Video Upload Options
Chemotherapy-induced peripheral neuropathy (CIPN) is a common adverse event of several first-line chemotherapeutic agents, including platinum compounds, taxanes, vinca alkaloids, thalidomide, and bortezomib, which negatively affects the quality of life and clinical outcome.
1. Introduction
Chemotherapy-induced peripheral neuropathy (CIPN) is among the most common adverse effects of chemotherapy, affects several millions of patients each year, and is characterized by tingling, numbness, increased sensitivity to cold and touch, and burning pain of the distal extremities [1][2]. Although the prevalence of CIPN varies depending on the anticancer agent, dose, duration of exposure, and method of assessment, it can be as high as 89% for acute symptoms and 85% for chronic CIPN [3]. The anticancer agents most commonly associated with CIPN include paclitaxel and oxaliplatin, although the side effects have also been reported after treatment with other taxanes (docetaxel) and platinum agents (cisplatin and carboplatin), vinca alkaloids (particularly vincristine), and bortezomib. CIPN is a tremendous health problem worldwide and remains one of the most important complications of contemporary oncology regimens as it may limit further use of curative-intent treatment and/or may cause the long-term quality of life concerns [4][5].
Despite having been the focus of intense investigation for several decades, management of CIPN continues to be challenging. According to the most recent 2020 American Society of Clinical Oncology (ASCO) CIPN guideline, no agents can be recommended for the prevention of CIPN due to lack of high-quality evidence in general, which remains unchanged since the initial 2014 guideline. Although the earlier guideline commented on venlafaxine as a preventative agent, the updated guideline does not recommend it based on a negative follow-up study, in which patients were treated for a longer time-period and evaluated with a more widely accepted CIPN measurement tool [6]. The ASCO guideline also supports duloxetine as the sole treatment option for established painful CIPN, for which additional supporting data has become available since the initial guideline in 2014, but no longer supports the utility of tricyclic antidepressants, gabapentinoids, or topical amitriptyline/ketamine/baclofen that were included as therapeutic approaches in the initial guideline.
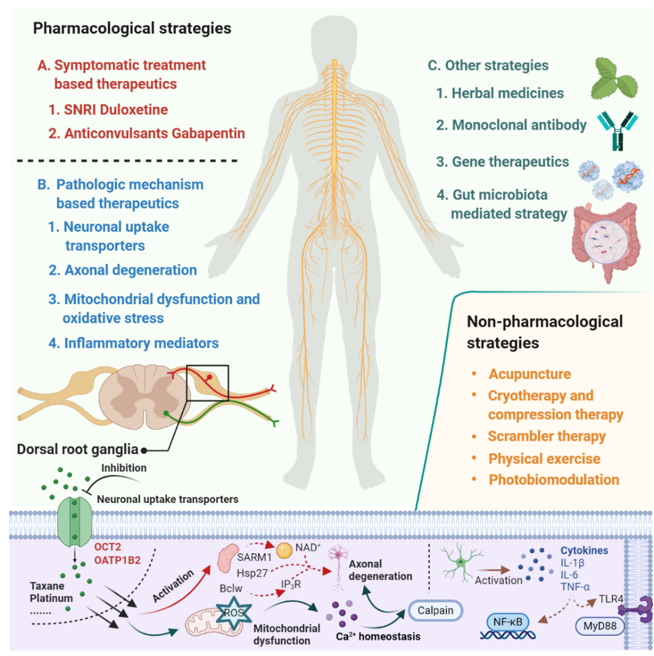
Despite the extensive research effort focused on understanding mechanisms involved in the development of CIPN, the translation of this mechanistic understanding into rationally-designed, clinical intervention studies remains problematic [7]. According to a study conducted by investigators at the National Cancer Institute, based on databases covering the period January 2011 to May 2019, only 3 of 35 clinical trials that investigated agents or devices to treat CIPN incorporated a mechanistic rationale to support the choice of the intervention. This not only speaks to the urgent need for collaborations between basic and clinical research teams but also emphasizes the thesis that a deeper understanding of the underlying mechanisms that initiate and cause the progression of CIPN will ultimately facilitate the development of effective prevention and treatment strategies [8][9][10]. In the current article, we provide an overview of emerging therapeutics for the prevention and treatment of CIPN and focus on pharmacological strategies that are derived from novel mechanistic insights and have the potential to be translated into clinically beneficial approaches (Figure 1).
Figure 1. Summary of emerging pharmacological and non-pharmacological therapeutics for prevention and treatment of chemotherapy-induced peripheral neuropathy.
