
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mohammad Shah Jahan | + 1892 word(s) | 1892 | 2021-02-15 13:37:28 | | | |
| 2 | Catherine Yang | + 7 word(s) | 1899 | 2021-02-22 10:21:43 | | | | |
| 3 | Catherine Yang | Meta information modification | 1899 | 2021-02-23 05:27:55 | | |
Video Upload Options
In recent years, research on spermine (Spm) has turned up a lot of new information about this essential polyamine, especially as it is able to counteract damage from abiotic stresses. Spm has been shown to protect plants from a variety of environmental insults, but whether it can prevent the adverse effects of drought has not yet been reported. Drought stress increases endogenous Spm in plants and exogenous application of Spm improves the plants’ ability to tolerate drought stress. Spm's role in enhancing antioxidant defense mechanisms, glyoxalase systems, methylglyoxal (MG) detoxification, and creating tolerance for drought-induced oxidative stress is well documented in plants. However, the influences of enzyme activity and osmoregulation on Spm biosynthesis and metabolism are variable. Spm interacts with other molecules like nitric oxide (NO) and phytohormones such as abscisic acid, salicylic acid, brassinosteroids, and ethylene, to coordinate the reactions necessary for developing drought tolerance. This review focuses on the role of Spm in plants under severe drought stress. We have proposed models to explain how Spm interacts with existing defense mechanisms in plants to improve drought tolerance.
1. Introduction
Polyamines (PAs) are water-soluble polycations that have important roles in the normal physiological and developmental functions of plants, as well as in the development of tolerance under conditions of abiotic stress [1][2]. Spermine (Spm), putrescine (Put), and spermidine (Spd) are low-molecular weight polyamines with aliphatic nitrogenous bases that are found in almost all types of living organisms [2]. They serve indispensable functions in physiological and developmental processes such as cell division, embryogenesis, floral emergence, leaf senescence, and responses to abiotic stress [3]. Spm is specifically involved in shoot and root development, floral induction, fruit set, leaf senescence, DNA synthesis, osmolyte balance, chlorophyll protection, gene transcription, and protein translation [4][5][6][7][8][9]. Spm is also crucial for mounting an effective response to environmental stresses such as those caused by drought [10][11][12][13], heavy metals [14][15][16], excessive heat [17], low temperatures [18], and high temperatures [19].
Drought is a major global threat to farming as the resulting stress severely alters key physiological and developmental processes [20][21][22][23][24][25], reducing production by as much as 25% [26]. Long-term drought leads to physiological and metabolic changes in plants including loss of cell turgor, water and mineral imbalances, and photosynthetic abnormalities [27]. However, Spm can significantly enhance plants’ resistance to several environmental stressors, including drought, salt, and heavy metals. Past studies reported that increasing the concentration of endogenous polyamines such as Spm in plants under water deficit conditions significantly increased tolerance [28]. The exogenous application of Spm upregulated the antioxidant systems involving superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), monodehydroascorbate reductase (MDHAR), dehydroascorbate reductase (DHAR), glutathione reductase (GR), glutathione S-transferase (GST), and glutathione peroxidase (GPX) [29][30].
We have reviewed the current literature on Spm’s biosynthesis, metabolism, and molecular interactions in response to drought stress in plants along with enhancement of drought stress resistance through regulation of Spm metabolism and external application of Spm. The purpose of this review was to clarify the mechanisms involved in stress resistance and Spm-mediated enhancement of plant tolerance through antioxidant activity and synergy with other molecules in plants under drought stress.
2. Spermine Biosynthesis and Metabolism in Plants
Spm biosynthesis is accomplished via two main pathways [20]. In the first pathway, the enzyme arginase converts arginine (arg) into ornithine, which is then transformed into putrescine by the enzyme, ornithine decarboxylase. Putrescine is a precursor of spermine. The second route comprises three pathways, which involve the conversion of arginine into agmatine by two enzymes, agmatine imidohydrolase and carbamoylputrescine amidohydrolase. Subsequently, spermidine synthase converts putrescine into spermidine, which is then transformed into spermine by spermine synthase [31][32][33].
