
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Bengu Ozturk | + 2367 word(s) | 2367 | 2021-03-12 03:41:42 | | | |
| 2 | Peter Tang | Meta information modification | 2367 | 2021-03-27 15:03:01 | | |
Video Upload Options
Bioactive lipids, such as fat-soluble vitamins, omega-3 fatty acids, conjugated linoleic acids, carotenoids and phytosterols play an important role in boosting human health and wellbeing. These lipophilic substances cannot be synthesized within the human body, and so people must include them in their diet. There is increasing interest in incorporating these bioactive lipids into functional foods designed to produce certain health benefits, such as anti-inflammatory, antioxidant, anticancer and cholesterol-lowering properties. However, many of these lipids have poor compatibility with food matrices and low bioavailability because of their extremely low water solubility. Nanotechnology is a promising technology that can be used to overcome many of these limitations. Different kinds of nanoscale delivery systems have been designed to encapsulate and protect bioactive lipids, thereby facilitating their handling, stability, food matrix compatibility, and bioavailability.
1. Introduction
Bioactive lipids are oil-soluble compounds that may provide certain health benefits to humans when consumed in appropriate amounts, i.e., doses and frequencies. Depending on their mechanisms of action, they may boost the immune system, reduce inflammation, improve bone health, eye and brain functions, reduce coronary heart diseases, and act as antioxidants and anticarcinogens [1][2][3][4]. Some of the most commonly studied bioactive lipids that may promote human health and well-being are phytosterols (e.g., β-sitosterol, stigmasterol, campesterol), fat soluble vitamins (e.g., vitamins A, D, E, K), carotenoids (e.g., β-carotene, lycopene, lutein, zeaxanthin, astaxanthin) and essential fatty acids (e.g., ALA, EPA, DHA, CLA,) [5][6] (Figure 1). As well as their traditional bioactivities (Section 2), a number of studies have recently shown that consumption of some of these bioactive lipids may also provide protection against human coronavirus SARS-CoV-2. For instance, ingestion of EPA and DHA has been reported to reduce inflammation, cytokine storm and blood coagulation, which might play role an important role in the recovery of patients with Covid-19 infection [7][8][9]. Health professionals have also recommended Covid-19 patients to take vitamin D to modulate respiratory tract disorders, boost immune cell proliferation and activity, and promote pulmonary ACE2 expression mechanisms [10].
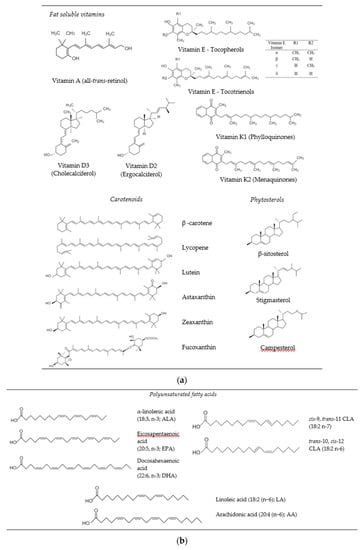
Figure 1. Chemical structures of bioactive lipids: (a) fat soluble vitamins, carotenoids, phytosterols, (b) polyunsaturated fatty acids.
Most bioactive lipids are not synthesized in the human body and so they have to be obtained from food sources, such as nuts, seeds, grains, eggs, meat, fish, tomato, fruits, and vegetables [1][11][12][13]. However, many people do not get enough of these bioactive compounds from their regular diets due to their low dietary levels, tendency to degrade during processing or storage, and their poor stability, solubility, and absorption characteristics within the human gut [14][15][16][17]. The food industry is therefore exploring methods of fortifying foods with stable and bioavailable forms of these bioactive lipids.
Nanotechnology has emerged as a powerful means of encapsulating, protecting, and delivering bioactive substances in foods, thereby improving their efficacy [18][19]. These nanoscale delivery systems should be constructed entirely from food grade ingredients and should be designed to provide resistance against the elevated temperatures, light levels, and oxygen levels they may be exposed to during food processing and storage. They should also be designed to be stable within the specific food matrix that they are utilized within.
