| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Nagatoshi Nishiwaki | + 873 word(s) | 873 | 2021-03-04 03:55:38 |
Video Upload Options
The ring transformation is a synthetic method for cyclic products including transfer of the partial structure of a cyclic substrate to a reagent, constructing a new ring system. When one substrate and two reagents are used to form a cyclic structure, it is called three-component ring transformation.
1. General Concept of TCRT
1.1. Ring transformation
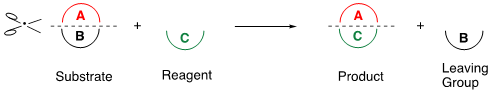
Ring transformation is a powerful synthetic method that accompanies “Scrap & Build” of cyclic compounds. The general concept of this method is shown in Scheme 1. When a substrate (A + B) is reacted with a reagent (C), the partial structure (A) of the substrate is transferred to the reagent to construct a new ring system (A + C), simultaneously eliminating the leaving group (B). This reaction facilitates the synthesis of functionalized compounds that are not easily afforded by alternative procedures.
Scheme 1. General concept of the ring transformation.
There are four types of ring transformations, namely, Diels-Alder-type, decarboxylative, degenerate, and nucleophilic-type ring transformations, among which the last nucleophilic-type ring transformation has not been studied extensively as compared to the other three ring transformations [1][2][3][4][5][6]. 1-methyl-3,5-dinitro-2-pyridone (1) serves as an excellent substrate for this reaction to afford functionalized 4-nitrophenols 3 upon treatment with enolate of 1,3-dicarbonyl compounds 2.
Table 1. Synthesis of functionalized 4-nitrophenols by ring transformation.
|
R1 |
R2 |
|
Solv. |
Temp./°C |
Yield/% |
|
OEt |
COOEt |
a |
pyridine |
50 |
91 |
|
OEt |
H |
b |
pyridine |
70 |
61 |
|
Me |
H |
c |
DMF |
70 |
53 |
|
COOEt |
H |
d |
pyridine |
110 |
42 |
1.2. General concept of TCRT
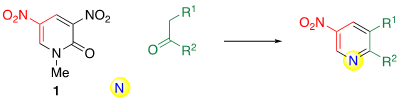
Although 1,3-dicarbonyl compounds 2 are excellent dinucleophilic reagents, only few products 3 are synthesized because of the low diversity of the available 2. If simple ketones 4 can be used instead of 2, the synthetic utility of the ring transformation should be improved. In such cases, it is necessary to use a nitrogen source as ketone is a mononucleophilic reagent. This process is referred to as three-component ring transformation (TCRT) (Scheme 2).
Scheme 2. The general concept of TCRT.
2. Synthesis of nitropyridines by TCRT
2.1. TCRT using ammonia as the nitrogen source
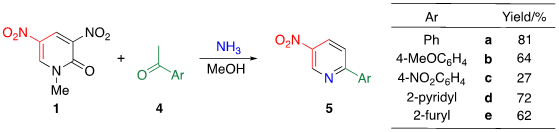
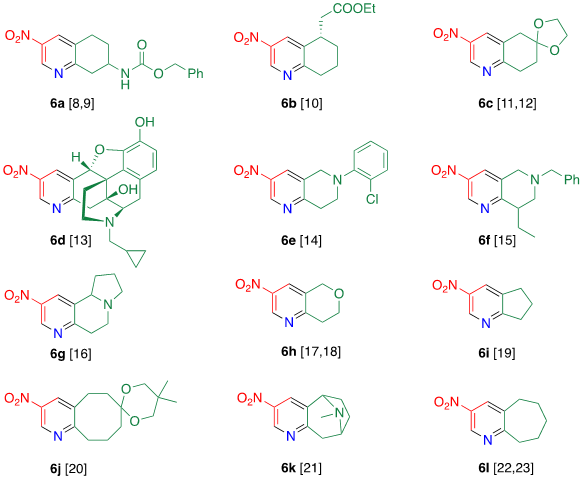
Tohda et al. reported the reaction of dinitropyridine 1 with ketones in the presence of ammonia (Table 2) [7]. When a methanol solution of pyridone 1 is heated with acetophenone 4a in the presence of larger amounts of ammonia (140 equiv.) at 120 °C in an autoclave, TCRT proceeds to afford 3-nitro-6-phenylpyridine 5a in 81% yield. This reaction is applicable to other aromatic ketones 4b–e to afford the corresponding 2-(het)aryl-5-nitropyridines 5b–e, respectively. This TCRT efficiently proceeds under mild conditions to afford [b]-fused 5-nitropyridines 6 only when cyclohexanone is used as the reagent (Figure 1).