In the final reaction, aminopropyl groups are added from decarboxylated S-adenosylmethionine (SAM), which is produced by SAM decarboxylase (SAMDC). These enzymes drive two types of reaction; terminal oxidation and back-conversion reaction. In terminal oxidation of Spm, 4-N-(3-aminopropyl)-4-aminobutanal, 1,3-diaminopropane, and H2O2 are produced. In the back-conversion reaction, Spm is transformed into Spd and Spd into Put, consequently leading to the generation of 3-aminopropanal and H2O2 [33] (Figure 1).
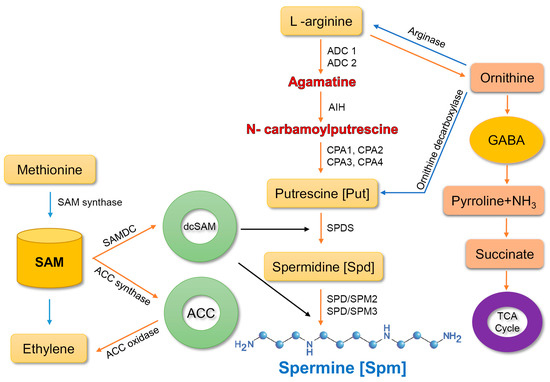
Figure 1. Spermine biosynthesis in plants. ADC, arginine decarboxylase; AIH, agmatine iminohydrolase; CPA-N, carbamoylputrescine amidohydrolase; SPDS, spermidine synthase; SPMS, spermine synthase; GABA, γ-aminobutyric acid; SAM-S, adenosylmethionine; SAMDC-S, adenosylmethionine decarboxylase; dcSAM, decarboxylated S-adenosylmethionine; ACC, 1-aminocyclopropane-1-carboxylic-acid synthase. Arrows represent synthesis and conversion.
3. Spermine Induced Drought Tolerance in Plants
Low water availability is one of the major abiotic stresses that severely affects plant growth and yield and leads to a decline in defense mechanisms [34]. Adequate soil water for short to long distance transport, osmoregulation, and single cell expansion through cellular membranes is vital for good crop production [35][36]. Drought negatively affects the movement of water in plants, but this can be partly overcome through the opening of membrane channels known as aquaporins (AQPs) that facilitate water permeability [36][37]. To maintain water balance, plants often synthesize polyamines like spermine that stabilize cell membranes and improve water use efficiency [38][39][40]. Recently, Li et al. (2020) [38] reported that Spm helped to maintain water balance under drought stress by increasing expression of the Ca2+-dependent AQPs, TrTIP2-1, TrTIP2-2, and TrPIP2-7.
However, the mechanism of spermine-mediated drought tolerance remained unclear. Spm regulates potassium channels and guard cells to control water loss by optimizing stomatal opening and closing [41][42]. Spm can regulate several abscisic acid-related genes, which in turn control stomatal closure, stress-response gene expression, and osmolyte production [43]. A significant positive correlation was seen between spermine levels and grain weight and filling rates in drought-tolerant wheat [44].
Increased production of Spm is a common stress response to drought in several plants such as rice [39], tomato [45], and yellow lupin [46]. Adamipour (2020) [28] found endogenous Spm accumulation in drought affected Rosa damascene seedlings and induction of defense mechanisms to mitigate drought stress. It has been confirmed that both endogenously produced and exogenously applied Spm are effective against drought stress [28][39], by enhancing drought-tolerance mechanisms (Figure 2).
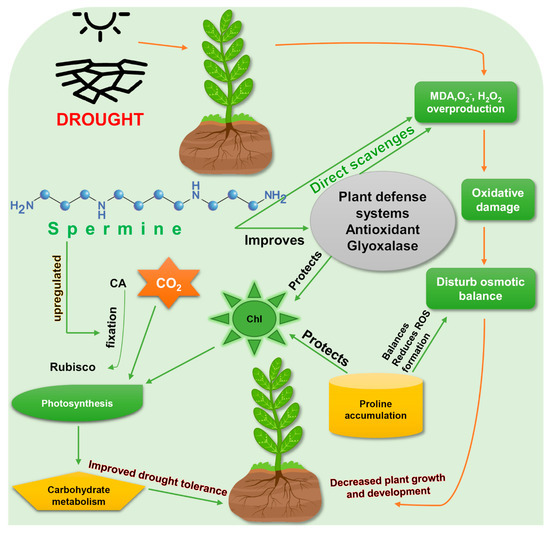
Figure 2. Enhancement of drought-stress tolerance by spermine. Exogenous application of spermine improves the drought tolerance in plants. Rubisco, ribulose-1,5-bisphosphate carboxylase/oxygenase; MDA, malondialdehyde; ROS, reactive oxygen species; Chl, chlorophyll; CO2, carbon dioxide; CA, carbonic anhydrase. Green arrows represent spermine’s actions to reduce drought stress, while red arrows show the direct effects of drought on plants.
