
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Shunsuke FUKUBA | + 2908 word(s) | 2908 | 2021-03-05 06:57:42 | | | |
| 2 | Vivi Li | Meta information modification | 2908 | 2021-03-16 10:30:00 | | |
Video Upload Options
Various bone graft products are commercially available worldwide. However, there is no clear consensus regarding the appropriate bone graft products in different clinical situations. This study is intended to summarize bone graft products, especially alloplastic bone substitutes that are available in multiple countries. It also provides dental clinicians with detailed and accurate information concerning these products. Furthermore, it discusses the prospects of alloplastic bone substitutes based on an analysis of the current market status, as well as a comparison of trends among countries.
1. Introduction
Dental bone graft materials have been commonly used with growth factors and/or barrier membranes in situations such as periodontal regeneration therapies and guided bone regeneration procedures before implant placements [1][2]. In recent years, these materials have also been applied to bone defects caused by peri-implantitis [3][4]. In the early 20th century, autologous bone from intraoral and extraoral sites was commonly used for periodontal and bone regeneration [5][6][7]. Autologous bone is considered the gold standard because it is the only bone graft that has the following three properties: osteogenesis, osteoinduction, and osteoconduction [8][9][10]. Osteogenesis is a property of autologous grafts, whereby new bone is formed by osteoblast cells derived from the graft. Osteoinduction is a property shared among autologous and allogeneic grafts, as well as intrinsic bone matrix proteins (e.g., bone morphogenetic proteins), that involves host stem cell differentiation into osteoblastic cells. Osteoconduction is a mechanical structure property comprising biocompatibility for the migration of osteogenic cells [11][12].
While periodontal and bone regeneration therapies using autologous bone have achieved predictable clinical outcomes, the harvesting of autologous bone graft requires a secondary surgical site (i.e., a donor site) and increases postoperative patient discomfort [13]. To address these problems, alternatives to autologous bone graft materials have been developed. The main advantages of using bone graft substitutes are unlimited availability and reduced morbidity [14][15]. Bone graft substitutes possess structural characteristics and/or chemical compositions similar to those of natural osseous tissue; accordingly, implantation of these substitutes promotes bone formation. Ideally, bone substitutes should be biocompatible (i.e., able to interface with the organism without eliciting an adverse response), osteoinductive, osteoconductive, absorbable (i.e., eventually be completely replaced by host tissues), safe, easy to use, and cost-effective [16]. There are several categories of dental bone graft substitutes such as allogeneic bone, xenogenic bone, and alloplastic materials; each has unique properties.
Allogeneic bone grafts are obtained from different individuals of the same species; these have full osteoconduction and partial osteoinduction capabilities [17]. Allogeneic bone grafts have been widely used and constitute an attractive alternative to autologous bone. Allogeneic bone grafts do not require a donor site or abundant supply, but exhibit variable regenerative abilities due to the absence of information concerning donor conditions (e.g., age and systemic health); they also may carry unknown infectious agents and are the focus of ethical and religious controversies [18][19]. In contrast, xenogenic bone grafts are obtained from different species, typically cattle or pigs; these only possess osteoconduction capability [20]. Xenogenic bone grafts have advantages similar to those of allogeneic bone grafts. However, xenogenic bone grafts carry a risk of infectious disease transmission (e.g., bovine spongiform encephalopathy and Creutzfeldt–Jakob disease); they are also the focus of ethical and religious controversies [21]. Finally, alloplastic bone substitutes are synthetic materials that contain some of the essential chemical components of natural bone (e.g., calcium and phosphate) and are known to promote bone regeneration, although they do not necessarily resemble its natural structure [22][23]. Common advantages of alloplastic bone substitutes are the standardized product quality and absence of infectious disease risk, compared with allogeneic and xenogenic bone grafts [24][25]. Since the regenerative abilities of alloplastic bone substitutes are weak, they are often applied with growth factors and/or membranes [22][25]. The main advantages of alloplastic bone substitutes involve their biological stability and volume maintenance that allow cell infiltration and remodeling [25]. Alloplastic bone substitutes have altered osteoconductive capabilities that depend on their compositions and manufacturing methods, as well as their mechanical properties, crystal structures, pore sizes, porosities, and absorption rates [26][27][28].
