Video Upload Options
The fertilised chick egg and particularly its chorioallantoic membrane (CAM) have drawn continuing interest in biomedicine and bioengineering fields, especially for research on vascular study, cancer, drug screening and development, cell factors, stem cells, etc.
1. Introduction
To produce new medications which are safe and efficacious, and can pass all regulatory requirements, pharmaceutical development must proceed through several stages: (1) drug candidate discovery, (2) molecule characterisation, (3) formulation for delivery, (4) pharmacokinetics and biodistribution, (5) efficacy and toxicology testing, (6) investigational new drug (IND) application, (7) bioanalytical testing and (8) phase I–IV clinical trials. The intermediate in vivo preclinical studies of the above stages 4 and 5 are traditionally conducted, by the laboratories of academia or industry, on experimental animals without and with human disease simulation. However, animal welfare and protection are raising more and more public concerns. The currently advocated guiding policy is to replace, reduce, and refine (3 Rs) animal use in research. Therefore, with plausible ethical, scientific, legal and economic reasons, it is necessary to develop scientific methods or platforms to reduce the need for animals or eventually to replace them entirely in scientific research and the pharmaceutical industry. Thus, fertilised eggs or chick embryos have become a good choice.
The egg embryo is considered a good pharmaceutical testing platform with the following advantageous features: (1) the egg embryo is a complete creature, with necessary organs within an isolated environment; (2) the size of the egg embryo is small and easy to handle; (3) the egg embryo contains rich nutrients and vigorous angiogenesis capacities; (4) eggs do not possess a complete immune system at certain stages of chick development and are much less expensive than immune-compromised animals; and (5) eggs are less restricted with animal welfare concerns because they are not considered animals yet.
Embryologically, during the development of fertilised eggs, an extremely rich vascular network is generated between the double layers of the chorioallantoic membrane (CAM). This vascular network fuses closely underneath the eggshell and connects to the embryonic circulation via the allantoic stalk. The CAM system has been widely used in in vivo assays for studying angiogenesis [1] and for human tumour growth and therapies [2]. Indeed, angiogenesis and anti-angiogenesis processes related to tissues, cells or soluble factors are tested by the CAM [2]. Many substances have been reported to boost or inhibit angiogenesis in the CAM, for instance growth factors, anti-cancer agents, pro-angiogenic molecules, natural and synthetic molecules, antibodies, organic-metallic compounds, antibiotics, etc. [3][4][5].
Meanwhile, the classical property of the CAM with a native vasculature already allows testing the pharmacological effects of certain compounds in biomedical research. Small-molecule vascular disrupting agents (VDAs) constitute a new therapy for cancer and can selectively affect the tumour vasculature via some pathways to inhibit blood flow and cause extensive necrosis within the tumour [3][4][5]. Therefore, VDAs are ideal candidates for demonstration of the efficiency and feasibility of the CAM platform in preclinical efficacy and toxicology testing of pharmaceuticals. To the best of our knowledge, only few studies have been conducted in this area.
The crucial step in evaluation is to observe and monitor vascular alterations, including angiogenesis, vascular disrupting processes following administration of tested medicines, etc. Researchers have developed different techniques to measure and quantify these properties in the CAM of the chick embryo. The techniques can be classified into destructive (ex ovo) and non-destructive (in ovo) and qualitative, semi-quantitative and quantitative ones [6][7].
2. CAM Assay
2.1. Development of Fertilised Chick Eggs in Incubation
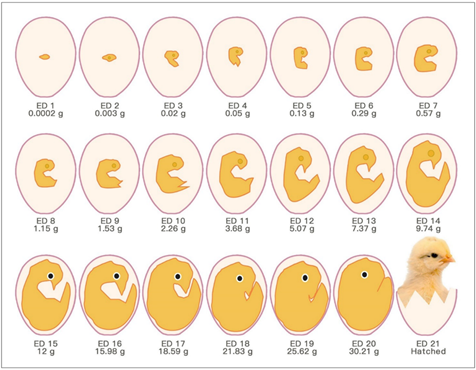
The chick embryo normally experiences 21 days of development before hatching, which corresponds to multiple stages (Figure 1). Usually, the first day of incubation is defined as embryonic day one (ED 1) [8].
Figure 1. Schematic drawings of chronological chick embryo development.
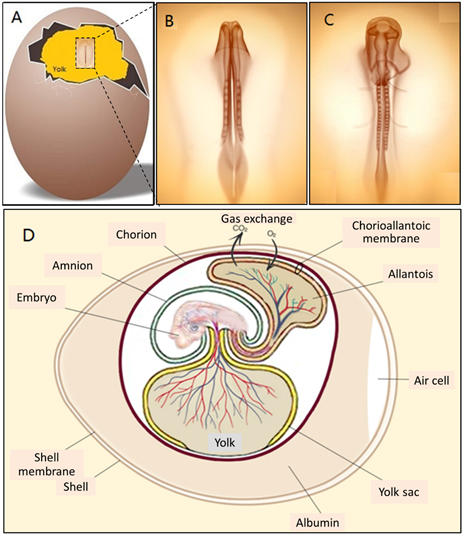
In fact, embryonic development starts in the chick egg before ED 1. The process of blastoderm generation was described long ago [9]. After mating, the female ovum meets with the male sperm cell to form a fertilised cell. Cell divisions initiate around 5 h after insemination, and then a cluster of cells is generated on the surface of the yolk (Figure 2A). This cluster of cells is called the blastoderm, which grows into the embryo (Figure 2B,C). Later, the blastoderm continues to divide into three germ layers: ectoderm, mesoderm and endoderm.