2. Bioactive Lipids
2.1. Fat Soluble Vitamins
Fat soluble vitamins are hydrophobic micronutrients that humans must consume to maintain various body functions and ensure health and wellbeing (Figure 1a) [6][20]. Vitamin A (all-trans retinol) is found in a variety of foods, with the chemical form depending on the source. Retinol is mainly found in plant-based foods while retinyl esters are found in animal-based foods. Vitamin A is known to improve visual health, help reproduction, boost the immune system, and promote growth [21]. Vitamin D is a steroid hormone that comes in two major forms: vitamin D2 (ergocalciferol) and D3 (cholecalciferol). In the body, the most bioactive form of vitamin D is 1,25-dihydroxyvitamin D3, which plays an important role in modulating the immune system, calcium metabolism, and the maintenance of bone and teeth health [22]. Vitamin E is comprised of a number of tocopherols (α-, β-, γ-, and δ-tocopherol) and tocotrienols (α-, β-, γ-, and δ-tocotrienol) that are essential for human health and performance. They also act as natural antioxidants and help prevent cancer, heart disease, diabetes, and skin diseases [23]. Vitamin K is a group of hydrophobic compounds (phylloquinones and menaquinones) that all have a methylated naphthoquinone ring system, which are substituted with isoprenoid and isoprene side chains, respectively. Vitamin K is a micronutrient whose ingestion helps prevent blood clotting and modulates bone health [24].
2.2. Carotenoids
Carotenoids are tetraterpenoid lipids that can be divided into two major classes according to their chemical structure: carotenes that are nonoxygenated (e.g., α-carotene, β-carotene, and lycopene) and xanthophylls (e.g., lutein, zeaxanthin, fucoxanthin, and β-cryptoxanthin) that are oxygenated (Figure 1a). Carotenoids are abundant in yellow/orange fruit and vegetables, dark leafy green vegetables, and algae. β-carotene has pro-vitamin A function, since it is converted into vitamin A in the human body through metabolic pathways. Lutein and zeaxanthin retard the progression of age-related cataracts and help to improve cognitive function and cardiovascular health [2][25]. Lycopene has been reported to inhibit breast and prostate cancer, reduced the risk of cardiovascular disease, and retard nerval degenerative diseases [26]. Fucoxanthin can be isolated from seaweed and is claimed to exhibit anticancer, antidiabetic, antioxidant, anti-inflammatory, and antimicrobial activities, was as providing protection against liver, brain, bone, skin, and eye diseases [27][28].
2.3. Phytosterols
Phytosterols (plant sterols and stanols) are important structural components of plant cell membranes (Figure 1a) [12]. Sterols can occur in free form or esterified and glycosylated bound form. The most common forms of phytosterols found in foods are β-sitosterol, stigmasterol and campesterol. Phytosterols have been reported to reduce the risk of inflammatory diseases, as well as arteriosclerosis and cardiovascular diseases by lowering serum LDL-cholesterol concentrations [3][29].
2.4. Essential Fatty Acids (Omega-3s and CLA)
Alpha-linolenic acid (ALA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA) are the major kinds of omega-3 polyunsaturated fatty acids (PUFAs) found in foods (Figure 1b) [11]. Fish oil is the main source of EPA and DHA in the human diet, although algae oil is also an important source of DHA [30]. Consumption of adequate amounts of omega-3 PUFAs has been reported to reduce the risk of cardiovascular diseases, boost neurological and visual functions, promote healthy aging, and improve cognitive functions in Alzheimer patients [4]. ALA is derived from plant seed oils and can be converted to EPA and DHA in vivo, but at a relatively low conversion rate [31]. Consequently, there is interest in fortifying plant-based foods with ALA as a non-animal source of healthy lipids [32]. Conjugated linoleic acids (CLA) are a group of positional and geometric isomers of linoleic acid. The most common bioactive isomers are cis-9, trans-11-CLA (9-CLA) and trans-10, cis-12-CLA (10-CLA), which belong to the n-7 and n-6 family, respectively. In vitro and animal models have shown that CLAs have various potential health benefits, including hypolipidemic, anti-carcinogenic, ostheosynthetic, anti-diabetagenic, and immunomodulatory effects. The major dietary sources of CLA are dairy and meat products from ruminants [33].