2. Discussion
2.1. Properties and Synthetic Routes of Each Composition of Alloplastic Bone Substitutes
The properties of alloplastic bone substitutes are known to vary according to their compositions, as follows.
CP is a generic term that loosely describes various compositions. LeGeros has described the following types of commercially available CP compounds: (1) calcium HA: Ca10(PO4)6(OH)2, either naturally derived (coralline or bovine) or synthetic; (2) β-TCP: Ca3(PO4)2; (3) BCP, consisting of a mixture of β-TCP and HA; and (4) unsintered CPs [29][30].
Pure HA (Ca10(PO4)6(OH)2) is among the least soluble of the CP compounds and is not found in biologic systems [31]. Synthetic HA is prepared by numerous techniques, broadly divided into (1) solid-state chemical reactions or (2) wet reactions. These preparations have distinct sintering temperatures.
β-TCP (β-Ca3[PO4]2) is one of the two polymorphs of TCP. Typically, β-TCP is prepared by sintering calcium-deficient HA to high temperatures [31]. It can be also be prepared at lower temperatures in water-free mediums or by solid-state acid–base chemical interactions.
Bioactive glasses (BGs) are amorphous materials, based on acid oxides (e.g., phosphorus pentoxide), silica (or alumina oxide), and alkaline oxides (e.g., calcium oxide, magnesium oxide, and zinc oxide). BGs possess an interconnective pore system and are available in both compact and porous forms [32]. The bioactivity of the BG surface enables the growth of osseous tissue [33].
CS is the oldest ceramic bone substitute material, first described by Dressman in 1892 for the filling of osseous defects in human patients [34]. Recent studies continue to demonstrate the bone healing properties of CS [35][36]. CS hemihydrate (CaSO4·1/2H2O) powder is hydrated to form CS dihydrate (CaSO4·2H2O), undergoing a slight exothermic reaction to set to a solid form.
The resorption rate of bone grafts is a feature that clinicians consider very important; there is substantial variability among alloplastic materials. HA is known to require a long interval for replacement by native bone due to its low substitution rate [37]. If socket grafting and early re-entry for implant placement is planned, there may be insufficient time for bone formation. Conversely, if the objective is correction of a contour defect (e.g., a buccal defect at a missing tooth site) and the majority of the implant is inserted into native bone for osseointegration, a slowly replaced material will presumably provide long-term space maintenance.
β-TCP is probably best known for its rapid resorption [38]. Lambert et al. compared the healing of rabbit sinuses augmented with xenograft, BCP, and pure β-TCP [39]. Each material supported the formation of new bone, but the bone architecture differed among materials. At 2 months after augmentation, the xenograft had formed an intimate bone bridge between the particles, while the β-TCP graft showed no bone formation. At 6 months after augmentation, there was nothing left in the β-TCP graft. These findings implied more rapid resorption of pure phase β-TCP compared to xenograft and BCP. In another study, Jensen et al. created defects in the mandibles of mini-pigs and grafted them with either autograft, xenograft, or β-TCP; they then harvested bone sections after 1, 2, 4, or 8 weeks [40]. Consistent with the results of other studies, they found that autografts and β-TCP produced slightly more new bone during initial healing (after 4 weeks).
BCP is a combination of two alloplastic materials, generally β-TCP and HA, with ratios adjusted to potentially manipulate their biomedical properties. Cordaro et al. carried out a randomized controlled trial comparing bone healing in grafted human sinuses with either BCP or xenograft at 6 to 8 months after engraftment [38]. The materials differed during later healing, such that less residual synthetic material remained, compared with xenograft material (26.6%). Mahesh et al. grafted human sockets with BGs, then compared bone formation with that achieved using xenografts. Significantly more new bone formed from the BG putty (36–57%) between 4 and 6 months after engraftment. Furthermore, the BG resorbed at approximately 20% per month [41]. Unlike the slower resorbing CP compounds, CS compounds resorb relatively quickly, generally within 8 weeks and certainly by 6 months after engraftment [42].
