
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Rossella Sartorius | + 4209 word(s) | 4209 | 2021-02-07 10:50:53 | | | |
| 2 | Vivi Li | + 6 word(s) | 4215 | 2021-02-20 02:56:56 | | |
Video Upload Options
The pharmaceutical use of bacteriophages as safe and inexpensive therapeutic tools is collecting renewed interest. The use of lytic phages to fight antibiotic-resistant bacterial strains is pursued in academic and industrial projects and is the object of several clinical trials. On the other hand, filamentous bacteriophages used for the phage display technology can also have diagnostic and therapeutic applications. Filamentous bacteriophages are nature-made nanoparticles useful for their size, the capability to enter blood vessels, and the capacity of high-density antigen expression. In the last decades, filamentous bacteriophage ‘fd’ was employed as antigen delivery system, able to trigger all arms of the immune response, with particular emphasis on the ability of the MHC class I restricted antigenic determinants displayed on phages to induce strong and protective cytotoxic responses.Moreover, fd bacteriophages, engineered to target mouse dendritic cells (DCs), activate innate and adaptive responses without the need of exogenous adjuvants, and more recently was employed for the display of immunologically active lipids.
1. Introduction
Bacteriophages only infect and multiply with their specific host and currently, as bacterial resistance to antibiotics becomes widespread, the therapeutic use of bacteriophages is back on the agenda [1]. Recently, based on successful treatments in five people with phage cocktails under a U.S. food and drug administration (FDA) process designed for emergencies, the University of California, San Diego (UCSD) is launching the Center for Innovative Phage Applications and Therapeutics (IPATH) to refine phage treatment and perform phage trials focused on patients with a single, known bacterial infection and without withholding other potentially beneficial treatments, including antibiotics [2]. In this context, an efficacious clinical treatment of Mycobacterium abscessus infection in a 15 years-old cystic fibrosis patient using a cocktail of three genetically engineered phages was recently reported [3], re-launching the use of bacteriophages as nanopharmaceuticals against antibiotic-resistant bacteria.
Besides the use of lytic phages as antibacterials, which has been extensively reviewed elsewhere [2], a collection of evidence was obtained in recent years on the potential translational usage of filamentous bacteriophages.
Filamentous phages are single-strand DNA virions belonging to the Inoviridae family; phages f1, fd, and M13 are a sub-group of rod-like shaped Inoviruses with a repeated and ordered capsid structure. They are closely related species, sharing almost the same genome (with only about 1–2% of difference) that infect and replicate in Escherichia coli bacterial cells and which are often collectively referred to as Ff phages.
Their peculiar proteic structure, together with the flexibility of the DNA genome and the easiness of purification has fostered their application in the phage display technology, with particular attention to the production of therapeutic antibodies first, and then as antigen delivery system for the development of new vaccine formulations [4][5].
Although practically every filamentous bacteriophage coat protein can be used to display foreign amino acidic sequences, the pVIII protein is the most used for the expression of exogenous peptides in high copy numbers. The pIII protein, instead, has been successfully used for the expression of up to five copies per virion of the receptor-ligand and single-chain antibody fragment (scFv) [6] (Figure 1).
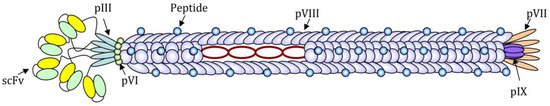
Figure 1. Schematic image of a filamentous bacteriophage nanoparticle engineered for the expression of a short antigenic peptide as a fusion with N-terminus of the pVIII protein and a single-chain antibody fragment (scFv) for the targeting, as a fusion with the N-terminus of the pIII protein. The circular single-strand DNA rich in CpG motifs can be recognized by Pattern Recognition Receptors (PPR) and acts as an adjuvant.
The foreign sequence expression is highly stable since the (poly)peptides of interest are genetically fused to phage proteins and not linked by chemical bonds. Small peptides can be easily expressed on all copies of the pVIII major coat protein, whereas for the expression of longer sequences (14–20 amino acids), such as immunologically relevant peptides, it is often necessary to construct hybrid phages, which express recombinant copies of the pVIII interspersed with wild-type copies, to guarantee a high expression of the exogenous sequence without affecting the stability of the phage lattice. Due to the highly-symmetrical and repeated structure, Ff phages are suitable for the high-density exposure of one or more epitopes on the coat surface. Overall phage nanoparticles are made of highly organized monomers represented mainly by the pVIII protein (5.5 kDa), an alpha-helix closely packed to compose a right-handed helical latex with a rod-like structure.
Combining a relatively simple surface structure with a particulate nature and with adjuvant properties, filamentous phages represent ideal nanoparticles compared to other carriers. Drugs can be easily conjugated to the phage surface by chemical modification of amino and carboxyl groups exposed in the amino terminus of the pVIII protein. Furthermore, the filamentous phages are extremely tolerant to variations in the size of their genome, which makes them versatile for engineering through the phage display technique, allowing the successful expression of B and T (CD4+ and CD8+) epitopes on the phage nanoparticles, and between the end of the 1990s and the first decade of 2000, filamentous phages were widely tested as immunogenic nanocarriers both in vitro and in vivo [6][7][8].
2. The Use of Filamentous Bacteriophages for the Induction and Analysis of Antibody Response
Filamentous bacteriophages can be utilized both to analyze the specificity of antibody responses and to induce a humoral immune reaction. In some instances, filamentous phages have been utilized to integrate epitope discovery and immunization functions into a single platform.