3. Nanoscale Delivery Systems for Encapsulation of Bioactive Lipids
Nanoscale delivery systems can be used to improve the handling, stability, and bioavailability of bioactive lipids [16][19][34][35]. For specific applications, nanotechnology has advantages over conventional encapsulation technologies, due to the smaller particle size and greater surface area of the carrier particles employed. Several lipid-based nanoscale delivery systems have been developed and to protect and deliver bioactive lipids, which differ in their compositions, structures, and functionalities (Figure 2) [16][36]. The functional performance of many of these delivery systems can be tailored for specific applications by changing the size, shape, charge, composition, or aggregation state of the nanoparticles they contain (Figure 3). A number of the most widely used nanoscale delivery systems for bioactive lipids are highlighted here.
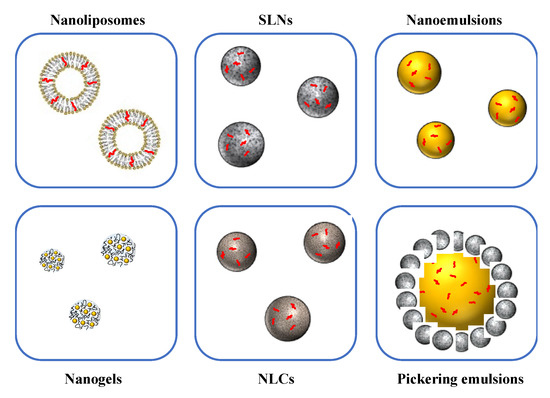
Figure 2. Various kinds of edible nanoparticles are available to encapsulate, protect, and delivery bioactive substances in foods.
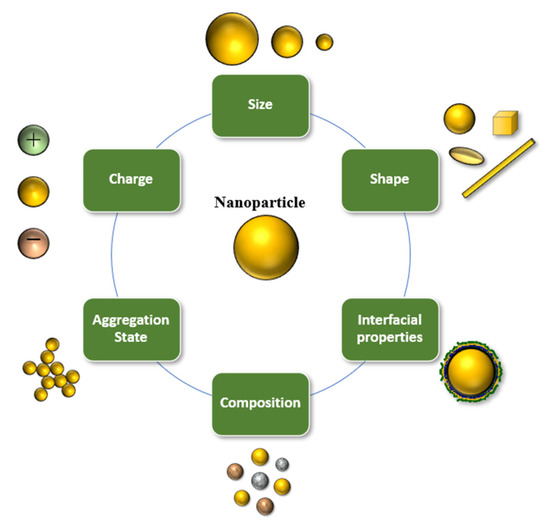
Figure 3. The properties of edible nanoscale delivery systems can be controlled to tailor their functionality.
4. Improved Food Matrix Compatibility and Handling
Encapsulation of bioactive lipids in nanoparticles is widely used to improve their compatibility with different kinds of food matrices. The exterior of these nanoparticles is usually hydrophilic, which means they can be readily dispersed into the aqueous environments found in many foods and beverages, such as soft drinks, milk analogs, yogurts, dressings, sauces, and condiments. Nevertheless, the nanoparticles must also be designed to remain stable under the specific solution conditions found within a particular food product, such as its pH, ionic strength, and ingredient formulation. This means that any repulsive interactions between the nanoparticles, typically electrostatic and/or steric, must be designed to be stronger than any attractive interactions, typically van der Waals, hydrophobic, bridging and/or depletion [37]. This can be achieved by controlling the interfacial composition and structure, particularly the charge, thickness, and polarity of the nanoparticle coating.
Nanotechnology can also be used to improve the handling and storage of bioactive lipids. For instance, the bioactive lipids can be converted into a liquid, gel, powdered, or solid form that can facilitate their transport and utilization during the manufacturing process. Moreover, trapping the bioactive lipids within nanoparticles can improve their resistance to creaming, sedimentation, flocculation, or coalescence during storage, as well as their resistance to chemical degradation, thereby increasing the shelf-life of the food product [38]. Nevertheless, nanoscale delivery systems must be carefully designed to retain the encapsulated bioactive lipids throughout storage, as well as to protect them from the specific environmental stressors they are exposed to, such as pH changes, heating, cooling, shearing, and dehydration. In the remainder of this section, examples of some of the nanoparticle-based systems used to improve the food matrix compatibility or handling of bioactive lipids are given.