2.2. Similarities and Differences in the Distributions of Alloplastic Bone Graft Products in Multiple Countries
The characteristics of alloplastic bone graft products in each country are shown in Figure 1. In summary, in Japan, there have been relatively few alloplastic bone substitutes approved by the PMDA. These products mainly consist of HA and β-TCP; none consist of CS, CP, or BGs. Recently, carbonate apatite (CA) and octacalcium phosphate (OCP) have been approved. Notably, human bone is carbonate apatite that contains 6–9% carbonate mass in its apatite structure. A previous study revealed that CA could upregulate osteoblast differentiation and was resorbed by osteoclasts [43][44]. OCP is a material that can be converted to HA in physiological conditions and is considered a mineral precursor to bone apatite crystals [45]. The performance of OCP as a bone substitute differs from that of HA materials in terms of its osteoconductivity and biodegradability. OCP elicits a cellular phagocytic response through osteoclast-like cells, similar to that elicited by the biodegradable material β-TCP [46][47][48]. Thus, CA and OCP may be promising alloplastic bone substitutes. Because of the strictness of PMDA approval, there are few other dental bone graft materials approved for periodontal and bone regeneration in Japan (two xenogenic bone graft and no allogeneic bone graft products), excluding bone graft products indicated for maxillofacial and orthopedic uses. Notably, allogeneic bone graft products are also regulated in most countries in Europe.
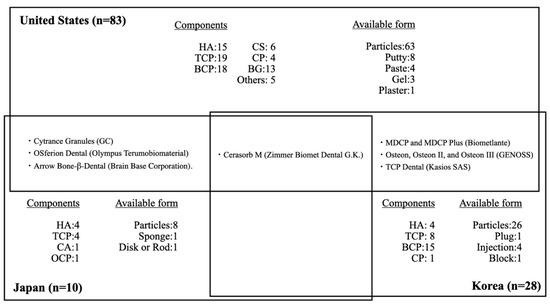
Figure 1. Distribution of dental alloplastic bone substitute products commercially available in the United States, Japan, and Korea. HA = hydroxyapatite, TCP = tricalcium phosphate, BCP = biphasic calcium phosphate, CS = calcium sulfate, CP = calcium phosphate, BG = bioglass, CA = carbonate apatite, OCP = octacalcium phosphate.
Only four kinds of alloplastic bone graft products are approved by both the PMDA and the FDA: Cytrance Granules (GC), OSferion Dental (Olympus Terumobiomaterial), Cerasorb M (Zimmer Biomet Dental G.K.), and Arrow Bone-β-Dental (Brain Base Corporation). Only one alloplastic bone graft product is approved by both the PMDA and the MOHW: Cerasorb M (Zimmer Biomet Dental G.K.). The products available in Japan are indicated mainly for periodontal defects, while only four products are indicated for GBR: Apaceram-AX-Dental (HOYA Technosurgical) for ridge preservation, Neobone (CoorsTek KK) for mineral bone augmentation, and Cytrance Granules (GC) and Bonarc (Toyobo) for GBR. Three of the four products were approved after 2019. Implant treatments after GBR included the sinus lift procedure are also widely performed by Japanese dentists under a self-pay care fee structure, based on clinical evidence and patient consent, using bone graft materials such as allogeneic and xenogenic bone grafts that are not approved by PMDA for off-label use. There were no products approved for the treatment of bone defects derived from peri-implantitis.
Approximately sixfold more alloplastic bone substitute products are approved by the FDA, compared with those approved by the PMDA, despite a previous report that allogeneic bone graft products comprise the major bone graft materials used in the United States. As in Japan, alloplastic bone substitute products approved by the FDA mainly consist of HA and β-TCP, as well as BCP; a few products consist of CS, CP, and BGs. Most alloplastic bone substitute products are indicated for periodontal defects, as well as ridge augmentation, ridge preservation, and sinus lift. Furthermore, 17 products have also been approved for treatment of bone defects derived from peri-implantitis.
