1000/1000
Hot
Most Recent

Despite the significant progress in anticancer drug development over recent years, there is a vital need for newer agents with unique, but still effective, mechanisms of action in order to treat the disease, particularly the highly aggressive and drug resistant types. Alkylating agents, in particular nitrogen-based alkylators, are commonly used to treat hematological and solid malignancies; they exert their antineoplastic effects at all phases of the cell cycle and prevent reproduction of tumor cells. Certain alkylating agents have been designed to be more lipophilic, enabling the compound to penetrate the cell and enhance its alkylating activity against tumors. This review details the evolution of currently available alkylating agents and their profiles, with a focus on nitrogen-based alkylating agents, as important anticancer therapy strategies.
It is a well-known fact that cancer is a major worldwide health issue, threatening human health with increasing incidence and mortality. According to the World Health Organization (WHO), cancer is the second leading cause of deaths globally, after cardiovascular disease [1]. There has been significant progress in the development of anticancer drugs in recent years; however, newer agents require unique, yet effective, mechanisms of action in order to treat cancers, including those cancers that are highly aggressive and drug-resistant [2].
Alkylating agents, which prevent cell reproduction through direct deoxyribonucleic acid (DNA) damage, are widely used for the treatment of hematological and solid malignancies, often in combination with other chemotherapy medicines. For example, combining camptothecin (or a camptothecin derivative) with an alkylating agent, such as melphalan or cyclophosphamide, has been shown to significantly reduce tumor development compared with single-agent use [3]. Alkylating agents are electrophilic and covalently bind to electron-rich functional groups of various targeted molecules. Alkylating agents exert their antineoplastic effects at all phases of the cell cycle and occur through the addition of an alkyl group, most often at the N7 position of guanine residues. This alkyl group addition causes DNA fragmentation, intra- and inter-strand DNA cross-linking, or DNA mutations due to nucleotide mispairing. These mechanisms disrupt DNA replication and eventually result in cell death (or apoptosis) [4]. Nitrogen mustard agents (or nitrogen-based alkylators), and their derivatives, were the first anticancer chemotherapy medicines. They were initially used for malignant lymphoma in the 1940s and are becoming increasingly attractive as a proven antineoplastic agent [2][4]. Over the past eight decades, a large number of compounds, particularly nitrogen mustard-based hybrid molecules, have been developed.
However, overcoming intrinsic and acquired resistance to alkylating agents and other chemotherapeutic drugs continues to be a major challenge, especially when chemoresistance may lead to disease recurrence, metastasis, or death [5][6]. Therefore, it is key to understand the molecular mechanisms at play to improve the overall clinical outcomes for cancer patients and minimize the potential of treatment failure. There are various mechanisms that may trigger alkylating drug resistance, including the action of O6-methylguanine methyltransferase (MGMT), which can reverse DNA methylation damage by removing alkylating lesions [5][7]. Studies have shown that the inhibition of MGMT with O6-benzylguanine promotes the antitumor activity of methylating agents (such as temozolomide) both in vitro and in vivo [7], highlighting this major mechanism of resistance to the cytotoxic effects of alkylating agents. Another major obstacle of anticancer treatment is the lack of site specificity, which may lead to adverse events (AEs) on non-tumor cells. Hence, the development of new alkylating agents has focused on reducing the toxicity observed with the early agents to improve selectivity and cytotoxicity [2][8].
Alkylating agents are broadly classified into nitrogen mustards, nitrosoureas, alkyl sulfonates, triazines, ethylenimines, hydrazines, benzoquinone-containing agents, illudins and platinum-containing agents.
Despite its name, mustard gas is not produced from the mustard plant, but from its chemical substances. Mustard gas in its impure form is yellow-brown in color, with an odor similar to mustard plants. When exposed, mustard gas can cause serious injury to the eyes, skin, and respiratory tract [4]. Sulfur mustards (bis [2-chloroethyl]-sulfide; 1,5-dichloro-3-thiapentane), which are related to the mustard gas group, may cause large skin blisters and, due to their high toxicity, are not suitable for medical use.