Exogenous foliar application of Spm increased survival rate, shoot length and weight, root length and weight, produced greener leaf tissues, and slowed water loss in Bermuda grass (Cynodon dactylon) [47]. Photosynthetic efficiency (FV/FM) and photosystem II (PSII) activity were found to be higher in Spm-treated plants under drought stress [48]. Levels of osmolytes such as proline and soluble sugars were also increased by spermine. Spm enhanced drought tolerance in creeping bentgrass (Agrostis stolonifera) through osmotic adjustment and hormonal regulation. Concentrations of gibberellic acid (GA1, GA4) and Abscisic acid (ABA) in Spm-treated creeping bentgrass were significantly increased under drought stress, which indicates a hormonal connection in Spm’s ability to promote drought tolerance [48] (Table 1).
Table 1. Spermine mediated growth, improved photosynthetic parameters and osmoregulation, and enhanced antioxidant defense in different plant species under drought stress.
| Species | Stress | Spermine Treatment | Effect | Outcome | References |
|---|---|---|---|---|---|
| Arabidopsis thaliana | Drought stress (1/2 MS agar plates) | 1 mM (exogenous pretreated seedlings) | Enhanced chlorophyll content, potential role in stomatal movement | Spm protected against drought stress | [49] |
| Cynodon dactilon | Drought stress (withholding water) | 5 mM (exogenous) | Proteins involved in ROS balance stimulated by spermine. Energy-related pathways stimulated by Spm treatment | Improved drought stress tolerance | [47] |
| Cucumber | Drought | 1 mM (pretreated seed) | Reduced ion leakage from the membrane and less lipid peroxidation | Nitric oxide acts downstream of Spm during drought stress to enhance stress tolerance | [50] |
| Creeping Bentgrass (Penn G2) | Drought (withholding water) | 1 mM (exogenous) | Spermine-treated plants maintained significantly higher turf quality (TQ), relative water content (RWC), and photochemical efficiency | Protected creeping bentgrass from drought stress | [48] |
| Chinese dwarf cherry (Cerasus humilis) | Drought stress (withholding water) | 0.2 mM (exogenous) | Increased RWC and prevented lipid peroxidation | Prevented drought-induced oxidative damage | [51] |
| Lettuce | Drought (10% polyethylene glycol, PEG) | 0.1 mM (exogenous) | Increased plant height and root length. Upregulated antioxidant activity | Significantly alleviated drought stress | [11] |
| Maize | Drought (50% and 75% field capacity) | 25 mgL (exogenous) | Increased content of protein, phenolic, flavonoids, and amino acids | Improved drought tolerance by increasing ethylene and polyamine synthesis | [52] |
| Maize (Giza 10 and Giza 129 cultivars) | Drought (50% and 75% field capacity) | 25 mgL (exogenous) | Stimulated synthesis of antioxidant enzymes, and promoted ROS scavenging | Enhanced drought tolerance and reduced ROS accumulation | [53] |
| Mung bean (Vigna radiata L. cv. BARI Mung-2) | Combined drought and high temperature stress | 0.2 mM (exogenous pretreated seedlings) | Upregulated antioxidant enzymes. Reduced methylglyoxal toxicity by stimulating glyoxalase systems | Improved tolerance to drought and high temperature stress | [29] |
| Orange (Poncirus trifoliata [L.] Raf.) | Combined heat and drought | 1 mmol L-1 (exogenous pretreated seedlings) | Activated antioxidant enzymes such as CAT, SOD, and peroxidases; induced heat shock proteins and abscisic acid-response element binding factors | Enhanced drought and heat tolerance in a perennial fruit crop | [16] |
| Oryza sativa | Drought (50% field capacity) | 10 µM (seed priming treatments and foliar application) | Activated antioxidant enzymes. Enhanced ROS scavenging and stress-related gene expression | Enhanced drought and heat tolerance in rice seedlings | [54] |
| Red tangerine (Citrus reticulata Blanco) | Drought (MS agar plates) | 1 mM (pretreated seed) | Increased enzymatic antioxidant activity such as SOD and peroxidase and ROS scavenging | Prevented oxidative damage and increased drought tolerance | [55] |