A previous study showed that 28 alloplastic bone substitute products were approved by the Korean MHOW through 2019: four HA, eight β-TCP, 15 BCP, and one CP [49]. Approximately four-fold more alloplastic bone substitute products have been approved by the MOHW, compared with those approved by the PMDA. In contrast to Japan, alloplastic bone substitute products approved by the MOHW mainly consist of BCP; none consist of CS or BGs. Most alloplastic bone substitute products are indicated for periodontal defects, as well as ridge augmentation, ridge preservation, and sinus lift. Furthermore, two products have been approved for bone defects derived from peri-implantitis. Only one product, Cerasorb M (Zimmer Biomet Dental G.K.) is approved in both Korea and Japan. Seven alloplastic bone graft products are approved by both the MHOW and the FDA: Cerasorb M (Zimmer Biomet Dental G.K.); MDCP and MDCP Plus (Biometlante); Osteon, Osteon II, and Osteon III (GENOSS); and TCP Dental (Kasios SAS). Compared with Japan and the United States, alloplastic bone graft substitute products that consist of BCP are the main such products in Korea.
Most studies involving clinical randomized controlled trials and split-mouth studies have used similar products: NovaBone (Jacksonville, FL, USA), Curasan (Research Triangle Park, NC, USA), or Biomet (3i) [50]. However, it is difficult to directly compare the product distribution with respect to indications because the descriptions of indications are not standardized among products; there is considerable ambiguity and inconsistency among products in Japan, the United States and Korea. The number of approved products varies among countries and products manufactured by companies tend to be most commonly used in their home countries.
2.3. Alloplastic Bone Graft Products for Periodontal and Bone Regeneration
In the context of periodontal regeneration, bone graft materials are required to increase space in patients with non-contained defects such as one-wall defects and class II furcation involvement [51][52]. Preferably, alloplastic bone substitutes will be completely resorbed. A previous study showed that non-resorbable products such as HA sintered at high temperatures tended not to be used for periodontal regeneration because of concerns that residual bone graft materials may cause long-term inhibition of periodontal tissue formation and weak resistance due to re-infection [22][26][27]. For complete bone substitute resorption, 3–6 months is an appropriate interval considering the speed of bone remodeling and creation of space [53][54][55]. In contrast, materials with slow resorption rates are required in situations involving GBR and sinus lift where robust space creation and primary implant stability are needed [54]. Although autologous bone is generally considered the gold standard, single-use autologous bone is not appropriate for GBR because of its high resorption rate [56]. Selection of a product with a suitable resorption rate is necessary for each clinical situation. We also emphasize that an appropriate surgical procedure should be considered in clinical situations. This procedure may include the concomitant use of alloplastic bone substitutes with growth factors, or the use of alternative surgical techniques such as onlay block grafting and distraction osteogenesis [57].
2.4. Available Forms of Alloplastic Bone Graft Products
The available forms of bone graft materials are mostly particles in the United States, Japan, and Korea. This trend may be changing in Japan. Since 2019, two products—ReFit Dental (HOYA Technosurgical) and Bonarc (Toyobo)—have been approved in sponge, disk, and rod forms to facilitate operability and handling. Materials with these forms are easy to trim to a size suitable for bone defect management and there is no need cause for concern regarding particles scattered around the defect. These products can also be fixed and sutured at the intended position. Furthermore, the efficiency of β-TCP coated with poly lactide-co-glycolide (β-TCP/PLGA) (Easy graft, Sunstar Inc.) has been demonstrated; this product can solidify after it fills in a bone defect, while retaining its shape. The moldable β-TCP/PLGA graft was effective for ridge preservation, while minimizing both linear and volumetric changes after tooth extraction in sockets with buccal bone deficiency in a dog model [58]. The second major available forms of products were putty in the United States and injection in Korea. The forms of alloplastic substitutes are determined by their chemical components and manufacturing methods. With further development of digital dentistry, alloplastic bone substitutes may be manufactured with forms completely fitted to bone defects before surgery [59][60][61][62]. Currently, customized alloplastic block bones are made using computer-aided design and computer-aided manufacturing or three-dimensional (3D) printing. The advantages of 3D printing include reduced material waste, enhanced optimizable surfaces and porous structures, and shorter operation time. Thus, there is great demand for 3D printing technology; many studies have been published concerning 3D printing technology. Although the evidence regarding 3D-printed alloplastic block bone grafts for ridge augmentation is currently limited to animal studies, the concept is very promising [63][64][65].
