
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Viktor Umansky | + 902 word(s) | 902 | 2020-04-13 15:07:44 | | | |
| 2 | Vicky Zhou | -37 word(s) | 865 | 2020-10-30 07:40:50 | | | | |
| 3 | Vicky Zhou | -37 word(s) | 865 | 2020-10-30 07:43:38 | | |
Video Upload Options
Despite of melanoma immunogenicity, this tumor develops immune escape mechanisms that stimulate a fast melanoma progression. Such mechanisms include impaired antigen presentation by tumor cells, accumulation of dysfunctional effector T cells and generation of the immunosuppressive TME represented by MDSC, TAN, CAF, TAM, and Treg. Therefore, numerous approaches were developed to reinvigorate the anti-tumor immune response. Recently approved immunotherapies with ICI (anti-PD-1, anti-PD-L1 and anti-CTLA-4 antibodies) have revolutionized the treatment of melanoma. This treatment significantly increased the survival of melanoma patients and provided a durable control of the disease [26,27,28]. However, the response rates to ICI are still restricted. Thus, further efforts should be undertaken to maximize the efficacy of ICI treatment. This aim could be achieved by improving the selection of patients who might benefit from the ICI therapy, by applying early radiological findings and by measuring predictive markers from tumor and liquid biopsies. Furthermore, the combination of different ICI (such as ipilimumab and nivolumab), their combination with targeting of the immunosuppressive TME or with other anti-cancer therapies could significantly improve the efficacy of tumor immunotherapy. Furthermore, targeting of other immune checkpoints (such as LAG-3, TIM-3, TIGIT) and its combination with approved ICI are currently under investigation.
1. Introduction
The concept of cancer immunosurveillance is based on the fact that tumor cells can be recognized and eliminated by immune system [1][2]. Immunogenicity of malignant melanoma is based on a high ultraviolet-driven mutational burden [3]. This leads to the overexpression of tumor specific antigens enabling the formation of the antigen specific immune response [4][5]. However, development of aggressive metastatic melanoma shows that tumors are edited by the immune system, and selected resistant variants could escape the immune control [6][7]. Therefore, several immune-based therapeutic approaches such as vaccination [8], adoptive transfers [9] and immune checkpoint-blockade [10] were applied, aiming at reinvigorating anti-tumor immune response and improving survival of advanced-stage melanoma patients [11].
The most studied negative immune checkpoint molecules and broadly accepted targets for immunotherapy are cytotoxic T lymphocyte-associated protein-4 (CTLA-4) and programmed cell death protein 1 (PD-1). CTLA-4 is upregulated on the T cell surface early during activation in lymph nodes, binds to CD80/CD86 reducing co-stimulation through CD28 and functions as a negative downstream loop for T cell receptor (TCR) signaling [12]. PD-1 interaction with its ligands PD-L1 and PD-L2 inhibits effector T cell functions in peripheral tissues [13]. Playing a pivotal role in the maintenance of self-tolerance under physiological conditions, these checkpoint molecules could be exploited by tumors to evade the immune responses. Hence, inhibiting such interactions could reactivate anti-tumor immune reactions [14]. Moreover, the combination of anti-CTLA-4 and anti-PD-1 antibodies was shown to work synergistically by expanding activated effector CD8 T cells [15][16]. Another approach was shown to implicate the combination of PD-L1-CD80 heterodimerization and the suppression of the CTLA-4/CD80 axis [17]. Currently used antibodies to target CTLA-4 are ipilimumab and tremelimumab, to target PD-1 are nivolumab, pembrolizumab, cemiplimab and to target PD-L1 are atezolizumab and avelumab [14][18][19].
2. Immunotherapy with Checkpoint Inhibitors in Melanoma
Despite of melanoma immunogenicity, this tumor develops immune escape mechanisms that stimulate a fast melanoma progression. Such mechanisms include impaired antigen presentation by tumor cells, accumulation of dysfunctional effector T cells and generation of the immunosuppressive TME represented by MDSC, TAN, CAF, TAM, and Treg. Therefore, numerous approaches were developed to reinvigorate the anti-tumor immune response. Recently approved immunotherapies with ICI (anti-PD-1, anti-PD-L1 and anti-CTLA-4 antibodies) have revolutionized the treatment of melanoma. This treatment significantly increased the survival of melanoma patients and provided a durable control of the disease [20][21][22]. However, the response rates to ICI are still restricted. Thus, further efforts should be undertaken to maximize the efficacy of ICI treatment. This aim could be achieved by improving the selection of patients who might benefit from the ICI therapy, by applying early radiological findings and by measuring predictive markers from tumor and liquid biopsies. Furthermore, the combination of different ICI (such as ipilimumab and nivolumab), their combination with targeting of the immunosuppressive TME or with other anti-cancer therapies could significantly improve the efficacy of tumor immunotherapy. Furthermore, targeting of other immune checkpoints (such as LAG-3, TIM-3, TIGIT) and its combination with approved ICI are currently under investigation (Table 1). Approved ICI, their targets, and targets for combined treatments are summarized in the Figure 1.
