
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Eduardo Luján-Soto | + 1467 word(s) | 1467 | 2021-02-26 03:56:02 | | | |
| 2 | Peter Tang | Meta information modification | 1467 | 2021-03-07 10:44:31 | | |
Video Upload Options
From embryo development through seedling growth, several molecular pathways control genome stability, environmental signal transduction and the transcriptional landscape.
1. Introduction
Seed is a fundamental entity in the life cycle of higher plants that functions to protect the embryo. It senses environmental cues to couple germination with optimal developmental conditions for the new plant. Timing of seed germination is a central trait controlled by complex network of biochemical and molecular mechanisms. The balance between inactive and germinating states impacts not only the offspring viability but also agronomical and industrial features like crop production and yield, harvesting period and post-harvesting processing. Molecular dissection of seed development phase transitions has revealed cell-signaling pathways, hormonal balance interactions, biotic or abiotic stress effects and biomechanical aspects leading to germination arrest and progression. Lately, attention has been directed to learning from large-scale genome reprogramming, where coordinated expression profiles are required for phase transitions during seed life cycle and are often associated with major changes in chromatin structure. Dynamic transcriptional control is endorsed by interactions between epigenetic effectors. Several mutants with known defects in seed maturation, dormancy and germination correspond to genes involved in chromatin structure, DNA methylation and sRNA pathways, evidencing the importance of epigenetic regulation and chromatin dynamic maintenance during these developmental stages.
2. Seed Dormancy and Germination
2.1. Securing the Future Plant
Several molecular, biochemical and physiological events take place in the embryo and surrounding tissues throughout seed development. These have been grouped into three major stages: embryo development, seed maturation and dormancy (Figure 1). At the late embryogenesis stage, seeds integrate signals and undergo final desiccation to become dormant [1], a state defined as the inability of intact viable seeds to complete germination for a given time period even under optimal environmental conditions [2].
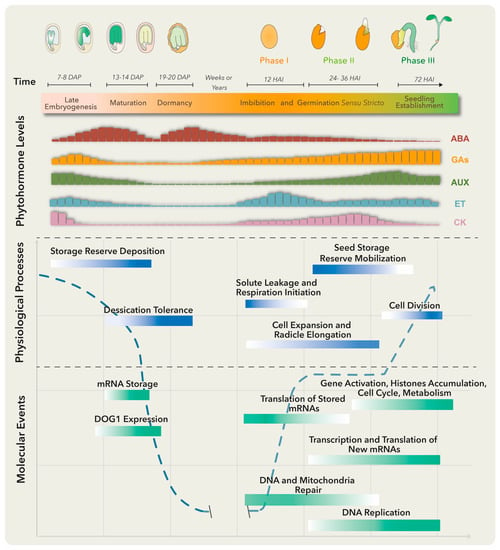
Figure 1. Molecular, biochemical and physiological processes occur from embryo maturation through dormancy to impact on seed germination. The upper panel represents the late seed developmental stages of a dicotiledoneous plant on the left and germination to seedling establishment stages on the right. The lower panels include, from top to bottom, changes in specific phytohormone levels; and physiological processes and molecular events that particularly represent the maturation, dormancy and germination stages. Longer bars refer to higher levels. Abscisic acid (ABA) positively regulates embryo maturation, dormancy induction and maintenance, while gibberellins (GAs) promote release from seed dormancy and germination. Ethylene (ET) production rises after seed soaking with a peak before radicle protrusion. The dashed blue line represents the water level throughout different developmental stages. DAP means days after pollination, and HAI means hours after imbibition.