3. Conclusions
To the best of our knowledge, this is the first descriptive report in the field of dentistry that attempts to identify all currently available alloplastic bone graft products approved for use in periodontal and bone regeneration in multiple countries, including the United States, Japan, and Korea. Detailed and accurate information concerning alloplastic bone products was available from three countries (i.e., the United States, Japan and Korea); trends and current statuses were identified. However, information concerning alloplastic bone products was unavailable in other countries and regions (i.e., the European Union, China). There is limited information available regarding the effectiveness and safety of alloplastic bone substitutes approved for use in dental practice. Overall, various alloplastic bone products are available, but this review could not show clear usage criteria for alloplastic bone graft products used in periodontal and bone regeneration. However, the comprehensive assessments in this study may greatly help dental clinicians and surgeons to understand the properties and indications of each alloplastic bone product. They may also aid in the selection of products in various clinical situations. Further studies (e.g., well-designed randomized controlled trials) are necessary to evaluate the clinical efficacies of dental alloplastic bone substitutes. Those studies should consider the current limited information and develop clinical evidence and guidelines that can benefit clinicians everywhere.
In the near future, alloplastic bone substitutes with high safety and standardized quality may be the first choice, instead of autologous bone, when they exhibit robust osteoconductive and osteoinductive capabilities. These products may be used because of their easier handling, high moldability form, and adequate resorption rate, as well as their abilities to be used with growth factors and/or cell transplantation.
References
- Darby, I. Periodontal materials. Aust. Dent. J. 2011, 56 (Suppl. 1), 107–118.
- Comprehensive periodontal therapy: A statement by the American Academy of Periodontology. J. Periodontol. 2011, 82, 943–949.
- Wen, S.C.; Huang, W.X.; Wang, H.L. Regeneration of Peri-implantitis Infrabony Defects: Report on Three Cases. Int. J. Periodontics Restor. Dent. 2019, 39, 615–621.
- Ramanauskaite, A.; Schwarz, F.; Sader, R.; Becker, J.; Obreja, K. Assessment of peri-implant tissue dimensions following surgical therapy of advanced ligature-induced peri-implantitis defects. Int. J. Implant Dent. 2021, 7, 4.
- Cortellini, P.; Bowers, G.M. Periodontal regeneration of intrabony defects: An evidence-based treatment approach. Int. J. Periodontics Restorative Dent. 1995, 15, 128–145.
- Mellonig, J.T.; Bowers, G.M.; Bright, R.W.; Lawrence, J.J. Clinical evaluation of freeze-dried bone allografts in periodontal osseous defects. J. Periodontol. 1976, 47, 125–131.
- Armitage, G.C. A brief history of periodontics in the United States of America: Pioneers and thought-leaders of the past, and current challenges. Periodontology 2000 2020, 82, 12–25.
- Giannoudis, P.V.; Chris Arts, J.J.; Schmidmaier, G.; Larsson, S. What should be the characteristics of the ideal bone graft substitute? Injury 2011, 42 (Suppl. 2), S1–S2.
- Fernandez de Grado, G.; Keller, L.; Idoux-Gillet, Y.; Wagner, Q.; Musset, A.M.; Benkirane-Jessel, N.; Bornert, F.; Offner, D. Bone substitutes: A review of their characteristics, clinical use, and perspectives for large bone defects management. J. Tissue Eng. 2018, 9, 2041731418776819.
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone tissue engineering: Recent advances and challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408.
- Dimitriou, R.; Tsiridis, E.; Giannoudis, P.V. Current concepts of molecular aspects of bone healing. Injury 2005, 36, 1392–1404.
- Delloye, C.; Cornu, O.; Druez, V.; Barbier, O. Bone allografts: What they can offer and what they cannot. J. Bone Jt. Surg. Br. 2007, 89, 574–579.
- Damien, C.J.; Parsons, J.R. Bone graft and bone graft substitutes: A review of current technology and applications. J. Appl. Biomater. 1991, 2, 187–208.
- Rawashdeh, M.A.; Telfah, H. Secondary alveolar bone grafting: The dilemma of donor site selection and morbidity. Br. J. Oral Maxillofac. Surg. 2008, 46, 665–670.