Nanoliposomes have been used to incorporate fish oil into yogurts so as to fortify them with health-promoting omega-3 fatty acids [39]. Nanoencapsulation of the fish oils significantly increased the overall acceptance and taste, aroma, texture, and appearance of the yogurts compared utilization of non-encapsulated (free) fish oil. Fish oil has also been encapsulated within caseinate-gum arabic nanocomplexes that were converted into a powdered form and then introduced into fruit juices [40]. Sensory analysis of these omega-3 enriched fruit juices (60 mg EPA+DHA/100 mL) indicated an acceptable taste by consumer panelists, suggesting that nanoencapsulation masked off-flavors from the fish oil. In another study, fish oil was encapsulated within nanoliposomes that were then incorporated into bread, without adversely affecting the texture or sensory acceptability [41]. Conjugated linoleic acid (CLA) has been encapsulated within NLCs that were then added to low-fat milk [42]. Encapsulation was shown to improve the oxidative stability of the CLA, thereby extending the shelf life of the product.
Nanoencapsulation has also been used to improve the food matrix compatibility of various kinds of carotenoids. For instance, astaxanthin has been encapsulated within oil-in-water nanoemulsions that were then successfully introduced into orange juice and milk to form stable carotenoid-fortified products [43]. Zeaxanthin has been encapsulated in biopolymer nanoparticles and nanoemulsions and then incorporated into yogurt [44]. Encapsulation of the carotenoids improved their chemical stability, while having no adverse effects on the appearance, color, flavor, consistency, and texture of the fortified yogurts. Carotenoids extracted from Cantaloupe melon have also been encapsulated in nanoemulsions stabilized by whey proteins and gelatin, which were then incorporated into yogurt [45]. In this case, nanoencapsulation improve the dispersibility and color stability of the carotenoids in the yogurt compared to a crude carotenoid extract. Lycopene has been encapsulated within SLN and NLC systems, which were then successfully introduced into an orange drink without adversely affecting its sensory attributes [46].
Powdered forms of bioactive-loaded nanoparticles can be produced by spray drying, which improves the handling and storage stability. The powders produced can be directly incorporated into dried food products, such as soup mixes, fruit powders, cereal powders, and infant formulas, by simple mixing, or they can be redispersed in water and then incorporated into wet food products, such as beverages, soups, or sauces [47]. Flaxseed oil (56% α-linolenic acid) has been encapsulated within nanocomplexes formulated from high amylose corn starch, which were then converted into a powdered form using spray drying [48]. The resulting powder was then used to fortify bread with omega-3 fatty acids. The encapsulated flaxseed oil had higher oxidative stability than free flaxseed oil during baking, which reduced off-flavor and acrylamide formation.
Vitamin D3 has been encapsulated within NLCs, which were then introduced into a yogurt-based beverage (“Lassi”), without adversely affecting the sensory attributes of the fortified product, such as appearance, flavor, aftertaste, and homogeneity [49]. Plant-based nanoemulsions have been used to encapsulate vitamin D and introduce them into plant-based milk analogs, such as almond and oat milks [50]. The nanoemulsions did not negatively alter the color or viscosity of the fortified milk analogs. Some examples of food and beverages that have been fortified with nanoencapsulated bioactive lipids are provided in Table 1.
Table 1. Effect of nanoencapsulation on applications of bioactive lipids in foods and beverages.