Precise determination of conformational epitopes of neutralizing antibodies represents a crucial step in the rational design of novel vaccines; the screening of random peptide libraries displayed on phages, and of gene or genome fragment phage libraries provides a powerful, cheap and quick technique for epitope mapping [9]. Phages bearing peptides that reproduce an epitope of interest can be selected from random peptide phage-display libraries, displaying more than 109 different peptides, utilizing monoclonal or polyclonal antibodies; in some cases, the selected peptide-displaying phage can then be used as an immunogen, to induce an antibody response [10][11]. Filamentous bacteriophage M13 is most commonly used to generate combinatorial libraries. Phages selected by phage library biopanning rarely display peptides that are identical in their primary sequence to a peptide of the protein antigen of interest; the method more commonly leads to the identification of mimotope peptides. A mimotope peptide can mimic both a linear epitope and a discontinuous conformational epitope [12]; it can also mimic a non-peptidic epitope, such as a lipopolysaccharide epitope or a glycan [13][14]. Genome-fragment phage display libraries are collections of phages engineered to express short polypeptides of a pathogen’s proteome. Phage libraries that contained fragments of the genome of the H5N1 strain responsible for an outbreak of human influenza in Vietnam in 2004–2005 were used to analyze the antibodies that made people recover from the infection [15]. The insert sizes, in the pIII gene, ranged between 50–200 base pairs (bp) and 200–1000 bp. This strategy allowed the identification of noncontinuous conformation-dependent epitopes—protein sequences that are not adjacent to one another in the polypeptide sequence of the protein, but that lie close together in space in the folded protein [15]. Moreover, whole-genome-fragment phage display libraries followed by surface plasmon resonance technologies were used to elucidate the effect of different adjuvants on the antibody repertoire against an H5N1 vaccine in humans [16]. A whole-genome phage display library spanning the entire Zika virus genome has been used for in-depth immune profiling of IgG and IgM antibody repertoires in longitudinal serum and urine samples from individuals acutely infected with Zika virus [17]. Furthermore, phage-displayed peptide libraries have been utilized to identify B epitopes on allergens, pathogens, and human proteins [18][19][20][21][22]. Thus, phage libraries are invaluable tools for the epitope specificity of antibody response analysis. In some cases, the phages selected from these libraries have been tested as vaccines.
The use of filamentous phages for the induction of antibody responses has been tested by researchers involved in the generation of vaccines against fungal infections. There is still no approved antifungal vaccine or antibody for use in humans; it is clear that humoral and cellular immunities are the most important host defense mechanisms against fungal infections; severe infections mainly occur in immunocompromised patients. In mice, vaccination with filamentous bacteriophages displaying a peptide from Fructose-bisphosphate aldolase1 (Fba1 epitope YGKDVKDLFDYAQE) of Candida albicans induced humoral and cellular immune responses, reduced fungal burden, and relieved kidney damage in infected mice, and significantly improved their survival rates. However, the protective efficacy of the phage vaccines was lower than the efficacy of vaccination with the whole Fba1 protein [23].
Another phage-based vaccination strategy against Candida albicans focused on heat shock protein (HSP) 90, a protein that plays a key pathogenic role in systemic infection. Hybrid-phage particles expressing the HSP90 DEPAGE epitope induced the specific antibody against HSP90, enhanced the cellular immune responses in mice, and afforded some protection from systemic candidiasis; hybrid-phage-immunized mice had fewer Colony-Forming Units in the kidneys compared with wild-type-immunized mice and vehicle-injected mice and had a statistically significant survival advantage over vehicle-injected group [24]. Furthermore, an fd bacteriophage functionalized with peptides from secreted aspartyl proteinase (Sap) 2 and Hsp90 was designed to capture or induce anti-Sap2 IgG and anti- Hsp90 IgG simultaneously since anti-Sap2 antibodies were found to be protective in people with systemic candidiasis [25].
Vaccination with filamentous bacteriophages has also been tested against another fungal pathogen, Sporothrix globosa. In this case, an epitope peptide (sequence KPVGHALLTPLGLDR) derived from a 70 KDa glycoprotein of S. globosa, that can induce a protective response, was displayed on the major coat protein pIII. Immunization with recombinant phage increased the survival rate of mice following S. globosa infection. The nature of the mechanisms underlying the resolution of infection in mice treated with recombinant phage is unresolved; it can be hypothesized that protective antibodies are elicited, which also increases the cell-mediated immune response [26].
Filamentous phages fd as nanocarriers of B cell epitopes proved useful for the induction of antibody responses to β-amyloid peptide, which is the main component of the plaques present in the brain of Alzheimer’s Disease (AD) patients. Immunization studies performed in transgenic mouse models of β-amyloid deposition have demonstrated that antibodies against β-amyloid can reduce amyloid load and improve cognition. The N-terminus of the beta-amyloid peptide is considered the most promising antibody target for inclusion in recombinant vaccines [27][28]. Antibody 6C6, a mouse monoclonal that disaggregates β-amyloid fibrils in vitro, was used to screen a random 15-mer peptide phage library, leading to the selection of several phages displaying the EFRH sequence, a B cell epitope corresponding to amino acids 3–6 within the human β-amyloid peptide [29]. Upon immunization, the phages displaying sequence EFRH induced antibodies with the same disaggregating properties as 6C6 [29]. Subsequent studies investigated the immunogenicity of phages that displayed varying numbers of copies of the EFRH epitope on the phage nanoparticles.
In particular, Solomon and collaborators generated phages containing around 150 recombinant copies of pVIII protein out of the 2700 copies of pVIII. The recombinant pVIII was modified to display either the EFRH sequence or the tandem repeat EFRHEFRH. The phages bearing the tandem repeat, and thus around 300 copies of epitope EFRH per nanoparticle, induced higher antibody titers than phages expressing 150 copies of the epitope, an observation that highlights the relevance of epitope density in phage immunizations [30]. Importantly, AD model mice treated with phages that express the EFRH epitope show a considerable improvement in their amyloid load and cognitive behavior [30][31].
We compared the immunogenicity of four different portions of the beta-amyloid peptide, displayed on filamentous phages fd, and observed that sequence AEFRH (corresponding to amino acids 2–6 within the human β-amyloid peptide) was the most immunogenic. As the first two amino acids of the processed N-terminus of the pVIII protein are an alanine (A) and a glutamic acid (E), we inserted in the pVIII only three extra amino acids, namely sequence FRH, to obtain sequence AEFRH. This strategy afforded the display of epitope 2–6 (AEFRH), at a density of 810 copies per phage particle [4][32]. AD model mice immunized monthly with AEFRH phages from age two months displayed a significant reduction in the number of β-amyloid plaques in the hippocampus and cortex at eight months. We observed however that the dosing protocol strongly affected the efficacy of vaccination with AEFRH phages; therefore, we hypothesize that the titer and affinity of the induced antibodies are affected by the dosing protocol and are crucial for efficacy [32][33][34][35]. Overall, the analysis of different epitopes of beta-amyloid suggests that the intensity of antibody response depends on characteristics of the displayed peptide, the method of its display and the dosing protocol. An epitope displayed in a high copy number on the surface of a phage is more effective in eliciting high antibody titers than the same epitope displayed in low copy number. A potential problem in single epitope anti-beta-amyloid vaccines is inter-individual variability in anti-beta-amyloid antibody titer and in the development of immunological memory to the B epitope of interest [36][37]. In this context, we are currently investigating the effect of the dosing protocol on these immunologic outcomes. We hypothesize that vaccines containing several epitopes may mitigate the problem of inter-individual variability in the immune response.