| Rosa damascena Miller var. trigintipetala Dieck | Drought (50% and 100% field capacity) | 0.5 mM (exogenous) | Improved growth (RWC), photosynthetic pigments and stomatal conductance(gs) | Mitigated drought stress | [56] |
| Soybean cultivars (Giza 111 and Gazi 21) | Drought (0, −0.1, −0.5, and −1.1 MPa) | 0.2 mM (pretreated seed) | Pigment enhancement, membrane stabilization, osmolyte accumulation, and water balance | Increased drought tolerance of soybean cultivar | [10] |
| Soybean | Drought (9% PEG) | 0.2 mM (exogenous) | Enhanced CAT, SOD, and POD activities; reduced lipid peroxidation | Improved drought tolerance of soybean | [57] |
| Valerian | Drought (withholding water) | 0.1 mM (exogenous) | Increased photosynthetic pigments and antioxidant enzyme activity | Improved drought tolerance | [58] |
| Wheat | Drought (withholding water) | 100 µM (exogenous) | Increased photosynthetic pigments, antioxidants, and Rubisco | Enhanced drought tolerance of wheat by reduction of oxidative injury | [9] |
| Wheat | Drought (withholding water) | 100 µM (exogenous) | Increased cell water status and accumulation of osmoprotectants | Improved drought tolerance | [32] |
| Wheat | Drought (soil water potential at −60 ± 5 kPa) | 1 mM (exogenous) | Relieved inhibition caused by drought stress | Enhanced grain filling and drought resistance | [44] |
| White clover | Drought stress (20% PEG 6000) | 0.5 mM (exogenous) | Improved sugar metabolism and dehydrin biosynthesis | Mitigated drought stress | [33] |
An increase in Spm has also been associated with drought tolerance in cherry tomatoes [59]. Overexpression of the DsADC gene in transgenic rice produced greater drought tolerance through conversion of Put to Spd and Spm [60]. The Arabidopsis acl5/spms mutant showed hypersensitivity to drought [49]. Liu et al. 2018 [11] treated lettuce plants with 0.1 mM Spm under drought stress induced by 10% PEG and observed significant improvement in morphological and physiological traits. Similar results were seen in mung bean seedlings with higher proline accumulation, osmotic protection, and increased chlorophyll synthesis under drought stress with Spm [29]. In soybean plants under drought stress, 0.2 mM Spm turned out to be the optimal concentration for increasing relative water content (RWC), osmoprotectant concentration, and mineral nutrients [10]. They also found that Spm alleviated drought stress in soybean plants by increasing endogenous spermine biosynthesis [30]. Other scientists reported that exogenous application of Spm to plants positively regulated photosynthetic activity [9][48][61].
Germination of seeds and survival of seedlings under environmental stress is a challenging goal for better crop yield [62]. Several studies have shown that Spm application to seeds is equally effective in promoting germination and early growth of seedlings. The crop yield can be significantly improved by treating seeds with Spm [30]. Seeds treated with Spm produced plants with improved PSII center activity, higher chlorophyll content, and balanced osmolyte accumulation [10]. Together, this body of evidence supports the idea that Spm treatment of seeds or plants can improve drought tolerance and osmoregulation, enhance antioxidant defense, and increase photosynthesis.
References
- Tsaniklidis, G.; Pappi, P.; Tsafouros, A.; Charova, S.N.; Nikoloudakis, N.; Roussos, P.A.; Paschalidis, K.A.; Delis, C. Polyamine Homeostasis in Tomato Biotic/Abiotic Stress Cross-Tolerance. Gene 2020, 727, 144230.
- Hussain, S.S.; Ali, M.; Ahmad, M.; Siddique, K.H. Polyamines: Natural and engineered abiotic and biotic stress tolerance in plants. Biotechnol. Adv. 2011, 29, 300–311.
- Sequera-Mutiozabal, M.; Antoniou, C.; Tiburcio, A.F.; Alcázar, R.; Fotopoulos, V. Polyamines: Emerging Hubs Promoting Drought and Salt Stress Tolerance in Plants. Curr. Mol. Bio. Rep. 2017, 3, 28–36.