Table 2. TCRT using dinitropyridine 1, ketones 4 and ammonia leading to nitropyridines 5.
Figure 1. Condensed nitropyridines [8][9][10][11][12][13][14][15][16][17][18][19][20][21][22][23].
2.2. TCRT using ammonium acetate as the nitrogen source
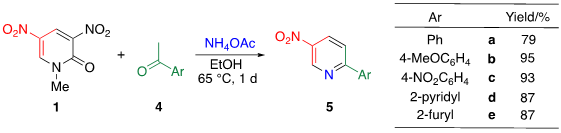
TCRT using ammonia as the nitrogen source is an effective approach to [b]-fused 5-nitropyridines 6. However, when less reactive ketones such as acetophenone 4a are used, both electrophilic sites of 1 are attacked by ammonia, which undergoes ammonolysis to consume pyridone 1 competitively. Le et al. mitigated this problem by using a less nucleophilic ammonium acetate as a nitrogen source instead of ammonia. Even when either electron-rich or -poor ketones 4a–e are used, TCRT efficiently proceeds under mild reaction conditions leading to nitropyridines 5a–e, respectively [24].
Table 3. TCRT with other aromatic ketones 5.
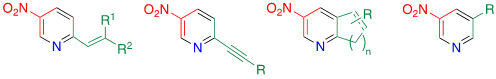
This protocol is applicable to a,b-unsaturated ketones [25], cycloalkanones [26], and aldehydes [24] to afford the corresponding nitropyridines (Figure 2). For the C–C bond formation on the pyridine framework, the Heck, Suzuki, Stille, and Sonogashira reactions are commonly used. However, these methods require the use of poisonous and expensive transition metals and a purification step to avoid metal contamination of the products. In addition, troublesome multistep reactions are necessary to prepare the substrates for these reactions (2-halo-5-nitropyridines). Thus, the TCRT is a metal-free supplementary method for the above-mentioned reactions.
Figure 2. Other types of nitropyridines.
When dinitropyridone 1 is subjected to a reaction with aliphatic ketones 7 in the presence of ammonium acetate, two types of TCRT occur to afford nitropyridines 8 and nitroanilines 9 (Table 4) [27]. Generally, 2,6-disubstituted 4-nitroanilines 9 are prepared from the corresponding anilines by nitration under harsh reaction conditions, wherein protection and deprotection of the amino groups are necessary [28]. Furthermore, the preparation of this compound suffers from limitation of Friedel–Crafts alkylation. There are several limitations for the Friedel–Crafts alkylation, such as the following: (1) the monoalkylated product undergoes further alkylation; (2) it is difficult to introduce two different alkyl groups; (3) primary alkyl groups longer than the ethyl group cannot be introduced; (4) a phenyl group cannot be introduced; and (5) nitrobenzene and aniline do not facilitate the alkylation. The TCRT overcomes these disadvantages.
Table 4. Two kinds of TCRT using aliphatic ketones 7.
|
Ketone |
|
Yield/% |
|||
|
R1 |
R2 |
|
9 |
8 |
8’ |
|
Me |
Me |
a |
83 |
13 |
— |
|
H |
H |
b |
51 |
47 |
— |
|
Et |
H |
c |
66 |
10 |
8 |
|
i-Pr |
H |
d |
58 |
0 |
31 |
|
Pr |
H |
e |
83 |
9 |
6 |
|
Et |
Et |
f |
67 |
24 |
— |
|
Pr |
Pr |
g |
74 |
22 |
— |
|
C6H5 |
Pr |
h |
62 |
24 |
13 |
|
C6H5 |
C6H5 |
i |
8 |
81 |
— |
3. Reaction mechanism of TCRT
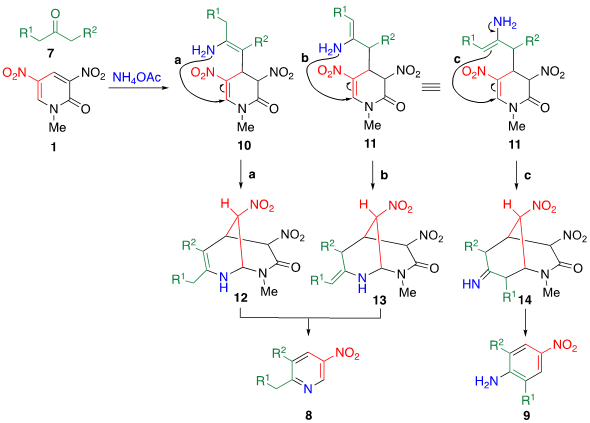
Scheme 2. Plausible mechanisms of TCRT when an aliphatic ketone 7 is employed as a reagent.