Then, the blastoderm evolves into the embryo as follows: The mesoderm separates into two layers, upper (somatic mesoderm) and lower (splanchnic mesoderm). Spaces between the two layers are surrounded and form the coelom. The ectoderm and the somatic mesoderm compose the somatopleure; the endoderm and the splanchnic mesoderm compose the splanchnopleure. The coelom merges the embryo body with the middle parts of the somatopleure and splanchnopleure.
The extra-embryonic parts grow into different membranes, including the yolk sac, the amnion, the chorion and the allantois. The chorion and the amnion are derived from the extra-embryonic somatopleure, and the yolk sac derives from the extra-embryonic splanchnopleure. The embryo body gets separated from the extra-embryonic tissues, only with the umbilicus connection. The endodermal and ectodermal tissue become epithelial cells of the membranes, and the mesodermal tissue creates the blood from and to this epithelium.
The yolk sac is the membrane enclosing the yolk, and digested yolk can pass into the embryonic blood system through it [10].
The amnion is a sac surrounding the embryo. It can secrete liquid to cushion the embryo and keep it from dehydration [10].
Chorioallantois is the membrane generated at the final stage and is formed by the fusion of the chorion and the allantois. The allantois becomes a balloon-like shape outside the embryo body by ED 4 and begins to fuse with the inside of the chorion by EDs 6–7, forming the chorioallantoic membrane (CAM).
Figure 2. (A) A blastoderm floating on the surface of yolk on ED 2, (B and C) magnified views of the blastoderm in development on ED 2 and (D) illustration of membranes and blood circulation system of an embryonic chick egg in the middle stage.
Three extra-embryonic membranes are there to support and nourish the embryo during growth: the yolk sac, the amnion and the CAM [11].
These membranes feature internal variable structures in eggs during embryo development. The size, morphology and position of the three membranes keep changing. All these changes are aimed to adapt the embryo development and physiological functions.
The extra-embryonic circulation has been distinguished from the intra-embryonic circulation [9]. The vitelline circulation and the allantoic circulation are extra-embryonic circulations. Major blood vessels connect different parts of the embryonic circulation (Figure 2D).
The circulating blood volume of the embryo from ED 4 until ED 18 has been determined (Table 1) [12]. The blood volume does not show an entire perfect curve of exponential growth. The blood volume reaches a peak value between ED 16 and ED 18 and decreases somewhat towards the end of hatching. This volume reduction is related to the degeneration of the extra-embryonic circulation system.
Table 1. Circulating blood volume (ml) in chick embryo on different embryonic days (EDs).
|
ED |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
12 |
14 |
16 |
18 |
|
Blood Volume (mL) |
0.04 |
0.10 |
0.17 |
0.26 |
0.37 |
0.51 |
0.68 |
1.15 |
2.15 |
3.13 |
2.58 |
Maina [13] comprehensively explained how the embryo absorbs vital nutrients from the albumen and the yolk to build new tissues and sustain existing organs during incubation before internal pipping (the chick pecks the CAM and the shell breaks). The calcium deposited in the eggshell provides the necessary source for bone formation in the chick embryo.
2.2. CAM Development and Physiological Function
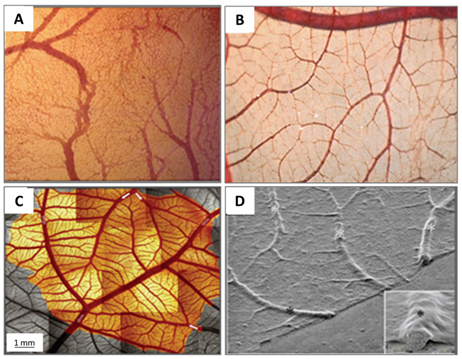
The CAM is a double-layer membrane fused by the chorion and the allantois and has an extremely rich vascular network. The vascular network in the CAM is connected with the embryonic circulation through the allantoic stalk (Figure 3A). The CAM is responsible for embryonic respiration, protecting and nourishing the embryo during most of the chick embryonic development.
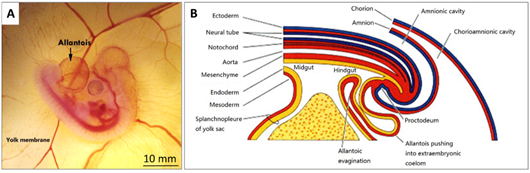
The formation of the allantois is shown in Figure 3B. The allantois of the embryo emerges at about 3.5 embryonic days (ED 3.5). The ventral wall of the endodermal hind gut of the embryo evaginates. This evagination pushes out a part of the embryo body into the extra-embryonic coelom. Its proximal portion (allantoic stalk) lies parallel and just next to the bottom of the yolk sac, and when the distal portion extends away from the embryo, it grows bigger. It is named the allantoic vesicle [14].