- Zhang, X.; Shen, L.; Li, F.; Meng, D.; Sheng, J. Methyl salicylate-induced arginine catabolism is associated with up-regulation of polyamine and nitric oxide levels and improves chilling tolerance in cherry tomato fruit. J. Agric. Food Chem. 2011, 59, 9351–9357.
- Tiburcio, A.F.; Altabella, T.; Bitrián, M.; Alcázar, R. The roles of polyamines during the lifespan of plants: From development to stress. Planta 2014, 240, 1–18.
- Feng, H.Y.; Wang, Z.M.; Kong, F.N.; Zhang, M.J.; Zhou, S.L. Roles of carbohydrate supply and ethylene, polyamines in maize kernel set. J. Integ. Plant Biol. 2011, 53, 388–398.
- Alet, A.I.; Sánchez, D.H.; Cuevas, J.C.; Marina, M.; Carrasco, P.; Altabella, T.; Tiburcio, A.F.; Ruiz, O.A. New insights into the role of spermine in Arabidopsis thaliana under long-term salt stress. Plant Sci. 2012, 182, 94–100.
- Tavladoraki, P.; Cona, A.; Federico, R.; Tempera, G.; Viceconte, N.; Saccoccio, S.; Battaglia, V.; Toninello, A.; Agostinelli, E. Polyamine catabolism: Target for antiproliferative therapies in animals and stress tolerance strategies in plants. Amino Acids 2012, 42, 411–426.
- Hassan, N.; Ebeed, H.; Aljaarany, A. Exogenous application of spermine and putrescine mitigate adversities of drought stress in wheat by protecting membranes and chloroplast ultra-structure. Physiol. Mol. Biol. Plants 2020, 26, 233–245.
- Dawood, M.F.; Abeed, A.H. Spermine-priming restrained water relations and biochemical deteriorations prompted by water deficit on two soybean cultivars. Heliyon 2020, 6, e04038.
- Liu, C.J.; Wang, H.R.; Wang, L.; Han, Y.Y.; Hao, J.H.; Fan, S.X. Effects of different types of polyamine on growth, physiological and biochemical nature of lettuce under drought stress. IOP Conf. Ser. Earth Environ. Sci. 2018, 185, 012010.
- Ebeed, H.T.; Hassan, N.M.; Aljarani, A.M. Exogenous applications of polyamines modulate drought responses in wheat through osmolytes accumulation, increasing free polyamine levels and regulation of polyamine biosynthetic genes. Plant Physiol. Biochem. 2017, 118, 438–448.
- Li, Z.; Jing, W.; Peng, Y.; Zhang, X.Q.; Ma, X.; Huang, L.K. Spermine alleviates drought stress in white clover with different resistance by influencing carbohydrate metabolism and dehydrins synthesis. PLoS ONE 2015, 10, e0120708.
- Taie, H.A.; El-Yazal, M.A.S.; Ahmed, S.M.; Rady, M.M. Polyamines modulate growth, antioxidant activity, and genomic DNA in heavy metal–stressed wheat plant. Environ. Sci. Pollut. Res. 2019, 1, 1–13.
- Benavides, M.P.; Groppa, M.D.; Recalde, L.; Verstraeten, S.V. Effects of polyamines on cadmium-and copper-mediated alterations in wheat (Triticum aestivum L.) and sunflower (Helianthus annuus L.) seedling membrane fluidity. Arch. Biochem. Biophys. 2018, 654, 27–39.
- Rady, M.M.; Hemida, K.A. Modulation of cadmium toxicity and enhancing cadmium-tolerance in wheat seedlings by exogenous application of polyamines. Ecotoxicol. Environ. Saf. 2015, 119, 178–185.
- Fu, X.Z.; Xing, F.; Wang, N.Q.; Peng, L.Z.; Chun, C.P.; Cao, L.; Ling, L.L.; Jiang, C.L. Exogenous spermine pretreatment confers tolerance to combined high-temperature and drought stress in vitro in trifoliate orange seedlings via modulation of antioxidative capacity and expression of stress-related genes. Biotech. Biotechnol. Equip. 2014, 28, 192–198.