A plausible mechanism for this TCRT is shown in Scheme 2. The enol form of 7 attacks the electrophilic 4-position of 1, then the adduct is converted enamines 10 and 11 by ammonium ion. Attack of the amino group attacks at the 6-position (routes a and b) furnishes bicyclic intermediates 12 and 13, from which nitroacetamide is eliminated to afford nitropyridine 8. On the other hand, the C-attack of enamine 11 at the 6-position (route c) leads to bicyclic intermediate 14, which affords nitroaniline 9.
References
- Glinkerman, C.M.; Boger, D.L. Synthesis, Characterization, and Rapid Cycloadditions of 5‐Nitro-1,2,3-triazine. Org. Lett. 2018, 18, 2628–2631.
- Nishiwaki, N.; Sugimoto, R.; Saigo, K.; Kobiro, K. Mechanistic Aspect of Ring Transformations in the Reaction of 5-Nitro-4-pyrimidinone with Acetophenone Derivatives and Cycloalkanones Depending on the Electron Density/Ring Size of the Ketone. Tetrahedron Lett. 2013, 54, 956–959.
- Nishiwaki, N.; Yamashita, K.; Azuma, M.; Adachi, T.; Tamura, M.; Ariga, M. Novel Synthesis of Bihetaryl Compounds. Synthesis 2004, 1996–2000.
- Nishiwaki, N.; Azuma, M.; Tamura, M.; Hori, K.; Tohda, Y.; Ariga, M. Facile Synthesis of Functionalized 4-Aminopyridines. Chem. Commun. 2002, 2170–2171.
- Gromov, S.P. Ring Transformation of Pyridines and Benzo Derivatives under the Action of C-Nucleophiles. Heterocycles 1995, 40, 441–475.
- Rusinov, V.L.; Chupakhin, O.N. Transformations of Nitropyrimidines by Action of C-Nucleophiles. Heterocycles 2000, 53, 1607–1630.
- Tohda, Y.; Eiraku, M.; Nakagawa, T.; Usami, Y.; Ariga, M.; Kawashima, T.; Tani, K.; Watanabe, H.; Mori, Y. The Nucleophilic Reaction upon Electron-Deficient Pyridone Derivatives. X. One-Pot Synthesis of 3-Nitropyridines by Ring Transformation of 1-Methyl-3,5-dinitro-2-pyridone with Ketones or Aldehydes in the Presence of Ammonia. Bull. Chem. Soc. Jpn. 1990, 63, 2820–2827.
- Drescher, K.; Haupt, A.; Unger, L.; Turner, S.C.; Braje, W.; Grandel, R.; Henry, C. Preparation of Aminotetrahydronaphtalenylbenzenesulfonamides and Related Compounds as Modulators of the Dopamine D3 Receptor. PCT Int. Appl. WO 2006040178 (2006).
- Yuan, J.; Han, N.; Yi, H.; Wang, Y.; Yang, S.; Wong, J.C. Preparation of Potent Small molecule inhibitors of Autophagy Useful in Treatment of Cancers and Acute Pancreatitis. PCT Int. Appl. WO 2014145512 (2014).
- Ge, M.; Yang, L.; Zhou, C.; Lin, S.; Cline, E. Preparation of Fused Pyridines as Antidiabetics. PCT Int. Appl. WO 2006083612 (2006).
- Bauta, W.E.; Cantrell, W.R.; Tidwell, M.W. Preparation of Hydroxyimino Tetrahydroquinoline Compounds as Reactivators of Organophosphorous-Inhibited Acetylcholinesterase. US Pat. US 20140350262 (2014).
- Kelly, M.G.; Kaub, C.J.; Kincaid, J.; Janagani, S.; Wu, G.; Wei, Z.-L.; Sahasrabudhe, K.; Duncton, M.; Upasani, R.B.; Fang, Y.; Cox, M. Preparation of Amide Derivatives as Ion-Channel Ligands. PCT Int. Appl. WO 2007100758 (2007).
- Ananthan, S.; Kezar, H.S. III; Saini, S.K.; Khare, N.K.; Davis, P.; Dersch, C.M.; Porreca, F.; Rothman, R.B. Synthesis, Opioid Receptor Binding, and Functional Activity of 5’-Substituted 17-Cyclopropylmethylpyrido[2’,3’:6,7]morphinas. Bioorg. Med. Chem. Lett. 2003, 13, 529–532.
- Guiadeen, D.; Kothandaraman, S.; Yang, L.; Mills, S.G.; MacCoss, M. An Expeditious Synthesis of 3-(Difluoromethoxy)- and 3-(Trifluoromethoxy)-5,6,7,8-tetrahydro-1,6-naphthyridines. Tetrahedron Lett. 2008, 49, 6368–6370.