|
Bioactive Lipid |
Nanocarrier System |
Food Application |
Benefits |
Reference |
|---|---|---|---|---|
|
Polyunsaturated omega-3 and omega-6 fatty acids |
||||
|
Flax seed oil (high in ALA) |
Starch nanocomplexes |
Bread |
Reduced lipid oxidation, reduced HMF and acrylamide during baking |
[48] |
|
Fish oil (EPA-DHA) |
Nanoliposomes |
Yogurt |
Increased bioactive retention, reduced lipid oxidation, high sensory score |
[39] |
|
Fish oil (EPA-DHA) |
Sodium caseinate/gum arabic nanocomplexes |
Fruit juice |
Acceptable taste scores, high bioaccessibility |
[40] |
|
Fish oil (EPA-DHA) |
Nanoliposomes |
Bread |
Improved oxidative stability, good textural and sensory quality |
[41] |
|
CLA |
Nanostructured lipid carriers |
Low fat milk |
Improved physical, oxidative and thermal stability |
[42] |
|
Carotenoids |
||||
|
Astaxanthin |
Nanodispersions |
Orange juice, skimmed milk |
Improved storage stability, high in vitro cellular uptake |
[43] |
|
Zeaxanthin |
Nanoparticles, nanoemulsions |
Yogurt |
High storage stability in food matrix, in vitro controlled release |
[44] |
|
Carotenoid extract (high in β-carotene) |
Nanoemulsion |
Yogurt |
Increased dispersibility, high storage stability |
[45] |
|
Lycopene |
Solid lipid nanoparticles, nanostructured lipid carriers |
Orange drink |
Increased dispersibility, better aftertaste scores, high overall acceptance |
[46] |
|
Lycopene |
Nanoemulsion |
Model beverage |
Improved storage stability |
[51] |
|
Fat soluble vitamins |
||||
|
Vitamin D3 |
Nanostructured lipid carrier |
‘Lassi’ Yogurt based beverage |
High sensory acceptance, sustained release in simulated intestinal fluid |
[49] |
|
Vitamin D3 |
Organic nanoparticles (Nanoemulsion, Nanocellulose), inorganic nanoparticle (TiO2) |
Plant-based milks |
Improved viscosity and color |
[50] |
|
Vitamin A, D3 |
Reassembled casein micelle |
Skim milk |
Improved storage stability |
[52] |
|
Vitamin D2 |
Na-caseinate nanocomplexes |
Milk |
Improved storage and thermal stability |
[53] |
|
Vitamin E |
Nanoemulsion |
Fruit juice |
Increased shelf life |
[54] |
|
Phytosterols |
||||
|
β-sitosterol |
Nanostructured lipid carriers |
Butter |
Improved storage stability, high antioxidant activity |
[55] |
|
Phytosterols |
Nanoporous starch aerogels |
Granola bars, pudding |
Improved bioaccessibility |
[56] |
ALA: α-linolenic acid, EPA: eicosapentaenoic acid, DHA: docosahexaenoic acid, CLA: conjugated linoleic acid.
References
- Sankar, R.; Ravisankar, P.; Reddy, A.A.; Nagalakshmi, B.; Koushik, O.S.; Vijaya Kumar, B.; Anvith, P.S. The comprehensive review on fat soluble vitamins. IOSR J. Pharm. 2015, 5, 12–28.
- Eggersdorfer, M.; Wyss, A. Carotenoids in Human Nutrition and Health. Arch. Biochem. Biophys. 2018, 652, 18–26.
- Trautwein, E.A.; Demonty, I. Phytosterols: Natural Compounds with Established and Emerging Health Benefits. Oléagineux Corps Gras Lipides 2007, 14, 259–266.
- Swanson, D.; Block, R.; Mousa, S.A. Omega-3 Fatty Acids EPA and DHA: Health Benefits throughout Life. Adv. Nutr. 2012, 3, 1–7.
- Chen, B.; McClements, D.J.; Decker, E.A. Design of Foods with Bioactive Lipids for Improved Health. Annu. Rev. Food Sci. Technol. 2013, 4, 35–56.
- Vázquez, L.; Corzo-Martínez, M.; Arranz-Martínez, P.; Barroso, E.; Reglero, G.; Torres, C. Bioactive Lipids. In Bioactive Molecules in Food. Reference Series in Phytochemistry; Mérillon, J.M., Ramawat, K.G., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 467–527.
- Das, U.N. Bioactive Lipids in COVID-19-Further Evidence. Arch. Med. Res. 2020.
- Rogero, M.M.; Leão, M.D.C.; Santana, T.M.; Pimentel, M.V.D.M.; Carlini, G.C.; Da Silveira, T.F.; Gonçalves, R.C.; Castro, I.A. Potential Benefits and Risks of Omega-3 Fatty Acids Supplementation to Patients with COVID-19. Free Radic. Biol. Med. 2020, 156, 190–199.
- Bistrian, B.R. Parenteral Fish-Oil Emulsions in Critically Ill COVID-19 Emulsions. J. Parenter. Enter. Nutr. 2020, 44, 1168.
- Meltzer, D.O.; Best, T.J.; Zhang, H.; Vokes, T.; Arora, V.; Solway, J. Association of Vitamin D Status and Other Clinical Characteristics with COVID-19 Test Results. JAMA Netw. Open 2020, 3, e2019722.
