J. Biol. Macromol. 2020, 154, 999–1006.
- Minoura, N.; Aiba, S.-I.; Gotoh, Y.; Tsukada, M.; Imai, Y. Attachment and Growth of Cultured Fibroblast Cells on Silk Protein Matrices. J. Biomed. Mater. Res. 1995, 29, 1215–1221.
- Inouye, K.; Kurokawa, M.; Nishikawa, S.; Tsukada, M. Use of Bombyx Mori Silk Fibroin as a Substratum for Cultivation of Animal Cells. J. Biochem. Biophys. Methods 1998, 37, 159–164.
- Sofia, S.; McCarthy, M.B.; Gronowicz, G.; Kaplan, D.L. Functionalized Silk-Based Biomaterials for Bone Formation. J. Biomed. Mater. Res. 2001, 54, 139–148.
- Dal Pra, I.; Freddi, G.; Minic, J.; Chiarini, A.; Armato, U. De Novo Engineering of Reticular Connective Tissue in Vivo by Silk Fibroin Nonwoven Materials. Biomaterials 2005, 26, 1987–1999.