- Aicher, T.D.; Skalitzky, D.J.; Toogood, P.L.; Vanhuis, C.A. Preparation of Dihydroisoquinoline-2(1H)-carboxamides and Related Compounds and Their Use in Treating Medical Conditions. PCT Int. Appl. WO 2019200120 (2019).
- Coulton, S.; Novelli, R.; Porter, R.; Thompson, M.; Ward, R.W. Preparation of Pyrido[2,1-a]isoquinolines and Pyrrolo[2,1-a]isoquinolines as Anticonsuvulsants.
- Stansfield, I.; Querolle, O.A.G.; Ligny, Y.A.E.; Gross, G.M.; Jacoby, E.; Meerpoel, L.; Green, S.R.; Hynd, G.; Kulagowski, J.J.; Macleod, C.; Mann, S.E. Cyanoindoline Derivatives as NIK Inhibitors and Their Preparation. PCT Int. Appl. WO 2018002219 (2018).
- Takada, S.; Sasatani, T.; Chomei, N.; Adachi, M.; Fujishita, T.; Eigyo, M.; Murata, S.; Kawasaki, K.; Matsushita, A. Synthesis and Structure – Activity Relationship of Fused Imidazopyridines: A New Series of Benzodiazepine Receptor Ligands. J. Med. Chem. 1996, 39, 2844–2851.
- Frank, R.; Christoph, T.; Lesch, B.; Lee, J. Preparation of Substituted Bicyclic Aromatic Carboxamide and Urea Derivatives as Vanilloid Receptor Ligands. PCT Int. Appl. WO 2013013816 (2013).
- Gpff, D.; Zhang, J.; Singh, R.; Holland, S.; Yu, J.; Heckrodt, T.; Ding, P.; Litvak, J. Preparation of Polycyclic Aryl and Heteroaryl Substituted Triazoles as AXL Receptor Tyrosine Kinase Inhibitors. PCT Int. Appl. WO 2009054864 (2009).
- Harling, J.D.; Harrington, F.P.; Thompson, M. A Facile Synthesis of the 3-Amino-5,6,7,8-tetrahydro[1,6]naphthyridine System and Some Alkylated and Polycyclic Homologues. Synth. Commun. 2001, 31, 787–797.
- Vara Prasad, J.V.N.; Boyer, F.E.; Chupak, L.; Dermyer, M.; Ding, Q.; Gavardinas, K.; Hagen, S.E.; Huband, M.D.; Jiao, W.; Kaneko, T.; Maiti, S.N.; Melnick, M.; Romero, K.; Patterson, M.; Wu, X. Synthesis and Structure–Acitivity Studies of Novel Benzocycloheptanone Oxazolidinone Antibacterial Agents. Bioorg. Med. Chem. Lett. 2006, 16, 5392–5397.
- Chupak, L.S.; Kaneko, T.; Josyula, V.PV.N.; Kim, J.-Y.; Choy, A.L.; Hagen, S.E.; Boyer, F.E. Jr. Preparation of Tricyclyl-Substituted Oxazolidinones and Related Compounds as Antibacterial Agents. PCT Int. Appl. WO 2004069832 (2004).
- Le, T.S.; Asahara, H.; Kobiro, K.; Sugimoto, R.; Saigo, K.; Nishiwaki, N. Synthesis of 2-Aryl-5-nitropyridines by Three-Component Ring Transformation of 3,5-Dinitro-2-pyridone. Asian J. Org. Chem. 2014, 3, 297–302.
- Le, T.S.; Asahara, H.; Nishiwaki, N. Metal-Free Synthesis of 2-Alkenyl/Alkynyl-5-nitropyridines Using Three-Component Ring Transformation. Chem. Lett. 2015, 44, 776–778.
- Le, T.S.; Asahara, H.; Nishiwaki, N. An Efficient Synthesis of Nitrated Cycloalka[b]pyridines. Synthesis. 2014, 46, 2175–2178.
- Le, T.S.; Asahara, H.; Nishiwaki, N. Tailor-Made Synthesis of N,N,2,6-Tetrasubstituted 4-Nitroanilines by Three-Component Ring Transformation of Dinitropyridone. Eur. J. Org. Chem. 2015, 1203–1206.
- Carver, F.J.; Hunter, C.A.; Livingstone, D.J.; McCabe, J.F.; Seward, E.M. Substituent Effects on Edge-to-Face Aromatic Interactions. Chem. Eur. J. 2002, 8, 2847–2859.