Figure 3. (A) A photo of a fertilised chick egg at day 4 post-incubation and (B) a schematic drawing displaying how the allantois is formed.
The allantoic vesicle enlarges very rapidly during EDs 4–10 [14]. As shown in Figure 3A, a chicken embryo at ED 4 forms the vascularised allantoic membrane. The background is the yolk sac membrane (YSM), also highly vascularised [15].
By the end of ED 7, most of the chorion is in contact with the shell membrane [16].
The process to form the CAM has been further described previously. Through the continuous enlargement of the allantoic vesicle, at about 100 h of incubation, the allantois starts to fuse with the chorion, and consequently, the CAM is formed. The CAM consists of three layers (chorion, stroma, allantoic membrane) and lies close to the eggshell (Figure. 4).
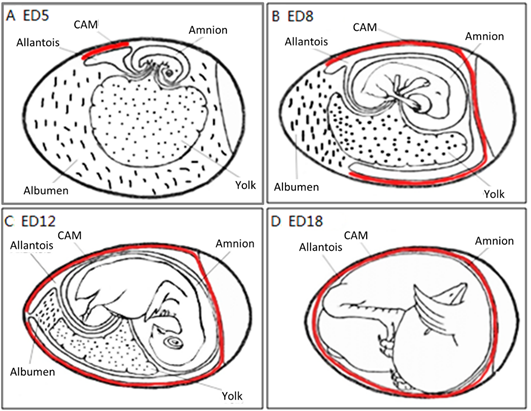
The CAM spreads over the yolk sac surface and covers it completely between ED 6 and ED 7 [16]. This is consistent with Romanoff’s description: around EDs 7–8, the CAM extends throughout the blunt half of the egg and reaches the middle line. He concluded that eventually, the CAM covers the entire egg around EDs 10–11.
As illustrated in Figure 4, the chorioallantois extends to embrace the contents of the whole egg at ED 12, attaching the entire surface of the inner shell membrane [17]. The CAM surface is 6 cm2 at ED 6, undergoing rapid extension, and becomes 65 cm2 at ED 14 [18].
Although the CAM becomes increasingly large, the two extra-embryonic circulations remain separate, but both are connected to the intra-embryonic circulation.
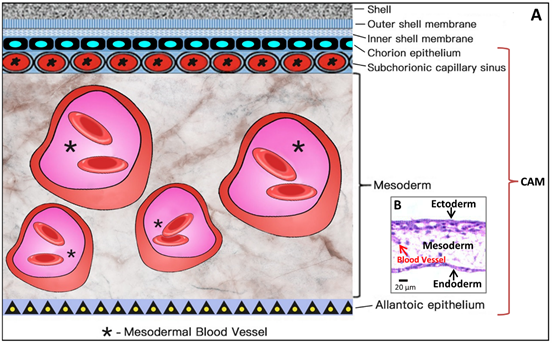
Coming to the end, apoptosis progresses in the CAM at ED 18, and apoptotic cells are found in the mesenchyme. Finally, during internal piping, chick lung ventilation initiates CAM degeneration [19]. The outer and inner shell membranes interface each other with the eggshell, the chorion epithelium and the subchorionic sinuses (Figure 5), which brings capillary blood close to the air via the air cell.
The CAM is a highly vascularised and transparent membrane. Arteries, veins and the capillary plexus exist in the CAM [20]. The chorioallantoic capillary volume and surface size increase during CAM development.
Figure 4. The development of the chorioallantoic membrane (CAM) (red lines) at different stages: (A) ED 5 initial coverage, (B) ED 8 half coverage, (C) ED 12 and (D) ED 18 full coverage of the chick embryo.
The CAM performs the gas-exchange function through the aerial vascular interface, when the chick embryo starts developing before ED 6.
Since the CAM is subjacent to the inner shell membrane at ED 5, this highly vascularised membrane serves as the respiratory system of the embryo and is solely responsible for gas exchange until ED 19 [20]. Here is the mechanism of gas exchange: the respiratory exchange of oxygen and carbon dioxide is a passive diffusion process between the embryo and the environment, the eggshell and the CAM confer a resistance function, and the vasculature in the CAM is correlated with the O2 uptake ability of the embryo in this diffusion process. The general trend of this function is a gradual increase from ED 6 to EDs 14–15, and then a plateau phase [21][22]. The demand for O2 increases throughout embryo development, with increased production of metabolic end products [23].
Meanwhile, the allantois works as a deposit for waste products excreted by embryo kidneys, mostly urea at the early stage and chiefly uric acid at a later stage.
The CAM has other physiological functions: transportation of electrolytes (sodium and chloride) from the allantois and calcium movement from shell to bone for mineralisation. Calcium movement can be certified by typical calcium-transporting cells in the chorionic cavity by ED 12, and the movement rate can reach 100 nmol of calcium per hour per 1 cm2 of the CAM surface [24].
2.3. Microcirculation and Morphology in the CAM
A full understanding of the vascular structure of the CAM is essential to investigate the mechanisms of the vascular response in the CAM assay platform.