- Saini, R.K.; Keum, Y.S. Omega-3 and Omega-6 Polyunsaturated Fatty Acids: Dietary Sources, Metabolism, and Significance—A Review. Life Sci. 2018, 203, 255–267.
- Moreau, R.A.; Nyström, L.; Whitaker, B.D.; Winkler-Moser, J.K.; Baer, D.J.; Gebauer, S.K.; Hicks, K.B. Phytosterols and Their Derivatives: Structural Diversity, Distribution, Metabolism, Analysis, and Health-Promoting Uses. Prog. Lipid Res. 2018, 70, 35–61.
- Maiani, G.; Castón, M.J.P.; Catasta, G.; Toti, E.; Cambrodón, I.G.; Bysted, A.; Granado-Lorencio, F.; Olmedilla-Alonso, B.; Knuthsen, P.; Valoti, M.; et al. Carotenoids: Actual Knowledge on Food Sources, Intakes, Stability and Bioavailability and Their Protective Role in Humans. Mol. Nutr. Food Res. 2009, 53, 194–218.
- Tabibian, M.; Torbati, M.; Mogaddam, M.R.A.; Mirlohi, M.; Sadeghi, M.; Mohtadinia, J. Evaluation of Vitamin D3 and D2 Stability in Fortified Flat Bread Samples during Dough Fermentation, Baking and Storage. Adv. Pharm. Bull. 2017, 7, 323–328.
- Dutta, D.; Chaudhuri, U.R.; Chakraborty, R. Structure, Health Benefits, Antioxidant Property and Processing and Storage of Carotenoids. Afr. J. Biotechnol. 2005, 4, 1510–1520.
- McClements, D.J. Nanoscale Nutrient Delivery Systems for Food Applications: Improving Bioactive Dispersibility, Stability, and Bioavailability. J. Food Sci. 2015, 80, N1602–N1611.
- Salminen, H.; Gömmel, C.; Leuenberger, B.H.; Weiss, J. Influence of Encapsulated Functional Lipids on Crystal Structure and Chemical Stability in Solid Lipid Nanoparticles: Towards Bioactive-Based Design of Delivery Systems. Food Chem. 2016, 190, 928–937.
- Yao, M.; McClements, D.J.; Xiao, H. Improving Oral Bioavailability of Nutraceuticals by Engineered Nanoparticle-Based Delivery Systems. Curr. Opin. Food Sci. 2015, 2, 14–19.
- Shin, G.H.; Kim, J.T.; Park, H.J. Recent Developments in Nanoformulations of Lipophilic Functional Foods. Trends Food Sci. Technol. 2015, 46, 144–157.
- Albahrani, A.A.; Greaves, R.F. Fat-Soluble Vitamins: Clinical Indications and Current Challenges for Chromatographic Measurement. Clin. Biochem. Rev. 2016, 37, 27–47.
- Clagett-Dame, M.; Knutson, D. Vitamin a in Reproduction and Development. Nutrients 2011, 3, 385–428.
- Prietl, B.; Treiber, G.; Pieber, T.R.; Amrein, K. Vitamin D and Immune Function. Nutrients 2013, 5, 2502–2521.
- Borel, P.; Desmarchelier, C. Annual Review of Nutrition Bioavailability of Fat-Soluble Vitamins and Phytochemicals in Humans: Effects of Genetic Variation. Annu. Rev. Nutr. 2018.
- Vermeer, C. Vitamin K: The Effect on Health beyond Coagulation—An Overview. Food Nutr. Res. 2012, 56, 5329.
- Boon, C.S.; McClements, D.J.; Weiss, J.; Decker, E.A. Factors Influencing the Chemical Stability of Carotenoids in Foods. Crit. Rev. Food Sci. Nutr. 2010, 50, 515–532.
- Liang, X.; Ma, C.; Yan, X.; Liu, X.; Liu, F. Advances in Research on Bioactivity, Metabolism, Stability and Delivery Systems of Lycopene. Trends Food Sci. Technol. 2019, 93, 185–196.