Primary dormancy, referred as dormancy in this review, is established by endogenous factors and influenced by the mother plant’s growing conditions during seed development. In addition, if nondormant or post-dormant seeds face unfavorable conditions for germination, like high temperatures, a secondary dormancy can be activated even after the seeds have been dispersed [3]. Embryo-induced dormancy relates to embryo immaturity or underdevelopment and/or the synthesis of dormancy promotion compounds by this structure [4]. On the other hand, seed-coat dormancy is conducted by tissues surrounding the embryo, such as endosperm, testa and coleorhiza, to restrain embryo growth, leaking of germination inhibitors, water uptake and radicle emergence [4][5]. Numerous treatments could release dormancy depending on the plant species. These include after-ripening (a certain period of storage at room temperature), cold and warm stratifications and seed treatments with smoke, light or nitrate [6]. Breaking seed dormancy promotes the necessary metabolic, hormonal and molecular conditions for germination (Figure 1).
There are several definitions for germination, the most accepted one defining it as the process starting with seed water uptake (imbibition) and concluding with the successful rupture of covering layers by the radicle [7]. Three phases are commonly distinguished during germination: phase I is characterized by rapid water uptake, seed swelling and reshaping, followed by perturbations in membrane structure and leakage of metabolites; phase II comprises a period of slower and stable water uptake concomitant with initial embryo expansion and covering layers weakening; finally, radicle protrusion (germination) marks the end of phase II and the beginning of phase III (a post germinative stage) distinguished by storage product mobilization from the endosperm to the embryo axis, as well as by triggering a second burst of water uptake and seedling growth [4].
2.2. Phytohormone Interplay for Seedling Success
Plant hormones are required at specific levels through dormancy, dormant state break and germination. Particularly, abscisic acid (ABA) and gibberellins (GAs) are the master regulators of these processes. ABA positively regulates dormancy induction and maintenance, while GAs promote release from seed dormancy and germination (Figure 1). Other phytohormones such as ethylene, cytokinin (CK), brassinosteroids (BRs), auxins (AUX) and jasmonic acid (JA) have been implicated in certain aspects of seed dormancy and/or germination regulation [6].
Before embryo maturation, a first ABA level increase comes mainly from maternal tissues and plays a key role in embryo growth, while during late maturation, a second peak is observed due to ABA supply from zygotic tissues [8]. When the dormant state is perturbed, the ABA level drops and the germination process starts. Treatments that stimulate dormancy loss can also trigger a substantial ABA reduction by activating genes involved in ABA catabolism [4].
The ABA counterplayers, GAs, comprise a group of compounds that regulate diverse plant processes, including germination and plant growth. The amount of bioactive GAs is reduced at stages where ABA peaks by inactivation reactions, to guarantee normal growth and development (Figure 1). GA excess translates into negative effects, like precocious seed germination and pre-harvesting sprouting or viviparity [9], while GA-deficient mutant seeds from Arabidopsis and tomato are unable to germinate unless external GA treatment is applied [10].
Another well-documented hormone involved in seed germination is ethylene (ET). This gaseous hormone plays a key role in seed dormancy release and germination in numerous species [11]. Ethylene antagonizes ABA effects through the regulation of ABA metabolism genes and signaling pathways. Mutants insensitive to ET are hypersensitive to ABA and present extended dormancy periods, while those showing increased ET production exhibit reduced sensitivity to ABA and a decrease in dormancy duration. Furthermore, ET promotes radicle protrusion, germination and seedling establishment by affecting and interacting with GA biosynthesis and signaling pathways [12].
JA and BRs could also impact on seed dormancy and germination. While their role has been poorly explored, evidence suggests that they might affect the ABA/GA balance and action under particular treatments. Briefly, JA exhibits a dual effect on germination depending on plant species. Nondormant Arabidopsis seeds display higher JA levels than dormant seeds and the hormone level further decreases upon seed imbibition [13]. On the other hand, cold stratification of wheat seeds triggers the increase in JA endogenous content, which positively regulates the activity of ABA biosynthesis repressors [14]. BRs could also promote seed germination through repressing ABA signaling in Arabidopsis. Treatment with BRs was able to rescue the low germination phenotype of both GA-biosynthetic and GA-insensitive mutants [15].