The vascular system is the first system built up in the chick embryo in order to facilitate respiration, because oxygen molecules only can diffuse about 100–200 μm in embryonic tissues [25].
As shown in Figure 5, the CAM holds a rich vascular network within its mesodermal layer, and paired allantoic (umbilical) arteries and veins supply blood to this system.
Figure 5. Images illustrating the maturation of the CAM vasculature. (A) Vascular remodelling, growth and anastomosis can be seen at ED 7; (B) hierarchic vascular structures and fully differentiated vessels are noted on ED 10; (C) vessel tree reconstruction and flow direction for major arterial and venous vessels (arrows); and (D) scanning electron microscope view of blood vessels on the CAM.
The different stages of the vascular development process in the CAM present specific characteristics [26]. On ED 4, all vessels remain undifferentiated capillaries, whose walls only have a single endothelial layer without a basal lamina. By ED 8, primary vessels grow and differentiate into an artery–venous system and thus create a capillary network. These small, thin-walled capillaries with a 10–15 µm lumenal diameter migrate and present at the superficial layer of the CAM, which is just beneath the chorionic epithelium (Figure 6).
Meanwhile, as the CAM is expanding, endothelium mitosis undergoes a rapid phase in the capillary network. Concurrently, the capillary endothelium in the CAM undergoes a sequence of structural changes. A capillary network develops via respective angiogenic processes, including sprouting, elongation, fusion to the capillary plexus and intussusceptive growth [27]. The total length of the CAM vessels is closely correlated with the entire area of the CAM [27].
Other vessels located in the mesodermal layer are bigger generally, with a 10–115 µm diameter, and the endothelium of the blood vessels is surrounded by a layer of mesenchymal cells and completely wrapped by a basal lamina (Figure 6).
The capillary plexus is formed and develops quickly from EDs 4–5 by vasculogenesis and sprouting angiogenesis [28]. After short-term sprouting, intussusceptive growth remains dominant and increases density and complexity. Intussusceptive growth is the main process from EDs 7–11, with lower endothelial cell proliferation [29], and the proliferation comes down after ED 11 [30]. Within this network, parts of the vessels become larger and extend laterally to form arteriolar and venular trees [27] (Figure 5C). EDs 10–12 is an important period for vascular development in the CAM. During EDs 10–12, the capillaries become adjacent to the chorionic epithelium (Figure 6).
Figure 6. Simplified schematic diagram (A) showing the structural components of the CAM, which amplifies the embedded microscopic view of the haematoxylin-eosin-stained slice of the CAM (B) where the ectoderm includes the chorion epithelium and sub-chorionic capillary sinus layers of the CAM, and the endoderm corresponds to the allantoic epithelium of the CAM. *Larger vessels in the mesoderm (stroma).
Now, arteries and veins are distinguished in the mesodermal vessels. For example, a piece of 1.7 cm2 CAM contains two major arteries and one major vein. Diameters of the two main arterioles are 261 µm and 172 µm, and the major venule diameter is 390 µm [31]. Arteries have walls containing one or two layers of mesenchymal cells with more connective tissue surrounding the endothelium, tending to develop a fibroblast-type adventitia. Veins have walls surrounded by connective tissues and incomplete mesenchymal cells, which develop into smooth muscle cells. CAM vessels grow rapidly up to ED 11, and then the endothelial cell mitosis dramatically reduces and stays at a minimal growth rate. The density and fractal dimension of the vascular network increase steadily from EDs 6 to 14 and stop at ED 15, for both complexity and branching patterns [32][33].
The successive development of the CAM has been studied [31]. At ED 14, the capillary plexus invades and is located at the surface of the ectoderm underneath the shell membrane. The larger vessels in the mesoderm can float freely and move with the spontaneous movement of the embryo, whereas the capillary plexus is embedded in the most superficial layer of the CAM, as illustrated in Figure 6.
The CAM microcirculation is supplied by two primary arteries and drained by a single vein. These primary vessels connect several vessels from the pre- and post-capillaries of next generations. The left and right allantoic arteries and vein remain apart during the fast growth of the CAM [31]. The interspace of major vessels allows inter-digitating arteriolar and venular trees to grow and let relatively few vessels cross.
In the assay platform to test the response of blood vessels, these large and primary vessels are the best target to be measured and evaluated in quantification by modern imaging techniques (medical and bioengineering).
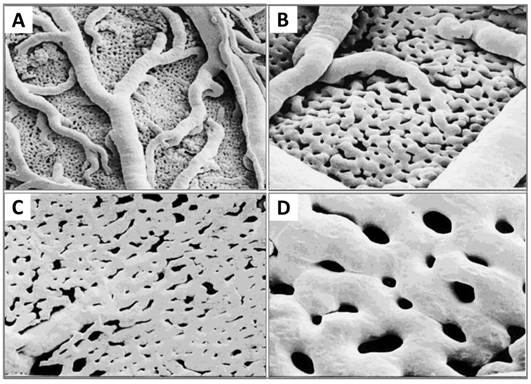
Now that the expansion of the CAM vasculature network is well established, branching patterns and microcirculation of pre- and post-capillaries of the CAM will be studied. The morphology and mechanism of such microcirculation are described in detail. The CAM consists of a superficial 2D network of very dense capillaries, named the capillary plexus, shown by scanning electron microscopy in Figure 7 as a mesh morphology [34]. The capillary mesh wraps a surrounding 3D space. The spatial configuration consists of two horizontal planes, which are connected by pre-capillary bridging vessels. These bridging vessels rise in an oblique-to-vertical direction towards the superficial capillary plane. The medium and large arterial and venous vessels, which supply and drain the superficial layer, are located within this 3D space.