Overall, filamentous phages proved very useful for the analysis of antibody specificity. As regards their use as immunogens, the data in the literature show that phage-displaying B cell epitopes in high copy on the pVIII coat protein are often able to induce an antibody response against the displayed antigenic determinant. One problem, however, is that the induction of an antibody response against a single peptide does not afford protection. Moreover, inter-individual variability in titer and memory may prove a general roadblock in the development of vaccines that focus the antibody response against a single B cell epitope; further research is required to address this problem, and the use of mixtures of phages displaying different epitopes being one of the ways to overcome it.
3. Filamentous Bacteriophage Nanocarriers for the Induction of Cellular Immune Responses
Hybrid filamentous virions expressing T helper (Th) peptides derived from HIV-1 (epitope Pep23, corresponding to residues 249–263 of the RTase) [38][39] or Human Cytomegalovirus (HCMV epitopes p128 and p30 of Pp65 protein) [40] as a fusion with the major coat protein pVIII were recognized by specific CD4+-restricted human T-cell lines and T cell clones and enhanced peptide antigenicity. Moreover, as mentioned in the previous paragraph, B and T cell epitopes from C. albicans HSP90 protein exposed on the pIII protein of recombinant bacteriophages and administered to mice, induced antibodies as well as cell-mediated immune responses and prolonged mice survival after in vivo systemic candidiasis challenge [24][41]. The protective response induced by the Candida antigen exposed on the phage surface was manly Th1 and Th17 oriented, with the production of cytokines like IL-2, IL-12, IFN-γ, and IL-17, conferring resistance to most fungal pathogens [23][42].
Indeed, the use of bacteriophages as nanocarriers of antigenic peptides demonstrated effective mainly in the induction of specific cytotoxic T cell responses.
Several cytotoxic T lymphocytes (CTL) epitopes were displayed on the filamentous phage lattice. Bacteriophages were shown to be effective in triggering a cytotoxic response to HLA-A2 restricted epitope ILKEPVHGV from HIV-1 reverse transcriptase (residues 309–317) [43][44] and Hepatitis B virus epitope S28–39 [45]. Mascolo et al. reported that the CTL response evoked in mice by administration of recombinant bacteriophage appeared to be mediated by the induction of T CD4+ lymphocytes stimulated by Th epitopes contained in the proteins of the phage capsid itself [46]. However, even if the co-display of an engineered helper peptide proved dispensable for the primary short-lived cytotoxic response, it was required for the induction of long-term memory CTLs [47].
It can be hypothesized that the success of filamentous bacteriophages as a nanodelivery system to induce cell-mediated responses against high-density expressed antigens is mainly due to the ability of the filamentous rod to be internalized and processed by antigen-presenting cells (APCs) and to the presentation of the displayed peptides in association with both MHC class II and MHC class I molecules [40][48]. Using macrophages to study recombinant phage particle intracellular fate, Wan et al. [49] demonstrated by confocal microscopy that the MHC class I-peptide exposed on bacteriophages are translocated from endosomes to ER during phagocytosis, suggesting that the mechanism of cross-presentation of phage particles occurs within the endocytic pathway: Endocytosed phage particles are transferred to the proteasome-mediated degradation pathway and peptides are then re-imported into phagosomes via the TAP complex for loading onto MHC class I molecules.
Phage-based nanovaccines have also been formulated for cancer treatment and prevention. The tumor-specific epitope 161–169 (EADPTGHSY) from melanoma-associated antigen (MAGE) A1 was successfully expressed on the surface of bacteriophage fd and used in vivo in a mouse model. MAGE-A1 bacteriophage injection elicited EADPTGHSY-specific cytotoxic T lymphocytes (CTL) responses and NK activity, protecting mice from tumor progression both in therapeutic and prophylactic schedule [50]. Similarly, phage expressing tumor antigen P1A35–43 prevented or suppressed tumor growth in a P815 murine mastocytoma model promoting CTL and Th1-oriented cellular responses [51]. Immunization with double hybrid filamentous bacteriophages co-expressing a tumor peptide from MAGE-A10 (amino acids 254–269) or MAGE-A3 (amino acids 271–279) together with a Th peptide of viral origin (Pep23) protected humanized HHD (HLA-A2.1/H2-Db) transgenic mice from tumor growth. In addition, human anti-MAGE-A3271–279-specific T cell clones isolated from bacteriophage-stimulated T cell lines showed high avidity for the MAGE-A3 epitope and were able to kill human MAGE-A3-tumor cell lines expressing a low amount of MAGE-A3271–279 peptide-HLA complex on the surface [52]. Immunization with a combination of wild-type bacteriophage fd in the presence of the Th and MAGE-A3271–279 CTL synthetic peptides did not induce CTL responses, demonstrating the ability of this nanocarrier to enhance the immunogenicity of the displayed peptides.
More recently, Roehnisch et al. developed an M13 bacteriophage nanovaccine against multiple myeloma (MM) and tested it in vivo in a phase I/II trial on human patients in the terminal stage of MM [53]. Bacteriophages chemically linked to idiotype (Id) proteins isolated from patients were generated and used to induce tumor-specific immune responses against MM cells. The results showed that intradermal immunization with Id-bacteriophages induced reduction of paraprotein levels in the blood, a symptom of responsiveness to the phage vaccination. Besides, several patients developed not only anti-Id antibodies but also a cellular response, as demonstrated by the positive delayed-type hypersensitivity (DTH) reaction induced in anti-Id positive patients. The DTH lesions revealed that phage administration in humans induces infiltration of neutrophils, macrophages, and CD8+ T cells. Moreover, peripheral blood mononuclear cells (PBMCs) from vaccinated patients were able to kill specifically myeloma target cells isolated from the bone marrow of the same patients. The phage vaccination was well tolerated by all the trial participants, demonstrating the safety and efficacy of bacteriophage nanovaccines.