- Jankovska-Bortkevič, E.; Gavelienè, V.; Šveikauskas, V.; Mockevičiutè, R.; Jankauskienè, J.; Todorova, D.; Sergiev, I.; Jurkonienè, S. Foliar application of polyamines modulates winter oilseed rape responses to increasing cold. Plants 2020, 9, 179.
- Nahar, K.; Hasanuzzaman, M.; Alam, M.M.; Rahman, A.; Mahmud, J.A.; Suzuki, T.; Fujita, M. Insights into spermine-induced combined high temperature and drought tolerance in mung bean: Osmoregulation and roles of antioxidant and glyoxalase system. Protoplasma 2017, 254, 445–460.
- Hasan, M.M.; Alharby, H.F.; Hajar, A.S.; Hakeem, K.R.; Alzahrani, Y. The effect of magnetized water on the growth and physiological conditions of Moringa species under drought stress. Pol. J. Environ. Stud. 2019, 28, 1145–1155.
- Hasan, M.M.; Alharby, H.F.; Uddin, M.N.; Ali, M.A.; Anwar, Y.; Fang, X.W.; Hakeem, K.R.; Alzahrani, Y.; Hajar, A.S. Magnetized water confers drought stress tolerance in Moringa biotype via modulation of growth, gas exchange, lipid peroxidation and antioxidant activity. Pol. J. Environ. Stud. 2020, 1, 29.
- Hasan, M.M.; Ali, M.A.; Soliman, M.H.; Alqarawi, A.A.; Abd Allah, E.F.; Fang, X.-W. Insights into 28-homobrassinolide (HBR)-mediated redox homeostasis, AsA–GSH cycle, and methylglyoxal detoxification in soybean under drought-induced oxidative stress. J. Plant Inter. 2020, 15, 371–385.
- Khan, A.; Anwar, Y.; Hasan, M.; Iqbal, A.; Ali, M.; Alharby, H.F.; Hakeem, K.R.; Hasanuzzaman, M. Attenuation of drought stress in Brassica seedlings with exogenous application of Ca2+ and H2O2. Plants 2017, 6, 20.
- Ahammed, G.J.; Li, X.; Wan, H.; Zhou, G.; Cheng, Y. SlWRKY81 reduces drought tolerance by attenuating proline biosynthesis in tomato. Scientia Hortic. 2020, 270. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Li, X.; Mao, Q.; Wan, H.; Zhou, G.; Cheng, Y. The SlWRKY81 transcription factor inhibits stomatal closure by attenuating nitric oxide accumulation in the guard cells of tomato under drought. Physiol. Plantarum 2020. [Google Scholar] [CrossRef]
- Vanani, F.R.; Shabani, L.; Sabzalian, M.R.; Dehghanian, F.; Winner, L. Comparative physiological and proteomic analysis indicates lower shock response to drought stress conditions in a self-pollinating perennial ryegrass. PLoS ONE 2020, 15, e0234317. [Google Scholar] [CrossRef]
- Li, K.; Xing, C.; Yao, Z.; Huang, X. Pbr MYB 21, a novel MYB protein of Pyrus betulaefolia, functions in drought tolerance and modulates polyamine levels by regulating arginine decarboxylase gene. Plant Biotechnol. J. 2017, 15, 1186–1203. [Google Scholar] [CrossRef]
- Adamipour, N.; Khosh-Khui, M.; Salehi, H.; Razi, H.; Karami, A.; Moghadam, A. Role of genes and metabolites involved in polyamines synthesis pathways and nitric oxide synthase in stomatal closure on Rosa damascena Mill. under drought stress. Plant Physiol. Biochem. 2020, 148, 53–61. [Google Scholar]
- Nahar, K.; Rahman, M.; Hasanuzzaman, M.; Alam, M.M.; Rahman, A.; Suzuki, T.; Fujita, M. Physiological and biochemical mechanisms of spermine-induced cadmium stress tolerance in mung bean (Vigna radiata L.) seedlings. Environ. Sci. Pollut. Res. 2016, 23, 21206–21218. [Google Scholar] [CrossRef]
- Rai, P.K. Heavy metals/metalloids remediation from wastewater using free floating macrophytes of a natural wetland. Environ. Technol. Innovation. 2019, 15, 100393.