The pre-capillary arterioles connect directly with the capillary network. However, the post-capillary venules connect with the superficial network via the venous sinus system. The small sinus is formed by the confluence of capillaries at the beginning of the post-capillary venules. The blood from the arterial tree streams into the superficial capillary mesh and then drains into the venous system. It is possible to distinguish the arterial and the venous blood in the circulatory system of the CAM by a smooth muscle layer surrounding the endothelium of the arterioles, but not that of the venules. Thus their morphology is different [34]. Another important conclusion is that blood vessels in the CAM have no terminal vessels, tips or sprouts and are always exhibited as a closed cycle [34].
More recently, morphology studies about microcirculation in the CAM have been deepened to discover how arterial and venous vessel trees integrate (connect) and communicate with the capillary bed [35]. These are described on a smaller scale by scanning electron microscopy. The interdigitating pattern derives from arterial and venous vessel trees, and long arterial pathways link with short venous pathways through the capillary mesh, and vice versa. A distinct feature is the presence of new connections between arteriole and venule trees, which feed and drain the underlying plexus not only at their termini but also along proximal segments of the vessel tree.
Figure 7. Resin corrosion cast (Mercox cast) of the developing CAM vasculature at ED 12. (A and B) A three-dimensional structure containing a capillary plexus and a layer of supplying and collecting vessels is recognisable. (C and D) Numerous pillars of different sizes caused by intussusceptive angiogenesis processes. Original magnification: (A) 3100, (B and C) 3200 and (D) 3400. Reproduced with permission from [11].
2.4. Growth and Regulation Factors of Angiogenesis in CAM
The angiogenesis process is controlled by the balance of multiple growth factors which have proliferative and inhibitory regulatory activity. A variety of growth factors have been revealed [36][37][38][39], which induce and promote CAM angiogenesis and undertake precise spatial and temporal regulations to form a mature vascular network (Table 2).
Vascular endothelial growth factor (VEGF) plays a leading role among these factors and is crucial for the angiogenic expansion at the early stage of CAM development. An endogenous VEGF-A presents two peaks at EDs 8–9 and 11–12 [40].
VEGF is the prime factor to attract migrating endothelial cells and stabilise vessels by bounding substrates during the formation of vascular tubes. Some researchers believe that VEGF causes vascular permeability, recruits endothelial cells and inhibits vessel stabilisation. The fibroblast growth factor (FGF) family is the most potent cytokine to stimulate mitogenesis of multiple cell types, such as endothelial cells, osteoblasts, bone marrow stromal cells, mesenchymal stem cells and immune cells. It directly causes fibrogenesis [37]. At present, there is a general consensus that VEGF and FGF are main factors for the vascular growth regulation in the chick CAM.
FGF-1 and FGF-2 are major prototypic members of the FGF family. Both of them initiate activation immediately after binding to their cell surface receptor, which is named fibroblast growth factor receptor-1 (FGFR1), one of the receptor tyrosine kinases. FGF-1 is considered as a standard angiogenesis stimulator [39]. Endogenous FGF-2 may affect the proliferation, movement, redistribution and invasion of endothelial cells. FGF-2 becomes detectable in the CAM since ED 6, and maximal concentrations occur between ED 10 and ED 14. Meanwhile, FGF located in the CAM can regulate angiogenesis [38].
Table 2. Representative growth factors for angiogenesis in the CAM.
|
Growth Factors |
Description |
Functions |
Ref. |
|
VEGF |
Vascular endothelial growth factor |
To exert vascular permeability and endothelial cell recruitment |
[37] |
|
FGF |
Fibroblast growth factor |
To elicit fibrocyte proliferation |
|
|
PDGF |
Platelet-derived growth factor |
To stimulate vascular stability |
[38] |
|
ANG |
Angiopoietin |
To act on endothelial sprouting, vessel wall remodelling and mural cell recruitment |
[37] |
|
HGF |
Hepatocyte growth factor |
As a cytokine to stimulate proliferation and morphogenesis of epithelia |
[37] |
|
HIF |
Hypoxia-inducible factor |
To induce expression of VEGF and its receptors |
[37] |
|
Endostain |
A proteolytic fragment of collagen XVIII (a component of the basement membrane) |
To act as an endogenous anti-angiogenic molecule |
[37] |
However, VEGF and FGF are not sufficient to finish angiogenesis, because they function not only as promoters of endothelial cell proliferation but also as inhibitors of vessel maturation. Through suppressing receptors on smooth muscle cells, VEGF inhibits pericyte coverage of vascular sprouts and renders existing vessels unstable, whereas platelet-derived growth factor (PDGF) creates vascular stability at the maturation stage (late stage) of angiogenesis .