The filamentous bacteriophage delivery system can overcome the induction of immune tolerance due to self-antigens in cancer. Anti-HER2 phage-based vaccination breaks self-tolerance and can delay tumor progression in HER2 transgenic mice, inducing not only a sustained humoral response but also a massive infiltration of CD3+ cells in the tumors [54]. This phenomenon could be due to the high self-immunogenicity of the carrier. The delivery of bacteriophages into the tumor bed using the display of tumor ligands on the phage scaffold strongly induces macrophages and neutrophils recruitment and the release of Th1 cytokines that promotes the proinflammatory environment and activates immune responses toward cancer cells [55][56]. Carcinoembryonic antigen [CEA]-targeted M13 bacteriophage in vivo injected in mice implanted with colorectal tumors induced tumor infiltration of both neutrophils and tumor-associated macrophages (TAM), maturation of dendritic cells (DCs), and strong CD8+ T cell-mediated antitumor responses [56].
These mechanisms were demonstrated to be MyD88-dependent [57] and regulated by Toll-like receptor 9 (TLR9) [58] via unmethylated CpG motif contained into the single-stranded DNA genome of the virion [59]. Recently, Gomes-Neto et al. have shown that the in vivo vaccination with filamentous bacteriophages fd expressing PA8 and TSKB20 CD8+ epitopes from human protozoan Trypanosoma cruzi induced both humoral and cytotoxic responses and protected mice against parasitemia and parasite burden induced by a high-dose inoculum of T. cruzi parasite [60]. This effect was completely abolished in TLR9-/- mice, reinforcing the idea that bacteriophages induce cellular and humoral responses through a TLR9-dependent mechanism.
3.1. Use of Filamentous Bacteriophages for Cell Targeting
The most efficient way to mount a sustained immune response is to deliver the antigens specifically to APCs to trigger both innate and adaptive immunity. This mission can be accomplished by the co-administration of both antigen and adjuvant to activate defined subsets of professional APCs such as DCs. Simultaneous delivery of antigen and adjuvant to the same antigen-presenting cell allows only cells internalizing the antigen to receive the proper activation signal, avoiding the induction of T-cell anergy due to the antigen presentation in the absence of co-stimuli.
A much-wanted option is thus to produce new generation vaccines able to combine pattern recognition receptors (PRR) ligands with antigenic proteins and to deliver them via specific targeting to DCs. In this context, we recently showed that filamentous bacteriophages fd could be engineered to target mouse DCs. Phage scaffold proteins are very tolerant of genetic modification, and their flexibility allows the generation of multivalent bacteriophages displaying two or more different exogenous peptides/proteins with targeting functionality and/or immunogenic proprieties. Phage display of an scFv directed to the DC surface receptor DEC205 significantly enhanced the virions uptake by DCs and strengthened cytotoxic T cell response against the ovalbumin (OVA)257–264 epitope co-expressed on the phage coat [61]. DEC205 is an endocytic receptor that internalizes its ligands into clathrin-coated vesicles and drives them into the deep endosomes or lysosomes, enhancing the MHC-antigen presentation [62]. Targeting the fd virion to this receptor leads to an improved and faster internalization. In addition, mice vaccinated with the hybrid fd virions inhibited the growth of the OVA-expressing tumors. The active delivery of bacteriophages into DEC205+ DCs favors DC maturation, inducing up-regulation of costimulatory molecules, cytokines, and chemokines release and DC migration to peripheral lymph nodes where adaptive immune responses will be initiated (Figure 2).
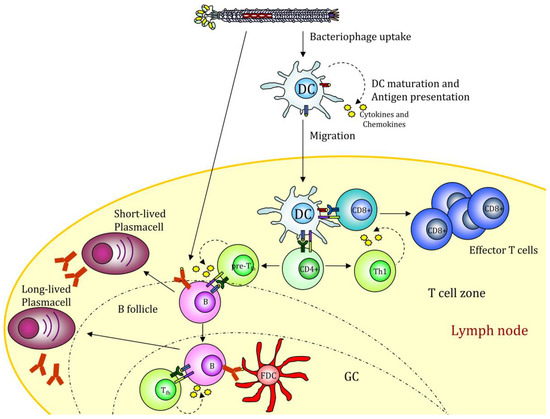
Figure 2. Filamentous bacteriophage nanovaccine can stimulate the humoral response or be taken up by antigen-presenting cells (APCs). The displayed antigenic peptides are processed and presented on MHCI and II molecules, leading to a CD4 and CD8 immune response. The presence of CpG sequences into the phage genome drives APC maturation. GC (Germinal Center); FDC (Follicular Dendritic Cell). The figure depicts the possible scenario of target mediated fd internalization based on scientific results reported in [38][48].
More recently, we demonstrated that the increase of proinflammatory cytokines and type I interferon by DCs targeted via DEC205 bacteriophages was due to the receptor-mediated internalization of bacteriophages into LAMP-1-positive late endosomal and lysosomal compartments of the DCs, where the active form of TLR9 resides. Thus, the DEC205 targeting strategy, enhancing the virion uptake, optimizes the TLR9 activation by the unmethylated CpG motifs contained into the single-strand DNA phage genome, increasing the presentation efficacy of the DCs [63].
The active delivery of bacteriophages to a target tissue or cell population via phage display of ligands for specific receptors can enhance bacteriophage therapeutic efficacy; currently, multidisciplinary approaches are used to identify tumor-binding ligands or vessel-specific homing peptides, including in vivo panning in cancer patients for the discovery of tumor-binding antibodies and peptides.
While injected, untargeted bacteriophages are distributed throughout the body, phages expressing peptides or antibodies against a specific target organ accumulate at the binding site, allowing the recovery of phages with high binding capability. In vivo phage-display library panning in human patients with stage IV cancer, including breast, malignant melanoma, and pancreas tumors, led to the recovery of tumor binding phages from the surgically excised cancer tissue and to the identification of tumor-homing peptides and binding antibodies that are unique targets for an individual patient and useful in diagnostic procedures or for drug delivery in cancer treatment [64][65]. Intravenous infusions of bacteriophages do not cause allergy or other immediate or delayed severe adverse reactions, supporting the safety of filamentous bacteriophage applications in humans [65].