- Younger, E.M.; Chapman, M.W. Morbidity at bone graft donor sites. J. Orthop. Trauma 1989, 3, 192–195.
- Cypher, T.J.; Grossman, J.P. Biological principles of bone graft healing. J. Foot Ankle Surg. 1996, 35, 413–417.
- Roberts, T.T.; Rosenbaum, A.J. Bone grafts, bone substitutes and orthobiologics: The bridge between basic science and clinical advancements in fracture healing. Organogenesis 2012, 8, 114–124.
- Dalkýz, M.; Ozcan, A.; Yapar, M.; Gökay, N.; Yüncü, M. Evaluation of the effects of different biomaterials on bone defects. Implant Dent. 2000, 9, 226–235.
- Winkler, T.; Sass, F.A.; Duda, G.N.; Schmidt-Bleek, K. A review of biomaterials in bone defect healing, remaining shortcomings and future opportunities for bone tissue engineering: The unsolved challenge. Bone Jt. Res. 2018, 7, 232–243.
- Khan, S.N.; Sandhu, H.S.; Parvataneni, H.K.; Girardi, F.P.; Cammisa, F.P., Jr. Bone graft substitutes in spine surgery. Bull. Hosp. Jt. Dis. 2000, 59, 5–10.
- Schroeder, J.E.; Mosheiff, R. Tissue engineering approaches for bone repair: Concepts and evidence. Injury 2011, 42, 609–613.
- Haugen, H.J.; Lyngstadaas, S.P.; Rossi, F.; Perale, G. Bone grafts: Which is the ideal biomaterial? J. Clin. Periodontol. 2019, 46 (Suppl. 21), 92–102.
- Rosen, P.S.; Reynolds, M.A.; Bowers, G.M. The treatment of intrabony defects with bone grafts. Periodontology 2000 2000, 22, 88–103.
- Stevenson, S.; Emery, S.E.; Goldberg, V.M. Factors affecting bone graft incorporation. Clin. Orthop. Relat. Res. 1996, 324, 66–74.
- Hsu, Y.T.; Wang, H.L. How to Select Replacement Grafts for Various Periodontal and Implant Indications. Clin. Adv. Periodontics 2013, 3, 167–179.
- Jin, Q.M.; Takita, H.; Kohgo, T.; Atsumi, K.; Itoh, H.; Kuboki, Y. Effects of geometry of hydroxyapatite as a cell substratum in BMP-induced ectopic bone formation. J. Biomed. Mater. Res. 2000, 51, 491–499.
- Klenke, F.M.; Liu, Y.; Yuan, H.; Hunziker, E.B.; Siebenrock, K.A.; Hofstetter, W. Impact of pore size on the vascularization and osseointegration of ceramic bone substitutes in vivo. J. Biomed. Mater. Res. A 2008, 85, 777–786.
- Tumedei, M.; Savadori, P.; Del Fabbro, M. Synthetic Blocks for Bone Regeneration: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2019, 20, 4221.
- LeGeros, R.Z. Biodegradation and bioresorption of calcium phosphate ceramics. Clin. Mater. 1993, 14, 65–88.
- LeGeros, R.Z. Properties of osteoconductive biomaterials: Calcium phosphates. Clin. Orthop. Relat. Res. 2002, 395, 81–98.
- Dorozhkin, S.V. Calcium Orthophosphate-Based Bioceramics. Materials (Basel) 2013, 6, 3840–3942.
- Schnürer, S.M.; Gopp, U.; Kühn, K.D.; Breusch, S.J. [Bone substitutes]. Orthopade 2003, 32, 2–10.
- Hollinger, J.O.; Brekke, J.; Gruskin, E.; Lee, D. Role of bone substitutes. Clin. Orthop. Relat. Res. 1996, 324, 55–65.
- Dressmann, H. Ueber knochenplombierung bei hohlenformigen defekten des knochens. Beitr Klin Chir 1892, 9, 804–810.
- Blaha, J.D. Calcium sulfate bone-void filler. Orthopedics 1998, 21, 1017–1019.