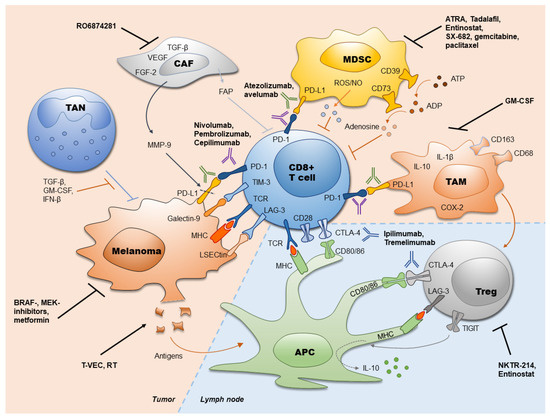
Figure 1. Immune checkpoint inhibitors in melanoma and their combination with other therapies. Currently used antibodies against PD-1 (atezolizumab, avelumab), PD-L1 (nivolumab, pembrolizumab, cepilimumab) and CTLA-4 (ipilimumab, tremelimumab) as well as strategies to increase the efficiency of immune checkpoint inhibitors (ICI) are presented. ADP: adenosine diphosphate; APC: antigen presenting cell; ATP: adenosine triphosphate; ATRA: all-trans retinoic acid; CAF: cancer-associated fibroblasts; COX-2: cyclooxygenase-2; CTLA-4: cytotoxic T lymphocyte-associated protein-4; FAP: fibroblast activation protein; FGF-2: fibroblast growth factor 2; GM-CSF: granulocyte-macrophage colony stimulating factor; IFN-β: interferon-β; IL: interleukin; LAG-3: lymphocyte activation gene-3; LSECtin: liver sinusoidal endothelial cell lectin; MDSC: myeloid-derived suppressor cells; MHC: major histocompatibility complex; MMP-9: matrix metallopeptidase 9; NO: nitric oxide; PD-1: programmed cell death protein 1; PD-L1: programmed cell death ligand 1; ROS: reactive oxygen species; RT: radiation therapy; TAM: tumor-associated macrophages; TAN: tumor associated neutrophils; TCR: T-cell receptor; TGF-β: transforming growth factor-β; TIGIT: T cell immunoreceptor with Ig and ITIM domains; TIM-3: T-cell immunoglobulin- and mucin domain- containing molecule 3; Treg; regulatory T cells; T-VEC: talimogen laherparepvec; VEGF: vascular endothelial growth factor.
References
- Dunn, G.P.; Bruce, A.T.; Ikeda, H.; Old, L.J.; Schreiber, R.D. Cancer immunoediting: From immunosurveillance to tumor escape. Nat. Immunol. 2002, 3, 991–998.
- Ribatti, D. The concept of immune surveillance against tumors. The first theories. Oncotarget 2017, 8, 7175–7180.
- Chalmers, Z.R.; Connelly, C.F.; Fabrizio, D.; Gay, L.; Ali, S.M.; Ennis, R.; Schrock, A.; Campbell, B.; Shlien, A.; Chmielecki, J.; et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017, 9, 34.
- Pitcovski, J.; Shahar, E.; Aizenshtein, E.; Gorodetsky, R. Melanoma antigens and related immunological markers. Crit. Rev. Oncol. Hematol. 2017, 115, 36–49.
- Faramarzi, S.; Ghafouri-Fard, S. Melanoma: A prototype of cancer-testis antigen-expressing malignancies. Immunotherapy 2017, 9, 1103–1113.
- Schreiber, R.D.; Old, L.J.; Smyth, M.J. Cancer immunoediting: Integrating immunity’s roles in cancer suppression and promotion. Science 2011, 331, 1565–1570.