Proteomics-based strategies [66] and next-generation sequencing (NGS) for in-depth analysis of the phage libraries [67] have further enhanced the phage display technique for the identification of pharmacological and diagnostic tools. Combining the screening of synthetic scFv library expressing on phage with the use of an NGS platform permits to increase the numbers of identified scFv candidates, covering a wide range of epitopes on the target protein, to drastically reduce the numbers of rounds of selection and also to follow the in vitro evolution of virtually all variable CDR3 sequences during the panning process leading to the identification of high-affinity antibodies [67].
3.2. Further Improvement by Coupling Immunologically Active Molecules to Filamentous Bacteriophages
Filamentous bacteriophages engineered for targeting of different tumors or tissue were used not only for the delivery of immunogenic epitopes as a fusion with the coat proteins but were also engineered for the targeted nanodelivery of therapeutic agents, drugs [68], fluorescent dyes for imaging and diagnostic [69], DNA cassettes [70], and siRNA [71].
Recently, the high-content of hydrophobic residues contained in the core domain of the pVIII protein [72][73] was exploited to promote the association of the surface major coat protein of the fd phage with the alpha-GalactosylCeramide (α-GalCer), a lipid stimulating invariant natural killer T (iNKT) cells [74]. It is likely that bacteriophage fd/α-GalCer conjugates are mainly internalized by DCs and the delivered α-GalCer is presented in association with the CD1d molecules expressed on the surface of DCs. α-GalCer-bacteriophages were demonstrated able to repeatedly stimulate iNKT to proliferate and to produce cytokines without the induction of iNKT anergy that is commonly found using the soluble lipid [75]. Moreover, therapeutic vaccination with recombinant bacteriophages functionalized with α-GalCer and decorated with a tumor-associated peptide increased the induction of antigen-specific CD8+ T cells and delayed tumor growth in mice, promoting the recruitment of tumor-specific CD8+ T cells inside the tumor bed [74].
References
- Reardon, S. Phage therapy gets revitalized. Nature 2014, 510, 15–16.
- Schmidt, C. Phage therapy’s latest makeover. Nat. Biotechnol. 2019, 37, 581–586.
- Dedrick, R.M.; Guerrero-Bustamante, C.A.; Garlena, R.A.; Russell, D.A.; Ford, K.; Harris, K.; Gilmour, K.C.; Soothill, J.; Jacobs-Sera, D.; Schooley, R.T.; et al. Engineered bacteriophages for treatment of a patient with a disseminated drug-resistant Mycobacterium abscessus. Nat. Med. 2019, 25, 730–733.
- Prisco, A.; De Berardinis, P. Filamentous Bacteriophage Fd as an Antigen Delivery System in Vaccination. Int. J. Mol. Sci. 2012, 13, 5179–5194.
- Hassapis, K.A.; Stylianou, D.C.; Kostrikis, L.G. Architectural Insight into Inovirus-Associated Vectors (IAVs) and Development of IAV-Based Vaccines Inducing Humoral and Cellular Responses: Implications in HIV-1 Vaccines. Viruses 2014, 6, 5047–5076.
- Sartorius, R.; Russo, D.; D’Apice, L.; De Berardinis, P.; Berardinis, P. Filamentous Bacteriophages: An Antigen and Gene Delivery System. In Innovation in Vaccinology; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2012; pp. 123–134.
- Henry, K.A.; Arbabi-Ghahroudi, M.; Scott, J.K. Beyond phage display: Non-traditional applications of the filamentous bacteriophage as a vaccine carrier, therapeutic biologic, and bioconjugation scaffold. Front. Microbiol. 2015, 6, 755.
- Aghebati-Maleki, L.; Bakhshinejad, B.; Baradaran, B.; Motallebnezhad, M.; Aghebati-Maleki, A.; Nickho, H.; Yousefi, M.; Majidi, J. Phage display as a promising approach for vaccine development. J. Biomed. Sci. 2016, 23, 66.
- Potocnakova, L.; Bhide, M.; Pulzova, L.B. An Introduction to B-Cell Epitope Mapping and In Silico Epitope Prediction. J. Immunol. Res. 2016, 2016, 6760830.
- Wang, L.F.; Yu, M. Epitope Identification and Discovery Using Phage Display Libraries: Applications in Vaccine Development and Diagnostics. Curr. Drug Targets 2004, 5, 1–15.
- Villa-Mancera, A.; Reynoso-Palomar, A.; Utrera-Quintana, F.; Carreon-Luna, L. Cathepsin L1 mimotopes with adjuvant quil a induces a th1/th2 immune response and confers significant protection against fasciola hepatica infection in goats. Parasitol. Res. 2014, 113, 243–250.
- Schiavone, M.; Fiume, G.; Caivano, A.; De Laurentiis, A.; Falcone, C.; Masci, F.F.; Iaccino, E.; Mimmi, S.; Palmieri, C.; Pisano, A.; et al. Design and Characterization of a Peptide Mimotope of the HIV-1 gp120 Bridging Sheet. Int. J. Mol. Sci. 2012, 13, 5674–5699.
- Xin, H.; Glee, P.; Adams, A.; Mohiuddin, F.; Eberle, K. Design of a mimotope-peptide based double epitope vaccine against disseminated candidiasis. Vaccine 2019, 37, 2430–2438.
- Mertens, P.; Walgraffe, D.; Laurent, T.; Deschrevel, N.; Letesson, J.J.; De Bolle, X. Selection of Phage-displayed Peptides Recognised by Monoclonal Antibodies Directed against the Lipopolysaccharide of Brucella. Int. Rev. Immunol. 2001, 20, 181–199.
- Khurana, S.; Suguitan, A.L.; Rivera, Y.; Simmons, C.P.; Lanzavecchia, A.; Sallusto, F.; Manischewitz, J.; King, L.R.; Subbarao, K.; Golding, H. Antigenic Fingerprinting of H5N1 Avian Influenza Using Convalescent Sera and Monoclonal Antibodies Reveals Potential Vaccine and Diagnostic Targets. PLoS Med. 2009, 6, e1000049.
- Khurana, S.; Chearwae, W.; Castellino, F.; Manischewitz, J.; King, L.R.; Honorkiewicz, A.; Rock, M.T.; Edwards, K.M.; Del Giudice, G.; Rappuoli, R.; et al. Vaccines with MF59 Adjuvant Expand the Antibody Repertoire to Target Protective Sites of Pandemic Avian H5N1 Influenza Virus. Sci. Transl. Med. 2010, 2.