PDGF drives pericytes and smooth muscle cells to recruit, which form a layer of cells around new capillaries, embed the endothelial lining and facilitate binding strongly to the extracellular matrix. These are necessary conditions to stabilise new blood vessels. Meanwhile, PDGF is functional to inhibit the recruitment of endothelial cells.
These facts show that a single factor is not sufficient to create a stable mature vasculature. However, the coordination and balance of multiple factors can induce a successful angiogenic response and mature vessel network in the CAM.
Not many concrete studies have been carried out on angiopoietins (ANGs). ANGs play an important role in endothelial sprouting, mural cell recruiting and vessel wall remodelling. Angiopoietin-1 (ANG-1), a 498-amino-acid glycoprotein, is a ligand of the endothelium-specific receptor Tie2 [41]. It recruits periendothelial cells, at the late stage of vascular maturation, in the presence of VEGF. Unlike VEGF, ANG-1 has no mitogenic effect in vivo [42]. However, another report indicated that ANG-2 can induce rapid initiation of blood vessels.
Hepatocyte growth factor (HGF), a specific growth factor for the liver, shows the highest expression at the beginning of chick embryo development. Meanwhile, HGF is a cytokine which stimulates the proliferation and morphogenesis of epithelia. HGF can also directly act on endothelial cells, including stimulation of proliferation, cell mobility, protease production and organisation of capillary-like tubes [43].
Hypoxia-inducible factor 1-alpha and 2-alpha (HIF-1α and HIF-2α) are activated by a hypoxic environment. HIF may switch on the expression of angiogenic genes, for example VEGF, which attract branching vessels to stretch towards hypoxic tissues [44]. A high expression level of HIF-1α correlates with a peak in the angiogenic process in the CAM.
Among anti-angiogenic molecules, endostatin, a proteolytic fragment of collagen XVIII (a component of the basement membrane), inhibits endothelial cell survival and migration. During angiogenesis in the CAM, endostatin progressively elevates its expression; meanwhile, most pro-angiogenic factors show a steadily downgrading expression.
In addition to these endocrine and paracrine molecules and growth factors mentioned above, the extracellular matrix of the CAM may modify its composition and express fibronectin, collagen type IV, laminin and specific glycosaminoglycans. These substances can assist the angiogenesis occurring in the matrix.
Recently, scientists have started to reveal cellular functional mechanisms and pathways of growth factors in CAM angiogenesis, for example EGFR, Ties receptor and AKT/PKB signalling and Ras-MEK-MAPK, AKT, P38 and PKC pathways.
Besides molecular regulation on endothelial cells, effects of some molecules on pericytes and the basement membrane in the CAM have also been studied. Netrin-4 is a laminin-disrupting molecule. Through disturbing the laminin network, it disrupts the full basement membranes surrounding pericytes, which are resultantly detached from the endothelium, leading to the collapse of the capillary network. Thus, netrin-4 treatment seriously impacts CAM angiogenesis and reduces the vascular area by altering the basement membrane and pericytes [45.
References
- Staton, C.A.; Stribbling, S.M.; Tazzyman, S.; Hughes, R.; Brown, N.J.; Lewis, C.E. Current methods for assaying angiogenesis in vitro and in vivo. Int. J. Exp. Pathol. 2004, 85, 233–248, doi:10.1111/j.0959-9673.2004.00396.x.
- Ribatti, D. The chick embryo chorioallantoic membrane in the study of tumor angiogenesis. Rom. J. Morphol. Embryol. 2008, 49, 131–135.
- Chase, D.M.; Chaplin, D.J.; Monk, B.J. The development and use of vascular targeted therapy in ovarian cancer. Gynecol. Oncol. 2017, 145, 393–406, doi:10.1016/j.ygyno.2017.01.031.
- Li, J. Research status mode Chicken chorioallantoic membrane applied for angiogenesis. Med. Front. 2015, 5, 4.
- Wang, S.; Liu, Y.; Feng, Y.; Zhang, J.; Swinnen, J.; Li, Y.; Ni, Y. A Review on Curability of Cancers: More Efforts for Novel Therapeutic Options Are Needed. Cancers (Basel) 2019, 11, 1782, doi:10.3390/cancers11111782.
- Ribatti, D.; Nico, B.; Perra, M.T.; Longo, V.; Maxia, C.; Annese, T.; Piras, F.; Murtas, D.; Sirigu, P. Erythropoietin is involved in angiogenesis in human primary melanoma. Int. J. Exp. Pathol. 2010, 91, 495–499, doi:10.1111/j.1365-2613.2010.00731.x.
- Richards, L.M.; Kazmi, S.M.; Davis, J.L.; Olin, K.E.; Dunn, A.K. Low-cost laser speckle contrast imaging of blood flow using a webcam. Biomed. Opt. Express 2013, 4, 2269–2283, doi:10.1364/BOE.4.002269.
- Makanya, A.N.; Dimova, I.; Koller, T.; Styp-Rekowska, B.; Djonov, V. Dynamics of the Developing Chick Chorioallantoic Membrane Assessed by Stereology, Allometry, Immunohistochemistry and Molecular Analysis. PLoS ONE 2016, 11, e0152821, doi:10.1371/journal.pone.0152821.