2. Pharmacological Strategies for Prevention/Treatment of CIPN
There are two overarching approaches in CIPN management: to target the underlying pathologic mechanism responsible for CIPN or to address the CIPN symptoms themselves [4].
2.1. Pathologic Mechanism-Based Therapeutics
The pathologic mechanism by which chemotherapeutics damage structures in the nervous system and cause CIPN is multifactorial [10]. Recent progress in preclinical studies has identified several new preventative and therapeutic targets and pathways, which have the potential to be translated into the clinic for improved management of CIPN.
2.1.1. Neuronal Uptake Transporters
Prior investigations have demonstrated that one of the initiating events leading to the development of CIPN is the extensive accumulation and retention of chemotherapeutic drugs in the peripheral sensory nerves present in dorsal root ganglia (DRG) [11][12]. For many small-molecule anticancer drugs, this process is mediated by uptake transporters located in DRG neurons, and ongoing efforts attempt to block these transporters, reduce intra-neuronal concentrations of the neurotoxic agents, and ultimately protect against a dose-limiting injury. The role of drug transporters in CIPN was recently extensively reviewed [13], and this field of research will be illustrated here for oxaliplatin and paclitaxel, two agents for which defined transporters have been identified that can be targeted pharmacologically.
2.1.2. Axonal Degeneration
Direct damage of peripheral nerves by axonal degeneration is the most significant final result from CIPN.
2.1.3. Mitochondrial Dysfunction and Oxidative Stress
Mitochondrial dysfunction and oxidative stress have been highlighted as key players in the pathophysiology of CIPN. Impairment of the mitochondrial physiological function is leading to increased production of reactive oxygen species (ROS) and oxidative stress.
2.1.4. Inflammatory Mediators
The activation of glial cells and the subsequent release and elevation of pro-inflammatory cytokines such as IL-1β, IL-6, TNF-α, and chemokines such as IL-8 and MCP-1 are common downstream events of neuropathic pain induced by chemotherapeutics [10].
2.2. Symptomatic treatment-based therapeutics
Due to the incomplete knowledge about the underlying pathophysiology of CIPN, symptomatic treatments have not been consistently successful.
2.3. Other strategies
Besides pathologic mechanism and symptomatic treatment-based therapeutics, a number of additional strategies have been proposed for the prevention and/or treatment against CIPN for which preliminarily supporting evidence has been generated.
3. Non-pharmacological interventions for prevention or treatment of CIPN
There is currently increasing interest in non-pharmacological strategies for prevention or treatment of CIPN due to the anticipation that avoidance of drugs may allow for benign intervention approaches. However, phase III evidence of benefit for several of these approaches, including acupuncture, physical exercise, cryotherapy/compression, and scrambler therapy is not yet available, and further research in larger clinical trials is needed to better delineate their utility.
4. Conclusions
CIPN is a common and persistent complication of commonly used chemotherapy drugs. The empiric identification of agents and interventions to mitigate CIPN has been disappointing, and presently there is no intervention available for prevention, and only one agent (duloxetine) has shown moderate evidence of treatment efficacy. Considering the debilitating consequences of CIPN on quality of life, it is imperative that future studies focus on details of events and biological pathways leading up to CIPN and that the development of effective clinical interventions should be derived from these mechanistic insights. Furthermore, the development, use and further refinement of appropriately predictive non-clinical models is urgently needed as this will be provide a critical first step in ultimately identifying safe and effective treatments for prevention or intervention. Indeed, it is likely that the failure to provide reproducible approaches and the current absence of effective strategies to combat CIPN is at least partially related to the prior use of model organisms without consideration of strain differences, age- and sex-dependence of phenotypes of interest, as well as the use of unstandardized behavioral tests, a failure to apply adequately-powered study designs that include appropriate controls and randomization.
Despite these study design problems, substantial progress has been made in recent years in our understanding of the pathogenesis for CIPN, and this work has provided important new mechanistic insights and a rationale for novel pharmacological and non-pharmacological strategies. Several of these emerging therapeutic strategies have focused on targeting neuronal transporters, neuroprotective mechanisms, neuro-inflammation, mitochondrial enzymes and oxidative stress, serotonin-norepinephrine reuptake, and nociceptor sodium channel inhibition, and many of these approaches are now under clinical evaluation. These clinical studies provide additional challenges that require careful consideration, and include selection of eligibility criteria, selection of outcome measures and endpoints, potential effects of the intervention on the efficacy of chemotherapy, and statistical issues related to sample sizes of randomized groups based on anticipated effect size and variability in the primary endpoints. In light of these considerations, it is our contention that the future development of improved, efficient intervention strategies for CIPN requires a developmental, collaborative, reverse-translational strategy that involves a multidisciplinary team of experienced pharmacologists, statisticians, and oncologists. Ultimately, such advances will help guide the care of millions of cancer survivors who are suffering from CIPN and its quality of life sequela.