- Ravichandran, S.; Hahn, M.; Belaunzarán-Zamudio, P.F.; Ramos-Castañeda, J.; Nájera-Cancino, G.; Caballero-Sosa, S.; Navarro-Fuentes, K.R.; Ruiz-Palacios, G.; Golding, H.; Beigel, J.H.; et al. Differential human antibody repertoires following Zika infection and the implications for serodiagnostics and disease outcome. Nat. Commun. 2019, 10, 1943.
- Luzar, J.; Štrukelj, B.; Lunder, M. Phage display peptide libraries in molecular allergology: From epitope mapping to mimotope based immunotherapy. Allergy 2016, 71, 1526–1532.
- Zahirović, A.; Lunder, M. Microbial Delivery Vehicles for Allergens and Allergen-Derived Peptides in Immunotherapy of Allergic Diseases. Front. Microbiol. 2018, 9, 1449.
- Jensen-Jarolim, E.; Leitner, A.; Kalchhauser, H.; Zürcher, A.; Ganglberger, E.; Bohle, B.; Scheiner, O.; Boltz-Nitulescu, G.; Breiteneder, H. Peptide mimotopes displayed by phage inhibit antibody binding to Bet v 1, the major birch pollen allergen, and induce specific IgG response in mice. FASEB J. 1998, 12, 1635–1642.
- Yang, Y.; Cao, M.J.; Alcocer, M.; Liu, Q.M.; Fei, D.X.; Mao, H.Y.; Liu, G.M. Mapping and characterization of antigenic epitopes of arginine kinase of Scylla paramamosain. Mol. Immunol. 2015, 65, 310–320.
- Tonelli, R.R.; Colli, W.; Alves, M.J. Selection of binding targets in parasites using phage-display and aptamer libraries in vivo and in vitro. Front. Immunol. 2012, 3, 419.
- Shi, H.; Dong, S.; Zhang, X.; Chen, X.; Gao, X.; Wang, L. Phage vaccines displaying YGKDVKDLFDYAQE epitope induce protection against systemic candidiasis in mouse model. Vaccine 2018, 36, 5717–5724.
- Wang, G.; Sun, M.; Fang, J.; Yang, Q.; Tong, H.; Wang, L. Protective immune responses against systemic candidiasis mediated by phage-displayed specific epitope of Candida albicans heat shock protein 90 in C57BL/6J mice. Vaccine 2006, 24, 6065–6073.
- Wang, Y.; Shi, H.; Dong, S.; Li, Y.; Wang, M.; Huai, Y.; Zhang, X.; Chen, X.; Mao, C.; Gao, X.; et al. Nontoxic engineered virus nanofibers as an efficient agent for the prevention and detection of fungal infection. Nano Res. 2018, 11, 2248–2255.
- Chen, F.; Jiang, R.; Wang, Y.; Zhu, M.; Zhang, X.; Dong, S.; Shi, H.; Wang, L. Recombinant phage elicits protective immune response against systemic s. Globosa infection in mouse model. Sci. Rep. 2017, 7, 42024.
- Mantile, F.; Basile, C.; Cicatiello, V.; De Falco, D.; Caivano, A.; De Berardinis, P.; Prisco, A. A multimeric immunogen for the induction of immune memory to beta-amyloid. Immunol. Cell Biol. 2011, 89, 604–609.
- Trovato, M.; De Berardinis, P. Novel antigen delivery systems. World J. Virol. 2015, 4, 156–168.
- Frenkel, D.; Katz, O.; Solomon, B. Immunization against Alzheimer’s β-amyloid plaques via EFRH phage administration. Proc. Natl. Acad. Sci. USA 2000, 97, 11455–11459.
- Lavie, V.; Becker, M.; Cohen-Kupiec, R.; Yacoby, I.; Koppel, R.; Wedenig, M.; Hutter-Paier, B.; Solomon, B. EFRH–Phage Immunization of Alzheimer’s Disease Animal Model Improves Behavioral Performance in Morris Water Maze Trials. J. Mol. Neurosci. 2004, 24, 105–114.
- Frenkel, D.; Dewachter, I.; Van Leuven, F.; Solomon, B. Reduction of beta-amyloid plaques in brain of transgenic mouse model of Alzheimer’s disease by EFRH-phage immunization. Vaccine 2003, 21, 1060–1065.
- Esposito, M.; Luccarini, I.; Cicatiello, V.; De Falco, D.; Fiorentini, A.; Barba, P.; Casamenti, F.; Prisco, A. Immunogenicity and therapeutic efficacy of phage-displayed beta-amyloid epitopes. Mol. Immunol. 2008, 45, 1056–1062.
- Castiglione, F.; Mantile, F.; De Berardinis, P.; Prisco, A. How the Interval between Prime and Boost Injection Affects the Immune Response in a Computational Model of the Immune System. Comput. Math. Methods Med. 2012, 2012, 842329.
- Mantile, F.; Trovato, M.; Santoni, A.; Barba, P.; Ottonello, S.; De Berardinis, P.; Prisco, A. Alum and Squalene-Oil-in-Water Emulsion Enhance the Titer and Avidity of Anti-Aβ Antibodies Induced by Multimeric Protein Antigen (1–11)E2, Preserving the Igg1-Skewed Isotype Distribution. PLoS ONE 2014, 9, e101474.
- Prisco, A.; De Berardinis, P. Immunogenicity of B and T epitopes displayed on bacteriophage fd. In Immunogenicity; Villanueva, C.J., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2011; pp. 205–216.
- Mantile, F.; Capasso, A.; De Berardinis, P.; Prisco, A. Identification of a Consolidation Phase in Immunological Memory. Front. Immunol. 2019, 10, 508.
- Prisco, A.; De Berardinis, P. Memory immune response: A major challenge in vaccination. Biomol. Concepts 2012, 3, 479–486.
- DeBerardinis, P. Recognition of HIV-derived B and T cell epitopes displayed on filamentous phages. Vaccine 1999, 17, 1434–1441.
- D’Apice, L.; Sartorius, R.; Caivano, A.; Mascolo, D.; Del Pozzo, G.; Di Mase, D.S.; Ricca, E.; Pira, G.L.; Manca, F.; Malanga, D.; et al. Comparative analysis of new innovative vaccine formulations based on the use of procaryotic display systems. Vaccine 2007, 25, 1993–2000.