2.3. Re-Shaping Quiescent Tissues towards Active Proliferation
To establish a balance between dormancy and germination, a complex regulatory network is built. For dormancy, ABA binds to its receptor proteins and the complex inhibits protein phosphatases 2C (PP2C; negative regulators of dormancy). This allows the activation of SNF1-related protein kinases (SnRK2; positive regulators of dormancy) and downstream target phosphorylation, including transcription factors ABA-insensitive ABI5, ABI3 (B3 family), and ABI4 (Ethylene response factor; ERF family) to activate plant responses [16]. A crosstalk between these and other signaling networks conducts the deposition of storage reserves, acquisition of desiccation tolerance and induction of dormancy. Upon dormancy state breaks in Arabidopsis thaliana, different gene expression programs become activated [17]. Genes involved in translation and protein assembly are upregulated [4][17], together with the transcriptional activation of enzymes involved in DNA replication, nitrogen metabolism, mobilization of storage products, cell wall modification, cytoplasmatic membrane-bound vesicle formation and hormone biosynthesis (Figure 1) [5][18]. Furthermore, changes in the seed proteome take place during dormancy release, comprising proteins involved in translation, cellular signaling, energy metabolism and redox status control [19].
During phase I of germination, protein synthesis is supported by pre-existing mRNA even before transcription re-activation. Stored mRNAs that are associated with single ribosomes in the dry seeds and become translationally upregulated in early germination encode proteins involved in redox reactions, glycolysis and translation [20][21]. Other stored mRNAs, accumulated in response to ABA and other environmental factors in late seed maturation and dormancy, are degraded to prevent translation of proteins that function as germination suppressors [21]. Phase I is also associated with DNA and mitochondria repair, required for the success of the germination process [22]. On the other hand, phase II is characterized by the synthesis of new mitochondria, proteins translated from newly transcribed mRNAs and continuous DNA repair [4]. Finally, phase III requires transcriptional activation of genes representing histone families, cell cycle and metabolic pathways to promote DNA synthesis, cell division and radicle elongation (Figure 1) [23]. Such gene expression program switches are finely regulated by the chromatin architecture and epigenetic modifications.
References
- Srivastava, L.M. Seed Development and Maturation. In Plant Growth and Development: Hormones and Environment; Srivastava, L.M., Ed.; Academic Press: San Diego, CA, USA, 2002; pp. 431–446.
- Gao, F.; Ayele, B.T. Functional genomics of seed dormancy in wheat: Advances and prospects. Front. Plant Sci. 2014, 5, 458.
- Buijs, G. A Perspective on Secondary Seed Dormancy in Arabidopsis thaliana. Plants 2020, 9, 749.
- Tuan, P.A.; Yamasaki, Y.; Kanno, Y.; Seo, M.; Ayele, B.T. Transcriptomics of cytokinin and auxin metabolism and signaling genes during seed maturation in dormant and non-dormant wheat genotypes. Sci. Rep. 2019, 9, 3983.
- Barrero, J.M.; Talbot, M.J.; White, R.G.; Jacobsen, J.V.; Gubler, F. Anatomical and transcriptomic studies of the coleorhiza reveal the importance of this tissue in regulating dormancy in barley. Plant Physiol. 2009, 150, 1006–1021.
- Carrera-Castano, G.; Calleja-Cabrera, J.; Pernas, M.; Gomez, L.; Onate-Sanchez, L. An Updated Overview on the Regulation of Seed Germination. Plants 2020, 9, 703.
- Weitbrecht, K.; Muller, K.; Leubner-Metzger, G. First off the mark: Early seed germination. J. Exp. Bot. 2011, 62, 3289–3309.
- Kanno, Y.; Jikumaru, Y.; Hanada, A.; Nambara, E.; Abrams, S.R.; Kamiya, Y.; Seo, M. Comprehensive hormone profiling in developing Arabidopsis seeds: Examination of the site of ABA biosynthesis, ABA transport and hormone interactions. Plant Cell Physiol. 2010, 51, 1988–2001.