- Shelp, B.J.; Bozzo, G.G.; Trobacher, C.P.; Zarei, A.; Deyman, K.L.; Brikis, C.J. Hypothesis/review: Contribution of putrescine to 4-aminobutyrate (GABA) production in response to abiotic stress. Plant Sci. 2012, 193, 130–135.
- Alcázar, R.; Altabella, T.; Marco, F.; Bortolotti, C.; Reymond, M.; Koncz, C.; Carrasco, P.; Tiburcio, A.F. Polyamines: Molecules with regulatory functions in plant abiotic stress tolerance. Planta 2010, 231, 1237–1249.
- Sequera-Mutiozabal, M.; Tiburcio, A.F.; Alcázar, R. Drought Stress Tolerance in Relation to Polyamine Metabolism in Plants. In Drought Stress Tolerance in Plants; Hossain, M., Wani, S., Bhattacharjee, S., Burritt, D., Tran, L.S., Eds.; Springer: Cham, Switzerland, 2016; Volume 1.
- Li, H.; Guo, Y.; Cui, Q.; Zhang, Z.; Yan, X.; Ahammed, G.J.; Yang, X.; Yang, J.; Wei, C.; Zhang, X. Alkanes (C29 and C31)-Mediated Intracuticular Wax Accumulation Contributes to Melatonin- and ABA-Induced Drought Tolerance in Watermelon. J. Plant Growth Reg. 2020.
- Mueller, N.D.; Gerber, J.S.; Johnston, M.; Ray, D.K.; Ramankutty, N.; Foley, J.A. Closing yield gaps through nutrient and water management. Nature 2012.
- Iwuala, E.; Odjegba, V.; Sharma, V.; Alam, A. Drought stress modulates expression of aquaporin gene and photosynthetic efficiency in Pennisetum glaucum (L.) R. Br. genotypes. Curr. Plant Biol. 2020, 21, 100131.
- Maurel, C.; Boursiac, Y.; Luu, D.T.; Santoni, V.R.; Shahzad, Z.; Verdoucq, L. Aquaporins in plants. Physiol. Rev. 2015, 95, 1321–1358.
- Li, Z.; Hou, J.; Zhang, Y.; Zeng, W.; Cheng, B.; Hassan, M.J.; Zhang, Y.; Pu, Q.; Peng, Y. Spermine regulates water balance associated with Ca2+-dependent aquaporins (TrTIP2-1, TrTIP2-2, and TrPIP2-7) expression in plants under water stress. Plant Cell Physiol. 2020, 61, 1576–1589.
- Yang, J.; Zhang, J.; Liu, K.; Wang, Z.; Liu, L. Involvement of polyamines in the drought resistance of rice. J. Exp. Bot. 2007, 58, 1545–1555.
- Tiburcio, A.F.; Alcázar, R. Potential Applications of Polyamines in Agriculture and Plant Biotechnology. In Polyamines. Methods in Molecular Biology; Alcázar, R., Tiburcio, A., Eds.; Humana Press: New York, NY, USA, 2018; Volume 1694.
- Misra, B.B.; Acharya, B.R.; Granot, D.; Assmann, S.M.; Chen, S. The guard cell metabolome: Functions in stomatal movement and global food security. Front. Plant Sci. 2015, 6, 1–13.
- Agurla, S.; Gayatri, G.; Raghavendra, A.S. Polyamines increase nitric oxide and reactive oxygen species in guard cells of Arabidopsis thaliana during stomatal closure. Protoplasma 2018, 255, 153–162.
- Fujita, Y.; Fujita, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. ABA-mediated transcriptional regulation in response to osmotic stress in plants. J. Plant Res. 2011, 124, 509–525.
- Liu, Y.; Liang, H.; Lv, X.; Liu, D.; Wen, X.; Liao, Y. Effect of polyamines on the grain filling of wheat under drought stress. Plant Physiol. Biochem. 2016, 100, 113–129.
- Sánchez-Rodríguez, E.; Romero, L.; Ruiz, J. Accumulation of free polyamines enhances the antioxidant response in fruits of grafted tomato plants under water stress. J. Plant Physiol. 2016, 190, 72–78.
- Juzoń, K.; Czyczyło-Mysza, I.; Marcińska, I.; Dziurka, M.; Waligórski, P.; Skrzypek, E. Polyamines in yellow lupin (Lupinus luteus L.) tolerance to soil drought. Acta Physiol. Plantarum. 2017, 39, 202.