- Romanoff, A. The Avian Embryo: Structural and Functional Development; Macmillan: New York, NY, USA, 1960.
- Bellairs, R.; Osmond, M. Extra-Embryonic Membranes. In Atlas of Chick Development; 2014; pp. 127–129, doi:10.1016/b978-0-12-384951-9.00013-7.
- Ribatti, D.; Nico, B.; Vacca, A.; Roncali, L.; Burri, P.H.; Djonov, V. Chorioallantoic membrane capillary bed: A useful target for studying angiogenesis and anti-angiogenesis in vivo. Anat. Rec. 2001, 264, 317–324, doi:10.1002/ar.10021.
- Kind, C. The development of the circulating blood volume of the chick embryo. Anat. Embryol. (Berl) 1975, 147, 127–132, doi:10.1007/BF00306727.
- Maina, J.N. Structure and Function of the Shell and the Chorioallantoic Membrane of the Avian Egg: Embryonic Respiration. In The Biology of the Avian Respiratory System, Springer, South Africa, 2017; pp. 219–247, doi:10.1007/978-3-319-44153-5_9.
- Ribatti, D. The chick embryo chorioallantoic membrane (CAM). A multifaceted experimental model. Mech. Dev. 2016, 141, 70–77, doi:10.1016/j.mod.2016.05.003.
- Nowak-Sliwinska, P.; Segura, T.; Iruela-Arispe, M.L. The chicken chorioallantoic membrane model in biology, medicine and bioengineering. Angiogenesis 2014, 17, 779–804, doi:10.1007/s10456-014-9440-7.
- Fuchs, A.; Lindenbaum, E.S. The two- and three-dimensional structure of the microcirculation of the chick chorioallantoic membrane. Acta Anat. (Basel) 1988, 131, 271–275, doi:10.1159/000146528.
- Mueller, C.A.; Burggren, W.W.; Tazawa, H. The Physiology of the Avian Embryo. In Sturkie's Avian Physiology; Elsevier, USA, 2015; pp. 739–766, doi:10.1016/b978-0-12-407160-5.00032-4.
- DeFouw, D.O.; Rizzo, V.J.; Steinfeld, R.; Feinberg, R.N. Mapping of the microcirculation in the chick chorioallantoic mem-brane during normal angiogenesis. Microvasc. Res. 1989, 38, 136–147, doi:10.1016/0026-2862(89)90022-8.
- Tazawa, H.; Whittow, G. Incubation Physiology: Sturkie’s Avian Physiology; Academic Press: Elsevier, USA,2000; pp. 617–637.
- Rahn, H.; Paganelli, C.V.; Ar, A. The avian egg: Air-cell gas tension, metabolism and incubation time. Respir. Physiol. 1974, 22, 297–309, doi:10.1016/0034-5687(74)90079-6.
- Kurz, H.; Ambrosy, S.; Wilting, J.; Marme, D.; Christ, B. Proliferation pattern of capillary endothelial cells in chorioallantoic membrane development indicates local growth control, which is counteracted by vascular endothelial growth factor appli-cation. Dev. Dyn. 1995, 203, 174–186, doi:10.1002/aja.1002030206.
- Piiper, J.; Tazawa, H.; Ar, A.; Rahn, H. Analysis of chorioallantoic gas exchange in the chick embryo. Respir. Physiol. 1980, 39, 273–284, doi:10.1016/0034-5687(80)90059-6.
- Rahn, H.; Ar, A. Gas exchange of the avian egg time structure and function. Am. Zool. 1980, 20, 477–487.
- Vargas, A.; Zeisser-Labouebe, M.; Lange, N.; Gurny, R.; Delie, F. The chick embryo and its chorioallantoic membrane (CAM) for the in vivo evaluation of drug delivery systems. Adv. Drug Deliv. Rev. 2007, 59, 1162–1176, doi:10.1016/j.addr.2007.04.019.
- Eichmann, A.; Yuan, L.; Moyon, D.; Lenoble, F.; Pardanaud, L.; Breant, C. Vascular development: From precursor cells to branched arterial and venous networks. Int. J. Dev. Biol. 2005, 49, 259–267, doi:10.1387/ijdb.041941ae.
- Ausprunk, D.H.; Knighton, D.R.; Folkman, J. Differentiation of vascular endothelium in the chick chorioallantois: A struc-tural and autoradiographic study. Dev. Biol. 1974, 38, 237–248, doi:10.1016/0012-1606(74)90004-9.
- Kurz, H. Physiology of angiogenesis. J. Neurooncol. 2000, 50, 17–35, doi:10.1023/a:1006485716743.
- Melkonian, G.; Munoz, N.; Chung, J.; Tong, C.; Marr, R.; Talbot, P. Capillary plexus development in the day five to day six chick chorioallantoic membrane is inhibited by cytochalasin D and suramin. J. Exp. Zool. 2002, 292, 241–254, doi:10.1002/jez.10014.