- Ogawa, M.; Hanada, A.; Yamauchi, Y.; Kuwahara, A.; Kamiya, Y.; Yamaguchi, S. Gibberellin biosynthesis and response during Arabidopsis seed germination. Plant Cell 2003, 15, 1591–1604.
- Hauvermale, A.L.; Steber, C.M. GA signaling is essential for the embryo-to-seedling transition during Arabidopsis seed germination, a ghost story. Plant Signal. Behav. 2020, 15, 1705028.
- Corbineau, F.; Xia, Q.; Bailly, C.; El-Maarouf-Bouteau, H. Ethylene, a key factor in the regulation of seed dormancy. Front. Plant Sci. 2014, 5, 539.
- Ahammed, G.J.; Gantait, S.; Mitra, M.; Yang, Y.; Li, X. Role of ethylene crosstalk in seed germination and early seedling development: A review. Plant Physiol. Biochem. 2020, 151, 124–131.
- Preston, J.; Tatematsu, K.; Kanno, Y.; Hobo, T.; Kimura, M.; Jikumaru, Y.; Yano, R.; Kamiya, Y.; Nambara, E. Temporal expression patterns of hormone metabolism genes during imbibition of Arabidopsis thaliana seeds: A comparative study on dormant and non-dormant accessions. Plant Cell Physiol. 2009, 50, 1786–1800.
- Xu, Q.; Truong, T.T.; Barrero, J.M.; Jacobsen, J.V.; Hocart, C.H.; Gubler, F. A role for jasmonates in the release of dormancy by cold stratification in wheat. J. Exp. Bot. 2016, 67, 3497–3508.
- Xi, W.; Liu, C.; Hou, X.; Yu, H. MOTHER OF FT AND TFL1 regulates seed germination through a negative feedback loop modulating ABA signaling in Arabidopsis. Plant Cell 2010, 22, 1733–1748.
- Finkelstein, R. Abscisic Acid synthesis and response. Arab. Book 2013, 11, e0166.
- Buijs, G.; Vogelzang, A.; Nijveen, H.; Bentsink, L. Dormancy cycling: Translation-related transcripts are the main difference between dormant and non-dormant seeds in the field. Plant J. 2020, 102, 327–339.
- Gao, F.; Jordan, M.C.; Ayele, B.T. Transcriptional programs regulating seed dormancy and its release by after-ripening in common wheat (Triticum aestivum L.). Plant Biotechnol. J. 2012, 10, 465–476.
- Gao, F.; Rampitsch, C.; Chitnis, V.R.; Humphreys, G.D.; Jordan, M.C.; Ayele, B.T. Integrated analysis of seed proteome and mRNA oxidation reveals distinct post-transcriptional features regulating dormancy in wheat (Triticum aestivum L.). Plant Biotechnol. J. 2013, 11, 921–932.
- Bai, B.; van der Horst, S.; Cordewener, J.H.G.; America, T.; Hanson, J.; Bentsink, L. Seed-Stored mRNAs that Are Specifically Associated to Monosomes Are Translationally Regulated during Germination. Plant Physiol. 2020, 182, 378–392.
- Sano, N.; Rajjou, L.; North, H.M. Lost in Translation: Physiological Roles of Stored mRNAs in Seed Germination. Plants 2020, 9, 347.
- Paszkiewicz, G.; Gualberto, J.M.; Benamar, A.; Macherel, D.; Logan, D.C. Arabidopsis Seed Mitochondria Are Bioenergetically Active Immediately upon Imbibition and Specialize via Biogenesis in Preparation for Autotrophic Growth. Plant Cell 2017, 29, 109–128.
- An, Y.Q.; Lin, L. Transcriptional regulatory programs underlying barley germination and regulatory functions of Gibberellin and abscisic acid. BMC Plant Biol. 2011, 11, 105.