- Lourenço-Lopes, C.; Garcia-Oliveira, P.; Carpena, M.; Fraga-Corral, M.; Jimenez-Lopez, C.; Pereira, A.G.; Prieto, M.A.; Simal-Gandara, J. Scientific Approaches on Extraction, Purification and Stability for the Commercialization of Fucoxanthin Recovered from Brown Algae. Foods 2020, 9, 1113.
- Peng, J.; Yuan, J.P.; Wu, C.F.; Wang, J.H. Fucoxanthin, a Marine Carotenoid Present in Brown Seaweeds and Diatoms: Metabolism and Bioactivities Relevant to Human Health. Mar. Drugs 2011, 9, 1806–1828.
- Hannan, M.A.; Sohag, A.A.M.; Dash, R.; Haque, M.N.; Mohibbullah, M.; Oktaviani, D.F.; Hossain, M.T.; Choi, H.J.; Moon, I.S. Phytosterols of Marine Algae: Insights into the Potential Health Benefits and Molecular Pharmacology. Phytomedicine 2020, 69, 153201.
- Ryan, L.; Symington, A.M. Algal-Oil Supplements Are a Viable Alternative to Fish-Oil Supplements in Terms of Docosahexaenoic Acid (22:6n-3; DHA). J. Funct. Foods 2015, 19, 852–858.
- Baker, E.J.; Miles, E.A.; Burdge, G.C.; Yaqoob, P.; Calder, P.C. Metabolism and Functional Effects of Plant-Derived Omega-3 Fatty Acids in Humans. Prog. Lipid Res. 2016, 64, 30–56.
- Sugasini, D.; Lokesh, B.R. Uptake of α-Linolenic Acid and Its Conversion to Long Chain Omega-3 Fatty Acids in Rats Fed Microemulsions of Linseed Oil. Lipids 2012, 47, 1155–1167.
- Benjamin, S.; Spener, F. Conjugated Linoleic Acids as Functional Food: An Insight into Their Health Benefits. Nutr. Metab. 2009, 6, 36.
- Akhavan, S.; Assadpour, E.; Katouzian, I.; Jafari, S.M. Lipid Nano Scale Cargos for the Protection and Delivery of Food Bioactive Ingredients and Nutraceuticals. Trends Food Sci. Technol. 2018, 74, 132–146.
- Assadpour, E.; Mahdi Jafari, S. A Systematic Review on Nanoencapsulation of Food Bioactive Ingredients and Nutraceuticals by Various Nanocarriers. Crit. Rev. Food Sci. Nutr. 2019, 59, 3129–3151.
- Sivakamasundari, S.K.; Leena, M.; Moses, J.A.; Anandharamakrishnan, C. Solid Lipid Nanoparticles: Formulation and Applications in Food Bioactive Delivery. In Innovative Food Processing Technologies; Elsevier: Amsterdam, The Netherlands, 2021; pp. 580–604.
- McClements, D.J. Food Emulsions, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2015; ISBN 9780429154034.
- McClements, D.J.; Rao, J. Food-Grade Nanoemulsions: Formulation, Fabrication, Properties, Performance, Biological Fate, and Potential Toxicity. Crit. Rev. Food Sci. Nutr. 2011, 51, 285–330.
- Ghorbanzade, T.; Jafari, S.M.; Akhavan, S.; Hadavi, R. Nano-Encapsulation of Fish Oil in Nano-Liposomes and Its Application in Fortification of Yogurt. Food Chem. 2017, 216, 146–152.
- Ilyasoglu, H.; El, S.N. Nanoencapsulation of EPA/DHA with Sodium Caseinate-Gum Arabic Complex and Its Usage in the Enrichment of Fruit Juice. LWT Food Sci. Technol. 2014, 56, 461–468.
- Ojagh, S.M.; Hasani, S. Characteristics and Oxidative Stability of Fish Oil Nano-Liposomes and Its Application in Functional Bread. J. Food Meas. Charact. 2018, 12, 1084–1092.
- Hashemi, F.S.; Farzadnia, F.; Aghajani, A.; Ahmadzadeh NobariAzar, F.; Pezeshki, A. Conjugated Linoleic Acid Loaded Nanostructured Lipid Carrier as a Potential Antioxidant Nanocarrier for Food Applications. Food Sci. Nutr. 2020, 8, 4185–4195.