- Djonov, V.; Schmid, M.; Tschanz, S.A.; Burri, P.H. Intussusceptive angiogenesis: Its role in embryonic vascular network formation. Circ. Res. 2000, 86, 286–292, doi:10.1161/01.res.86.3.286.
- Burton, G.J.; Palmer, M.E. Development of the chick chorioallantoic capillary plexus under normoxic and normobaric hy-poxic and hyperoxic conditions: A morphometric study. J. Exp. Zool. 1992, 262, 291–298, doi:10.1002/jez.1402620309.
- Maibier, M.; Reglin, B.; Nitzsche, B.; Xiang, W.; Rong, W.W.; Hoffmann, B.; Djonov, V.; Secomb, T.W.; Pries, A.R. Structure and hemodynamics of vascular networks in the chorioallantoic membrane of the chicken. Am. J. Physiol. Heart Circ. Physiol. 2016, 311, H913–H926, doi:10.1152/ajpheart.00786.2015.
- Wagner-Amos, K.; Seymour, R.S. Effect of local shell conductance on the vascularisation of the chicken chorioallantoic membrane. Respir. Physiol. Neurobiol. 2003, 134, 155–167, doi:10.1016/s1569-9048(02)00209-4.
- Kirchner, L.M.; Schmidt, S.P.; Gruber, B.S. Quantitation of angiogenesis in the chick chorioallantoic membrane model using fractal analysis. Microvasc. Res. 1996, 51, 2–14, doi:10.1006/mvre.1996.0002.
- Shumko, J.Z.; Defouw, D.O.; Feinberg, R.N. Vascular histodifferentiation in the chick chorioallantoic membrane: A mor-phometric study. Anat. Rec. 1988, 220, 179–189, doi:10.1002/ar.1092200209.
- Smith, A.F.; Nitzsche, B.; Maibier, M.; Pries, A.R.; Secomb, T.W. Microvascular hemodynamics in the chick chorioallantoic membrane. Microcirculation 2016, 23, 512–522, doi:10.1111/micc.12301.
- Marinaccio, C.; Nico, B.; Ribatti, D. Differential expression of angiogenic and anti-angiogenic molecules in the chick em-bryo chorioallantoic membrane and selected organs during embryonic development. Int. J. Dev. Biol. 2013, 57, 907–916, doi:10.1387/ijdb.130317dr.
- Bai, Y.; Bai, L.; Zhou, J.; Chen, H.; Zhang, L. Sequential delivery of VEGF, FGF-2 and PDGF from the polymeric system en-hance HUVECs angiogenesis in vitro and CAM angiogenesis. Cell Immunol. 2018, 323, 19–32, doi:10.1016/j.cellimm.2017.10.008.
- Ribatti, D. Endogenous Basic Fibroblast Growth Factor Is Implicated in the Vascularization of chick embryo chorioallantoic membrane. Dev. Biol. 1995, 170, 39–49.
- Forough, R.; Weylie, B.; Patel, C.; Ambrus, S.; Singh, U.S.; Zhu, J. Role of AKT/PKB signaling in fibroblast growth factor-1 (FGF-1)-induced angiogenesis in the chicken chorioallantoic membrane (CAM). J. Cell. Biochem. 2005, 94, 109–116, doi:10.1002/jcb.20274.
- Baum, O.; Suter, F.; Gerber, B.; Tschanz, S.A.; Buergy, R.; Blank, F.; Hlushchuk, R.; Djonov, V. VEGF-A promotes intussuscep-tive angiogenesis in the developing chicken chorioallantoic membrane. Microcirculation 2010, 17, 447–457, doi:10.1111/j.1549-8719.2010.00043.x.
- Asahara, T.; Chen, D.; Takahashi, T.; Fujikawa, K.; Kearney, M.; Magner, M.; Yancopoulos, G.D.; Isner, J.M. Tie2 receptor ligands, angiopoietin-1 and angiopoietin-2, modulate VEGF-induced postnatal neovascularization. Circ. Res. 1998, 83, 233–240, doi:10.1161/01.res.83.3.233.
- Drenkhahn, M.; Gescher, D.M.; Wolber, E.M.; Meyhoefer-Malik, A.; Malik, E. Expression of angiopoietin 1 and 2 in ectopic endometrium on the chicken chorioallantoic membrane. Fertil. Steril. 2004, 81 (Suppl. 1), 869–875, doi:10.1016/j.fertnstert.2003.09.040.
- Bussolino E, D.R.M., Ziche M, Bocchietto E, Olivero M, Naldini L,; Gaudino G, T.L., Coffer A, Comoglio, P. Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth. J. Cell Biol. 1992, 119, 629–641.
- Eva, V. Structural Analysis of the Angiogenesis in the Chicken Chorioallantoic Membrane; KU LEUVEN, Doctoral Thesis, Leuven, 2011.
- Reuten, R.; Patel, T.R.; McDougall, M.; Rama, N.; Nikodemus, D.; Gibert, B.; Delcros, J.G.; Prein, C.; Meier, M.; Metzger, S.; et al. Structural decoding of netrin-4 reveals a regulatory function towards mature basement membranes. Nat. Commun. 2016, 7, 13515, doi:10.1038/ncomms13515.