References
- Jaggi, A.S.; Singh, N. Mechanisms in cancer-chemotherapeutic drugs-induced peripheral neuropathy. Toxicology 2012, 291, 1–9, doi:10.1016/j.tox.2011.10.019.
- Quasthoff, S.; Hartung, H.P. Chemotherapy-induced peripheral neuropathy. Neurol. 2002, 249, 9–17, doi:10.1007/pl00007853.
- Seretny, M.; Currie, G.L.; Sena, E.S.; Ramnarine, S.; Grant, R.; MacLeod, M.R.; Colvin, L.A.; Fallon, M. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain 2014, 155, 2461–2470, doi:10.1016/j.pain.2014.09.020.
- Hu, S.; Huang, K.M.; Adams, E.J.; Loprinzi, C.L.; Lustberg, M. Recent Developments of Novel Pharmacologic Therapeutics for Prevention of Chemotherapy-Induced Peripheral Neuropathy. Cancer Res. 2019, 25, 6295–6301, doi:10.1158/1078-0432.ccr-18-2152.
- Cavaletti, G.; Alberti, P.; Argyriou, A.A.; Lustberg, M.; Staff, N.P.; Tamburin, S.; on behalf of the Toxic Neuropathy Consortium of the Peripheral Nerve Society. Chemotherapy‐induced peripheral neurotoxicity: A multifaceted, still unsolved issue. Peripher. Nerv. Syst. 2019, 24, S6–S12, doi:10.1111/jns.12337.
- Loprinzi, C.L.; Lacchetti, C.; Bleeker, J.; Cavaletti, G.; Chauhan, C.; Hertz, D.L.; Kelley, M.R.; Lavino, A.; Lustberg, M.B.; Paice, J.A.; et al. Prevention and Management of Chemotherapy-Induced Peripheral Neuropathy in Survivors of Adult Cancers: ASCO Guideline Update. Clin. Oncol. 2020, 38, 3325–3348, doi:10.1200/jco.20.01399.
- Germain, D.S.; O’Mara, A.M.; Mph, J.L.R.; Torres, A.D.; Minasian, L.M. Chemotherapy-induced peripheral neuropathy: Identifying the research gaps and associated changes to clinical trial design. Cancer 2020, doi:10.1002/cncr.33108.
- Dorsey, S.G.; Kleckner, I.R.; Barton, D.; Mustian, K.; O’Mara, A.; St. Germain, D.; Cavaletti, G.; Danhauer, S.C.; Hershman, D.L.; Hohmann, A.G. The National Cancer Institute clinical trials planning meeting for prevention and treatment of chemo-therapy-induced peripheral neuropathy. JNCI J. Natl. Cancer Inst. 2019, 111, 531–537.
- Gadgil, S.; Ergün, M.; Heuvel, S.A.V.D.; Van Der Wal, S.E.; Scheffer, G.J.; Hooijmans, C.R. A systematic summary and comparison of animal models for chemotherapy induced (peripheral) neuropathy (CIPN). PLoS ONE 2019, 14, e0221787, doi:10.1371/journal.pone.0221787.
- Zajączkowska, R.; Kocot-Kępska, M.; Leppert, W.; Wrzosek, A.; Mika, J.; Wordliczek, J. Mechanisms of chemotherapy-induced peripheral neuropathy. J. Mol. Sci. 2019, 20, 1451.
- Brewer, J.R.; Morrison, G.; Dolan, M.E.; Fleming, G.F. Chemotherapy-induced peripheral neuropathy: Current status and progress. Oncol. 2016, 140, 176–183, doi:10.1016/j.ygyno.2015.11.011.
- Wozniak, K.M.; Vornov, J.J.; Wu, Y.; Liu, Y.; Carozzi, V.A.; Rodriguez-Menendez, V.; Ballarini, E.; Alberti, P.; Pozzi, E.; Semperboni, S.; et al. Peripheral Neuropathy Induced by Microtubule-Targeted Chemotherapies: Insights into Acute Injury and Long-term Recovery. Cancer Res. 2018, 78, 817–829, doi:10.1158/0008-5472.can-17-1467.
- Stage, T.B.; Hu, S.; Sparreboom, A.; Kroetz, D.L. Role for Drug Transporters in Chemotherapy‐Induced Peripheral Neuropathy. Transl. Sci. 2020, doi:10.1111/cts.12915.