- Ulivieri, C.; Citro, A.; Ivaldi, F.; Mascolo, D.; Ghittoni, R.; Fanigliulo, D.; Manca, F.; Baldari, C.T.; Li Pira, G.; Del Pozzo, G. Antigenic properties of hcmv peptides displayed by filamentous bacteriophages vs. Synthetic peptides. Immunol. Lett. 2008, 119, 62–70.
- Yang, Q.; Wang, L.; Lu, D.N.; Gao, R.J.; Song, J.N.; Hua, P.Y.; Yuan, D.W. Prophylactic vaccination with phage-displayed epitope of C. albicans elicits protective immune responses against systemic candidiasis in C57BL/6 mice. Vaccine 2005, 23, 4088–4096.
- Wang, Y.; Su, Q.; Dong, S.; Shi, H.; Gao, X.; Wang, L. Hybrid phage displaying SLAQVKYTSASSI induces protection againstCandida albicanschallenge in BALB/c mice. Hum. Vaccines Immunother. 2014, 10, 1057–1063.
- De Berardinis, P.; Sartorius, R.; Fanutti, C.; Perham, R.N.; Del Pozzo, G.; Guardiola, J. Phage display of peptide epitopes from HIV-1 elicits strong cytolytic responses. Nat. Biotechnol. 2000, 18, 873–876.
- De Berardinis, P.; Sartorius, R.; Caivano, A.; Mascolo, D.; Domingo, G.; Pozzo, G.; Gaubin, M.; Perham, R.; Piatier-Tonneau, D.; Guardiola, J. Use of Fusion Proteins and Procaryotic Display Systems for Delivery of HIV-1 Antigens: Development of Novel Vaccines for HIV-1 Infection. Curr. HIV Res. 2003, 1, 441–446.
- Wan, Y.; Wu, Y.; Bian, J.; Wang, X.; Zhou, W.; Jia, Z.; Tan, Y.; Zhou, L. Induction of hepatitis B virus-specific cytotoxic T lymphocytes response in vivo by filamentous phage display vaccine. Vaccine 2001, 19, 2918–2923.
- Mascolo, D.; Barba, P.; De Berardinis, P.; Di Rosa, F.; Del Pozzo, G. Phage display of a CTL epitope elicits a long-termin vivocytotoxic response. FEMS Immunol. Med. Microbiol. 2007, 50, 59–66.
- Del Pozzo, G.; Mascolo, D.; Sartorius, R.; Citro, A.; Barba, P.; D’Apice, L.; De Berardinis, P. Triggering DTH and CTL activity by fd filamentous bacteriophages: Role of CD4+ T cells in memory responses. J. Biomed. Biotechnol. 2010, 2010, 894971.
- Gaubin, M.; Fanutti, C.; Mishal, Z.; Durrbach, A.; De Berardinis, P.; Sartorius, R.; Del Pozzo, G.; Guardiola, J.; Perham, R.N.; Piatier-Tonneau, D. Processing of Filamentous Bacteriophage Virions in Antigen-Presenting Cells Targets Both HLA Class I and Class II Peptide Loading Compartments. DNA Cell Biol. 2003, 22, 11–18.
- Wan, Y.; Wu, Y.; Zhou, J.; Zou, L.; Liang, Y.; Zhao, J.; Jia, Z.; Engberg, J.; Bian, J.; Zhou, W. Cross-presentation of phage particle antigen in MHC class II and endoplasmic reticulum marker-positive compartments. Eur. J. Immunol. 2005, 35, 2041–2050.
- Fang, J.; Wang, G.; Yang, Q.; Song, J.; Wang, Y.; Wang, L. The potential of phage display virions expressing malignant tumor specific antigen MAGE-A1 epitope in murine model. Vaccine 2005, 23, 4860–4866.
- Wu, Y.; Wan, Y.; Bian, J.; Zhao, J.; Jia, Z.; Zhou, L.; Zhou, W.; Tan, Y. Phage display particles expressing tumor-specific antigens induce preventive and therapeutic anti-tumor immunity in murine p815 model. Int. J. Cancer 2002, 98, 748–753.
- Sartorius, R.; Pisu, P.; D’Apice, L.; Pizzella, L.; Romano, C.; Cortese, G.; Giorgini, A.; Santoni, A.; Velotti, F.; De Berardinis, P. The use of filamentous bacteriophage fd to deliver MAGE-A10 or MAGE-A3 HLA-A2-restricted peptides and to induce strong antitumor CTL responses. J. Immunol. 2008, 180, 3719–3728.
- Roehnisch, T.; Then, C.; Nagel, W.; Blumenthal, C.; Braciak, T.; Donzeau, M.; Böhm, T.; Flaig, M.; Bourquin, C.; Oduncu, F.S. Phage idiotype vaccination: First phase I/II clinical trial in patients with multiple myeloma. J. Transl. Med. 2014, 12, 119.
- Bartolacci, C.; Andreani, C.; Curcio, C.; Occhipinti, S.; Massaccesi, L.; Giovarelli, M.; Galeazzi, R.; Iezzi, M.; Tilio, M.; Gambini, V.; et al. Phage-Based Anti-HER2 Vaccination Can Circumvent Immune Tolerance against Breast Cancer. Cancer Immunol. Res. 2018, 6, 1486–1498.
- Eriksson, F.; Culp, W.D.; Massey, R.; Egevad, L.; Garland, D.; Persson, M.A.; Pisa, P. Tumor specific phage particles promote tumor regression in a mouse melanoma model. Cancer Immunol. Immunother. 2007, 56, 677–687.
- Murgas, P.; Bustamante, N.; Araya, N.; Cruz-Gomez, S.; Duran, E.; Gaete, D.; Oyarce, C.; Lopez, E.; Herrada, A.A.; Ferreira, N.; et al. A filamentous bacteriophage targeted to carcinoembryonic antigen induces tumor regression in mouse models of colorectal cancer. Cancer Immunol. Immunother. 2018, 67, 183–193.
- Eriksson, F.; Tsagozis, P.; Lundberg, K.; Parsa, R.; Mangsbo, S.M.; Persson, M.A.A.; Harris, R.A.; Pisa, P. Tumor-Specific Bacteriophages Induce Tumor Destruction through Activation of Tumor-Associated Macrophages. J. Immunol. 2009, 182, 3105–3111.