- Anarjan, N.; Tan, C.P. Chemical Stability of Astaxanthin Nanodispersions in Orange Juice and Skimmed Milk as Model Food Systems. Food Chem. 2013, 139, 527–531.
- de Campo, C.; Queiroz Assis, R.; Marques da Silva, M.; Haas Costa, T.M.; Paese, K.; Stanisçuaski Guterres, S.; de Oliveira Rios, A.; Hickmann Flôres, S. Incorporation of Zeaxanthin Nanoparticles in Yogurt: Influence on Physicochemical Properties, Carotenoid Stability and Sensory Analysis. Food Chem. 2019, 301.
- Medeiros, A.K.D.O.C.; de Carvalho Gomes, C.; de Araújo Amaral, M.L.Q.; de Medeiros, L.D.G.; Medeiros, I.; Porto, D.L.; Aragão, C.F.S.; Maciel, B.L.L.; de AraújoMorais, A.H.; SouzaPassos, T. Nanoencapsulation Improved Water Solubility and Color Stability of Carotenoids Extracted from Cantaloupe Melon (Cucumis Melo L.). Food Chem. 2019, 270, 562–572.
- Akhoond Zardini, A.; Mohebbi, M.; Farhoosh, R.; Bolurian, S. Production and Characterization of Nanostructured Lipid Carriers and Solid Lipid Nanoparticles Containing Lycopene for Food Fortification. J. Food Sci. Technol. 2018, 55, 287–298.
- Chopde, S.; Datir, R.; Deshmukh, G.; Dhotre, A.; Patil, M. Nanoparticle Formation by Nanospray Drying & Its Application in Nanoencapsulation of Food Bioactive Ingredients. J. Agric. Food Res. 2020, 2, 100085.
- Gökmen, V.; Mogol, B.A.; Lumaga, R.B.; Fogliano, V.; Kaplun, Z.; Shimoni, E. Development of Functional Bread Containing Nanoencapsulated Omega-3 Fatty Acids. J. Food Eng. 2011, 105, 585–591.
- Maurya, V.K.; Aggarwal, M. Fabrication of Nano-Structured Lipid Carrier for Encapsulation of Vitamin D3 for Fortification of ‘Lassi’; A Milk Based Beverage. J. Steroid Biochem. Mol. Biol. 2019, 193.
- Zhou, H.; Liu, J.; Dai, T.; Muriel Mundo, J.L.; Tan, Y.; Bai, L.; McClements, D.J. The Gastrointestinal Fate of Inorganic and Organic Nanoparticles in Vitamin D-Fortified Plant-Based Milks. Food Hydrocoll. 2021, 112.
- Kim, S.O.; Ha, T.V.A.; Choi, Y.J.; Ko, S. Optimization of Homogenization-Evaporation Process for Lycopene Nanoemulsion Production and Its Beverage Applications. J. Food Sci. 2014, 79.
- Loewen, A.; Chan, B.; Li-Chan, E.C.Y. Optimization of Vitamins A and D3 Loading in Re-Assembled Casein Micelles and Effect of Loading on Stability of Vitamin D3 during Storage. Food Chem. 2018, 240, 472–481.
- Syama, M.A.; Arora, S.; Gupta, C.; Sharma, A.; Sharma, V. Enhancement of Vitamin D 2 Stability in Fortified Milk during Light Exposure and Commercial Heat Treatments by Complexation with Milk Proteins. Food Biosci. 2019, 29, 17–23.
- Dasgupta, N.; Ranjan, S.; Mundra, S.; Ramalingam, C.; Kumar, A. Fabrication of Food Grade Vitamin E Nanoemulsion by Low Energy Approach, Characterization and Its Application. Int. J. Food Prop. 2016, 19, 700–708.
- Bagherpour, S.; Alizadeh, A.; Ghanbarzadeh, S.; Mohammadi, M.; Hamishehkar, H. Preparation and Characterization of Betasitosterol-Loaded Nanostructured Lipid Carriers for Butter Enrichment. Food Biosci. 2017, 20, 51–55.
- Ubeyitogullari, A.; Ciftci, O.N. In Vitro Bioaccessibility of Novel Low-Crystallinity Phytosterol Nanoparticles in Non-Fat and Regular-Fat Foods. Food Res. Int. 2019, 123, 27–35.