Mustard gas was initially developed as a devastating chemical warfare agent in World War I and was subsequently banned as part of the 1925 Geneva Protocol [9]. However, during World War II, a ship explosion resulted in a vast number of military personnel being accidentally exposed to 2000 mustard gas bombs [4][8]. To prevent any further harm at the time, research to discover more efficient agents and protective measures was conducted, and it was found that nitrogen mustard agents were more stable (compared with sulfur mustards) [9]. In 1942, Gilman, Goodman, and co-workers at Yale discovered that bis(2-chloromethyl) methylamine (or chlormethine) possessed anticancer effects in animal models [10], and subsequent clinical trials showed encouraging results. Further clinical trials in patients with terminal malignant diseases resulted in successful outcomes. It is interesting to note that these findings were kept confidential due to the war, and were not disclosed for almost two decades when they were revealed by one of the discoverers [11].
Nitrogen-based alkylators are the most frequently used alkylating agents in cancer therapy [4][12]. Following uptake, the nitrogen-based alkylator is metabolized to a highly reactive aziridinium derivative that alkylates DNA and inhibits DNA reduplication [4]. There are several generations of nitrogen-based alkylating drugs (Table 1, Figure 1).
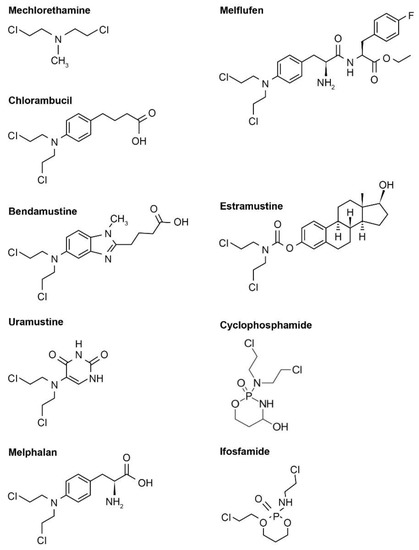
Figure 1. Examples of various nitrogen-based alkylator chemical structures. Note that these chemical structures are publicly available and adapted from online resources (such as ChemDraw).
Table 1. Examples of nitrogen-based alkylators.
|
Generation |
Compound (Chemical Formula) |
First FDA Approval (yr) |
Molecular Mass |
Partition Coefficient (log P) |
Dosage |
|---|---|---|---|---|---|
|
First-generation |
Mechlorethamine (C5H11Cl2N) |
1949 |
192.5 |
−1.24 |
0.4 mg/kg |
|
Second-generation |
Chlorambucil (C14H19Cl2NO2) |
1957 |
304.2 |
3.21 |
0.1–0.2 mg/kg/day |
|
Bendamustine (C16H21Cl2N3O2) |
2008 |
358.3 |
3.09 |
100–120 mg/m2 |
|
|
Melphalan (C13H18Cl2N2O2) |
1964 |
305.2 |
1.79 |
6–10 mg/day |
|
|
Third-generation |
Uramustine (C8H11Cl2N3O2) |
1962 |
252.1 |
1.13 |
150 μg/kg |
|
Melflufen (Melphalan flufenamide; C24H30Cl2FN3O3) |
2020 a |
498.5 |
4.04 |
40 mg (fixed dose) |
|
|
Steroid-coupled mustards |
Estramustine (C23H32Cl2NO6P) |
1981 |
520.4 |
4.97 |
14 mg/kg |
|
Phosphoramide mustards |
Cyclophosphamide (C7H15Cl2N2O2P) |
1959 |
261.1 |
0.63 |
200 mg/day |
|
Ifosfamide (C7H15Cl2N2O2P) |
1987 |
261.1 |
0.86 |
1.2 g/m2 |
a Submitted for approval. Adapted from sources: https://chemoth.com/types/alkylating; https://Chemicalsafety.Com/Sds-Search/; http://www.chemspider.com/; Diethelm-Varela et al. [13] (FDA: US Food and Drug Administration; yr: year).