- Hashiguchi, S.; Yamaguchi, Y.; Takeuchi, O.; Akira, S.; Sugimura, K. Immunological basis of M13 phage vaccine: Regulation under MyD88 and TLR9 signaling. Biochem. Biophys. Res. Commun. 2010, 402, 19–22.
- Mori, K.; Kubo, T.; Kibayashi, Y.; Ohkuma, T.; Kaji, A. Anti-vaccinia virus effect of M13 bacteriophage DNA. Antivir. Res. 1996, 31, 79–86.
- Gomes-Neto, J.F.; Sartorius, R.; Canto, F.B.; Almeida, T.S.; Dias, A.A.; Barbosa, C.H.D.; Melo, G.A.; Oliveira, A.C.; Aguiar, P.H.N.; Machado, C.R.; et al. Vaccination With Recombinant Filamentous fd Phages Against Parasite Infection Requires TLR9 Expression. Front. Immunol. 2018, 9, 1173.
- Sartorius, R.; Bettua, C.; D’Apice, L.; Caivano, A.; Trovato, M.; Russo, D.; Zanoni, I.; Granucci, F.; Mascolo, D.; Barba, P.; et al. Vaccination with filamentous bacteriophages targeting DEC-205 induces DC maturation and potent anti-tumor T-cell responses in the absence of adjuvants. Eur. J. Immunol. 2011, 41, 2573–2584.
- Mahnke, K.; Guo, M.; Lee, S.; Sepulveda, H.; Swain, S.L.; Nussenzweig, M.; Steinman, R.M. The Dendritic Cell Receptor for Endocytosis, Dec-205, Can Recycle and Enhance Antigen Presentation via Major Histocompatibility Complex Class II–Positive Lysosomal Compartments. J. Cell Biol. 2000, 151, 673–684.
- Sartorius, R.; D’Apice, L.; Trovato, M.; Cuccaro, F.; Costa, V.; De Leo, M.G.; Marzullo, V.M.; Biondo, C.; D’Auria, S.; De Matteis, M.A.; et al. Antigen delivery by filamentous bacteriophage fd displaying an anti-DEC-205 single-chain variable fragment confers adjuvanticity by triggering a TLR 9-mediated immune response. EMBO Mol. Med. 2015, 7, 973–988.
- Krag, D.N.; Shukla, G.S.; Shen, G.P.; Pero, S.; Ashikaga, T.; Fuller, S.; Weaver, D.L.; Burdette-Radoux, S.; Thomas, C. Selection of Tumor-binding Ligands in Cancer Patients with Phage Display Libraries. Cancer Res. 2006, 66, 7724–7733.
- Shukla, G.S.; Krag, D.N.; Peletskaya, E.N.; Pero, S.C.; Sun, Y.J.; Carman, C.L.; McCahill, L.E.; Roland, T.A. Intravenous Infusion of Phage-displayed Antibody Library in Human Cancer Patients: Enrichment and Cancer-Specificity of Tumor-Homing Phage-Antibodies. Cancer Immunol. Immunother. 2013, 62, 1397–1410.
- Shadidi, M.; Sørensen, D.; Dybwad, A.; Furset, G.; Sioud, M. Mucosal vaccination with phage-displayed tumour antigens identified through proteomics-based strategy inhibits the growth and metastasis of 4T1 breast adenocarcinoma. Int. J. Oncol. 2008, 32, 241–247.
- Ravn, U.; Gueneau, F.; Baerlocher, L.; Osteras, M.; Desmurs, M.; Malinge, P.; Magistrelli, G.; Farinelli, L.; Kosco-Vilbois, M.H.; Fischer, N. By-passing in vitro screening—Next generation sequencing technologies applied to antibody display and in silico candidate selection. Nucleic Acids Res. 2010, 38, e193.
- Ghosh, D.; Kohli, A.G.; Moser, F.; Endy, D.; Belcher, A.M. Refactored M13 Bacteriophage as a Platform for Tumor Cell Imaging and Drug Delivery. ACS Synth. Biol. 2012, 1, 576–582.
- Lee, K.J.; Lee, J.H.; Chung, H.K.; Ju, E.J.; Song, S.Y.; Jeong, S.Y.; Choi, E.K. Application of peptide displaying phage as a novel diagnostic probe for human lung adenocarcinoma. Amino Acids 2016, 48, 1079–1086.
- Namdee, K.; Khongkow, M.; Boonrungsiman, S.; Nittayasut, N.; Asavarut, P.; Temisak, S.; Saengkrit, N.; Puttipipatkhachorn, S.; Hajitou, A.; Ruxrungtham, K.; et al. Thermoresponsive Bacteriophage Nanocarrier as a Gene Delivery Vector Targeted to the Gastrointestinal Tract. Mol. Ther. Nucleic Acids 2018, 12, 33–44.
- Bedi, D.; Gillespie, J.W.; Petrenko, V.A.; Ebner, A.; Leitner, M.; Hinterdorfer, P.; Petrenko, V.A. Targeted Delivery of siRNA into Breast Cancer Cells via Phage Fusion Proteins. Mol. Pharm. 2013, 10, 551–559.
- Stopar, D.; Spruijt, R.B.; Wolfs, C.J.A.M.; Hemminga, M.A. Protein–lipid interactions of bacteriophage m13 major coat protein. Biochim. Biophys. Acta (BBA) Biomembr. 2003, 1611, 5–15.
- Marvin, D.A.; Symmons, M.F.; Straus, S.K. Structure and assembly of filamentous bacteriophages. Prog. Biophys. Mol. Biol. 2014, 114, 80–122.
- Sartorius, R.; D’Apice, L.; Barba, P.; Cipria, D.; Grauso, L.; Cutignano, A.; De Berardinis, P. Vectorized Delivery of Alpha-GalactosylCeramide and Tumor Antigen on Filamentous Bacteriophage fd Induces Protective Immunity by Enhancing Tumor-Specific T Cell Response. Front. Immunol. 2018, 9, 1496.
- Parekh, V.V.; Wilson, M.T.; Olivares-Villagómez, D.; Singh, A.K.; Wu, L.; Wang, C.R.; Joyce, S.; Van Kaer, L. Glycolipid antigen induces long-term natural killer T cell anergy in mice. J. Clin. Investig. 2005, 115, 2572–2583.