- Kelly, C.M.; Wilkins, R.M.; Gitelis, S.; Hartjen, C.; Watson, J.T.; Kim, P.T. The use of a surgical grade calcium sulfate as a bone graft substitute: Results of a multicenter trial. Clin. Orthop. Relat. Res. 2001, 382, 42–50.
- Bosshardt, D.D.; Bornstein, M.M.; Carrel, J.P.; Buser, D.; Bernard, J.P. Maxillary sinus grafting with a synthetic, nanocrystalline hydroxyapatite-silica gel in humans: Histologic and histomorphometric results. Int. J. Periodontics Restorative Dent. 2014, 34, 259–267.
- Cordaro, L.; Bosshardt, D.D.; Palattella, P.; Rao, W.; Serino, G.; Chiapasco, M. Maxillary sinus grafting with Bio-Oss or Straumann Bone Ceramic: Histomorphometric results from a randomized controlled multicenter clinical trial. Clin. Oral Implants Res. 2008, 19, 796–803.
- Lambert, F.; Leonard, A.; Lecloux, G.; Sourice, S.; Pilet, P.; Rompen, E. A comparison of three calcium phosphate-based space fillers in sinus elevation: A study in rabbits. Int. J. Oral Maxillofac. Implants 2013, 28, 393–402.
- Jensen, S.S.; Broggini, N.; Hjørting-Hansen, E.; Schenk, R.; Buser, D. Bone healing and graft resorption of autograft, anorganic bovine bone and beta-tricalcium phosphate. A histologic and histomorphometric study in the mandibles of minipigs. Clin. Oral Implants Res. 2006, 17, 237–243.
- Mahesh, L.; Venkataraman, N.; Shukla, S.; Prasad, H.; Kotsakis, G.A. Alveolar ridge preservation with the socket-plug technique utilizing an alloplastic putty bone substitute or a particulate xenograft: A histological pilot study. J. Oral Implantol. 2015, 41, 178–183.
- Tay, B.K.; Patel, V.V.; Bradford, D.S. Calcium sulfate- and calcium phosphate-based bone substitutes. Mimicry of the mineral phase of bone. Orthop. Clin. N. Am. 1999, 30, 615–623.
- Doi, Y.; Shibutani, T.; Moriwaki, Y.; Kajimoto, T.; Iwayama, Y. Sintered carbonate apatites as bioresorbable bone substitutes. J. Biomed. Mater. Res. 1998, 39, 603–610.
- Brown, W.E. Crystal growth of bone mineral. Clin. Orthop. Relat. Res. 1966, 44, 205–220.
- Suzuki, O.; Shiwaku, Y.; Hamai, R. Octacalcium phosphate bone substitute materials: Comparison between properties of biomaterials and other calcium phosphate materials. Dent. Mater. J. 2020, 39, 187–199.
- Moore, W.R.; Graves, S.E.; Bain, G.I. Synthetic bone graft substitutes. ANZ J. Surg. 2001, 71, 354–361.
- Galea, L.; Alexeev, D.; Bohner, M.; Doebelin, N.; Studart, A.R.; Aneziris, C.G.; Graule, T. Textured and hierarchically structured calcium phosphate ceramic blocks through hydrothermal treatment. Biomaterials 2015, 67, 93–103.
- Ishikawa, K. Bone Substitute Fabrication Based on Dissolution-Precipitation Reactions. Materials (Basel) 2010, 3, 1138–1155.
- Ku, J.K.; Hong, I.; Lee, B.K.; Yun, P.Y.; Lee, J.K. Dental alloplastic bone substitutes currently available in Korea. J. Korean Assoc. Oral Maxillofac. Surg. 2019, 45, 51–67.
- Salem, D.; Natto, Z.; Elangovan, S.; Karimbux, N. Usage of Bone Replacement Grafts in Periodontics and Oral Implantology and Their Current Levels of Clinical Evidence—A Systematic Assessment. J. Periodontol. 2016, 87, 872–879.
- Reynolds, M.A.; Kao, R.T.; Camargo, P.M.; Caton, J.G.; Clem, D.S.; Fiorellini, J.P.; Geisinger, M.L.; Mills, M.P.; Nares, S.; Nevins, M.L. Periodontal regeneration-intrabony defects: A consensus report from the AAP Regeneration Workshop. J. Periodontol. 2015, 86, S105–S107.