- Shi, H.; Ye, T.; Chan, Z. Comparative proteomic and physiological analyses reveal the protective effect of exogenous polyamines in the bermudagrass (Cynodon dactylon) response to salt and drought stresses. J. Proteome Res. 2013, 12, 4951–4964.
- Krishnan, S.; Merewitz, E.B. Polyamine application effects on gibberellic acid content in creeping bentgrass during drought stress. J. Amer. Soc. Hortic. Sci. 2017, 142, 135–142.
- Yamaguchi, K.; Takahashi, Y.; Berberich, T.; Imai, A.; Takahashi, T.; Michael, A.J.; Kusano, T. A protective role for the polyamine spermine against drought stress in Arabidopsis. Biochem. Biophy.Res. Commun. 2007, 352, 486–490.
- Arasimowicz-Jelonek, M.; Floryszak-Wieczorek, J.; Kubiś, J. Interaction between polyamine and nitric oxide signalling in adaptive responses to drought in cucumber. J. Plant Growth Reg. 2009, 28, 177–186.
- Yin, Z.P.; Li, S.; Ren, J.; Song, X.S. Role of spermidine and spermine in alleviation of drought-induced oxidative stress and photosynthetic inhibition in Chinese dwarf cherry (Cerasus humilis) seedlings. Plant Growth Reg. 2014, 74, 209–218.
- Talaat, N.B.; Shawky, B.T. Dual application of 24-epibrassinolide and spermine confers drought stress tolerance in maize (Zea mays L.) by modulating polyamine and protein metabolism. J. Plant Growth Reg. 2016, 35, 518–533.
- Talaat, N.B.; Shawky, B.T.; Ibrahim, A.S. Alleviation of drought-induced oxidative stress in maize (Zea mays L.) plants by dual application of 24-epibrassinolide and spermine. Environ. Exp. Bot. 2015, 113, 47–58.
- Farooq, M.; Wahid, A.; Lee, D.-J. Exogenously applied polyamines increase drought tolerance of rice by improving leaf water status, photosynthesis and membrane properties. Acta Physiol. Plant. 2009, 31, 937–945.
- Shi, J.; Fu, X.-Z.; Peng, T.; Huang, X.-S.; Fan, Q.-J.; Liu, J.-H. Spermine pre-treatment confers dehydration tolerance of citrus in vitro plants via modulation of antioxidative capacity and stomatal response. Tree Physiol. 2010, 30, 914–922.
- Hassan, F.A.; Ali, E.F.; Alamer, K.H. Exogenous application of polyamines alleviates water stress-induced oxidative stress of Rosa damascena Miller var. trigintipetala Dieck. S. Afr. J. Bot. 2018, 116, 96–102.
- Radhakrishnan, R.; Lee, I.J. Spermine promotes acclimation to osmotic stress by modifying antioxidant, abscisic acid, and jasmonic acid signals in soybean. J. Plant Growth Reg. 2013, 32, 22–30.
- Mustafavi, S.H.; Shekari, F.; Maleki, H.H. Influence of exogenous polyamines on antioxidant defence and essential oil production in valerian (Valeriana offcinalis L.) plants under drought stress. Acta Agric. Slov. 2016, 107, 81–91.
- Montesinos-Pereira, D.; Barrameda-Medina, Y.; Romero, L.; Ruiz, J.M.; Sánchez-Rodríguez, E. Genotype differences in the metabolism of proline and polyamines under moderate drought in tomato plants. Plant Biol. 2014, 16, 1050–1057.
- Do, P.T.; Drechsel, O.; Heyer, A.G.; Hincha, D.K.; Zuther, E. Changes in free polyamine levels, expression of polyamine biosynthesis genes, and performance of rice cultivars under salt stress: A comparison with responses to drought. Front. Plant Sci. 2014, 5, 182.
- Seifi, H.S.; Shelp, B.J. Spermine differentially refines plant defense responses against biotic and abiotic stresses. Front. Plant Sci. 2019, 10, 117.
- Ma, Y.; Zhang, J.; Li, X.; Zhang, S.; Lan, H. Effects of environmental stress on seed germination and seedling growth of Salsola ferganica (Chenopodiaceae). Acta Ecol. Sin. 2016, 36, 456–463.