- Ko, J.S. The Immunology of Melanoma. Clin. Lab. Med. 2017, 37, 449–471.
- Ott, P.A.; Hu, Z.; Keskin, D.B.; Shukla, S.A.; Sun, J.; Bozym, D.J.; Zhang, W.; Luoma, A.; Giobbie-Hurder, A.; Peter, L.; et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 2017, 547, 217–221.
- Rohaan, M.W.; Van Den Berg, J.H.; Kvistborg, P.; Haanen, J.B.A.G. Adoptive transfer of tumor-infiltrating lymphocytes in melanoma: A viable treatment option. J. Immunother. Cancer 2018, 6, 102.
- Ottaviano, M.; De Placido, S.; Ascierto, P.A. Recent success and limitations of immune checkpoint inhibitors for cancer: A lesson from melanoma. Virchows Arch. 2019, 474, 421–432.
- Luke, J.J.; Flaherty, K.T.; Ribas, A.; Long, G.V. Targeted agents and immunotherapies: Optimizing outcomes in melanoma. Nat. Rev. Clin. Oncol. 2017, 14, 463–482.
- Sansom, D.M. CD28, CTLA-4 and their ligands: Who does what and to whom? Immunology 2000, 101, 169–177.
- Alsaab, H.O.; Sau, S.; Alzhrani, R.; Tatiparti, K.; Bhise, K.; Kashaw, S.K.; Iyer, A.K. PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: Mechanism, combinations, and clinical outcome. Front. Pharmacol. 2017, 8, 561.
- Gellrich, F.F.; Schmitz, M.; Beissert, S.; Meier, F. Meier Anti-PD-1 and Novel Combinations in the Treatment of Melanoma—An Update. J. Clin. Med. 2020, 9, 223.
- Wei, S.C.; Anang, N.A.A.S.; Sharma, R.; Andrews, M.C.; Reuben, A.; Levine, J.H.; Cogdill, A.P.; Mancuso, J.J.; Wargo, J.A.; Pe’er, D.; et al. Combination anti–CTLA-4 plus anti–PD-1 checkpoint blockade utilizes cellular mechanisms partially distinct from monotherapies. Proc. Natl. Acad. Sci. USA 2019, 116, 22699–22709.
- Gide, T.N.; Quek, C.; Menzies, A.M.; Tasker, A.T.; Shang, P.; Holst, J.; Madore, J.; Lim, S.Y.; Velickovic, R.; Wongchenko, M.; et al. Distinct Immune Cell Populations Define Response to Anti-PD-1 Monotherapy and Anti-PD-1/Anti-CTLA-4 Combined Therapy. Cancer Cell 2019, 35, 238–255.
- Zhao, Y.; Lee, C.K.; Lin, C.H.; Gassen, R.B.; Xu, X.; Huang, Z.; Xiao, C.; Bonorino, C.; Lu, L.F.; Bui, J.D.; et al. PD-L1:CD80 Cis-Heterodimer Triggers the Co-stimulatory Receptor CD28 While Repressing the Inhibitory PD-1 and CTLA-4 Pathways. Immunity 2019, 51, 1059–1073.
- Urwyler, P.; Earnshaw, I.; Bermudez, M.; Perucha, E.; Wu, W.; Ryan, S.; Mcdonald, L.; Karagiannis, S.N.; Taams, L.S.; Powell, N.; et al. Mechanisms of checkpoint inhibition induced adverse events. Clin. Exp. Immunol. 2020.
- Wessely, A.; Steeb, T.; Erdmann, M.; Heinzerling, L.; Vera, J.; Schlaak, M.; Berking, C.; Heppt, M.V. Wessely; Steeb; Erdmann; Heinzerling; Vera; Schlaak; Berking; Heppt The Role of Immune Checkpoint Blockade in Uveal Melanoma. Int. J. Mol. Sci. 2020, 21, 879.
- Hamid, O.; Robert, C.; Daud, A.; Hodi, F.S.; Hwu, W.J.; Kefford, R.; Wolchok, J.D.; Hersey, P.; Joseph, R.; Weber, J.S.; et al. Five-year survival outcomes for patients with advanced melanoma treated with pembrolizumab in KEYNOTE-001. Ann. Oncol. 2019, 30, 582–588.
- Hodi, F.S.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Rutkowski, P.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1480–1492.
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N. Engl. J. Med. 2019, 381, 1535–1546.