- Reddy, M.S.; Aichelmann-Reidy, M.E.; Avila-Ortiz, G.; Klokkevold, P.R.; Murphy, K.G.; Rosen, P.S.; Schallhorn, R.G.; Sculean, A.; Wang, H.L. Periodontal regeneration-furcation defects: A consensus report from the AAP Regeneration Workshop. J. Periodontol. 2015, 86, S131–S133.
- Morand, D.N.; Davideau, J.L.; Clauss, F.; Jessel, N.; Tenenbaum, H.; Huck, O. Cytokines during periodontal wound healing: Potential application for new therapeutic approach. Oral Dis. 2017, 23, 300–311.
- Chappuis, V.; Rahman, L.; Buser, R.; Janner, S.F.M.; Belser, U.C.; Buser, D. Effectiveness of Contour Augmentation with Guided Bone Regeneration: 10-Year Results. J. Dent. Res. 2018, 97, 266–274.
- Sculean, A.; Chapple, I.L.; Giannobile, W.V. Wound models for periodontal and bone regeneration: The role of biologic research. Periodontol. 2000 2015, 68, 7–20.
- Lambert, F.; Léonard, A.; Drion, P.; Sourice, S.; Layrolle, P.; Rompen, E. Influence of space-filling materials in subantral bone augmentation: Blood clot vs. autogenous bone chips vs. bovine hydroxyapatite. Clin. Oral Implants Res. 2011, 22, 538–545.
- Cicciù, M.; Fiorillo, L.; Cervino, G.; Habal, M.B. BMP Application as Grafting Materials for Bone Regeneration in the Craniofacial Surgery: Current Application and Future Directions by an RCT Analysis. J. Craniofac. Surg. 2020.
- Okada, M.; Matsuura, T.; Akizuki, T.; Hoshi, S.; Shujaa Addin, A.; Fukuba, S.; Izumi, Y. Ridge preservation of extraction sockets with buccal bone deficiency using poly lactide-co-glycolide coated β-tricalcium phosphate bone grafts: An experimental study in dogs. J. Periodontol. 2019, 90, 1014–1022.
- Rasperini, G.; Pilipchuk, S.P.; Flanagan, C.L.; Park, C.H.; Pagni, G.; Hollister, S.J.; Giannobile, W.V. 3D-printed Bioresorbable Scaffold for Periodontal Repair. J. Dent. Res. 2015, 94, 153s–157s.
- Park, C.H. Biomaterial-Based Approaches for Regeneration of Periodontal Ligament and Cementum Using 3D Platforms. Int. J. Mol. Sci. 2019, 20, 4364.
- Ma, Y.; Xie, L.; Yang, B.; Tian, W. Three-dimensional printing biotechnology for the regeneration of the tooth and tooth-supporting tissues. Biotechnol. Bioeng. 2019, 116, 452–468.
- Yen, H.H.; Stathopoulou, P.G. CAD/CAM and 3D-Printing Applications for Alveolar Ridge Augmentation. Curr. Oral Health Rep. 2018, 5, 127–132.
- Kim, J.W.; Yang, B.E.; Hong, S.J.; Choi, H.G.; Byeon, S.J.; Lim, H.K.; Chung, S.M.; Lee, J.H.; Byun, S.H. Bone Regeneration Capability of 3D Printed Ceramic Scaffolds. Int. J. Mol. Sci. 2020, 21, 4837.
- Spath, S.; Drescher, P.; Seitz, H. Impact of Particle Size of Ceramic Granule Blends on Mechanical Strength and Porosity of 3D Printed Scaffolds. Materials (Basel) 2015, 8, 4720–4732.
- Lim, H.K.; Hong, S.J.; Byeon, S.J.; Chung, S.M.; On, S.W.; Yang, B.E.; Lee, J.H.; Byun, S.H. 3D-Printed Ceramic Bone Scaffolds with Variable Pore Architectures. Int. J. Mol. Sci. 2020, 21, 6942.