Among these highly reactive agents, the bis(2-chloroethyl) group is the main chemical constituent, and they all share a common mechanism of action, an in-situ generation of highly electrophilic cyclopropyl through an intramolecular nucleophilic substitution reaction [12][13]. The resulting aziridinium cation serves as the direct alkylating agent and, once formed, it undergoes nucleophilic attack by DNA bases, most often N-7 of guanine, to form a covalent bond (Figure 2). The remaining molecule is important in determining the physical properties of the agent, including transport, distribution, lipophilicity, and reactivity [12]. It is worth noting that a number of agents are prodrugs that have been designed to be selectively activated inside tumor cells. Overall, the underlying evolution of the nitrogen-based alkylator class is the design and development of molecules that are increasingly lipophilic and distributed effectively.
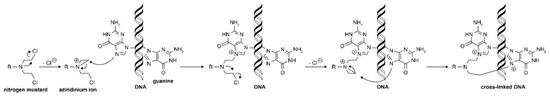
Figure 2. Nitrogen mustard alkylation reaction process. Reprinted (adapted) with permission from [9], Copyright American Chemical Society, 2020.
Alkylating agents, in particular nitrogen mustards, have been important anticancer treatments for many decades, and continue to evolve as researchers seek to overcome drug resistance and lack of selectivity [4][13]. By the late 1950s, approximately 500 nitrogen-based alkylators had been produced, where around 30 had progressed to the clinical trial stage [14]. As a group, alkylating agents are often used in combination with other anticancer drugs. Cyclophosphamide in combination with docetaxel has been demonstrated as an adjuvant therapy in breast cancer patients, with a favorable safety profile and mild AEs reported in postoperative patients [3]. The rationale of conjugating antitumor drugs with nitrogen-based alkylators may present a novel strategy for antitumor agents with enhanced antitumor effect, selectivity, and lowered toxicity (such as brefeldin A, a macrolide antibiotic with an antitumor, antiviral, and antifungal profile) [2].
Lipophilicity is an important physicochemical property in medicinal chemistry and has a major influence on the pharmacokinetics and pharmacodynamics of a drug substance. Some studies have shown a significant increase of lipophilic nature for the N-mustard construct compared with the parent compound [15]. To reduce the toxicity associated with nitrogen mustards, steroids have been tested as a vehicle to deliver the mustard drugs to a specific target tissue via interaction with steroid receptors, and over time, further developed conjugated alkylating agents have shown improved lipophilicity and solubility [2]. By improving lipophilicity, the active metabolite can sufficiently diffuse from its site of formation [3] and enhance certain agent characteristics, including rapid cellular uptake, cytotoxicity, and selectivity. This shift presents these modified agents as potentially novel and exciting alkylators that may improve clinical outcomes, possibly transforming the treatment landscape for patients with malignancies. However, it must be noted that lipophilicity can limit the dissolution of an antitumor agent. For orally administered agents, which need to be readily absorbed, their pharmacokinetic profile is dependent on possessing a hydrophilic–lipophilic balance to ensure that the agent is adequately distributed.
While this article covers currently approved drugs, preclinical and clinical progress has been made in the targeted delivery of nitrogen-based alkylator agents, including DNA-directed nitrogen mustards, antibody-directed enzyme prodrug therapy (ADEPT), gene-directed enzyme prodrug therapy (GDEPT), and CNS-targeted nitrogen mustards. DNA-targeted alkylating agents help to overcome the sequence-specific challenges with the majority of alkylating anticancer agents by using DNA intercalating carriers that improve the binding component of the DNA interaction. ADEPT involves linking an enzyme to an antibody that catalyzes prodrug conversion into the active form. This approach allows the alkylating agent to be cell cycle-independent and less likely to induce acquired resistance. For the GDEPT method, specific genes encoding an enzyme are introduced into cancer cells capable of catalyzing and activating the non-toxic prodrug into cytotoxic forms. Compared to ADEPT, which occurs extracellularly, prodrug activation occurs intracellularly in GDEPT [8]. Strategies that focus on stimuli-responsive (such as high levels of reactive oxygen species [ROS]) nitrogen mustard prodrugs are also gaining traction as anticancer therapies. For example, a ROS-activated nitrogen mustard prodrug encapsulated in liposomes has shown to specifically target and kill hematoma tumor cells [16]. In some cases, DNA carrier molecules have been used to address the loss of drug by reaction limitation associated with mustard alkylators and have been shown to bind intrinsically to the DNA core with both region and sequence specificity [17].