| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ramin Zibaseresht | + 1165 word(s) | 1165 | 2021-02-08 19:40:30 | | | |
| 2 | Ramin Zibaseresht | Meta information modification | 1165 | 2021-02-09 09:51:25 | | |
Video Upload Options
The ligand, 4'-(4-(2,2,2-tris(1H-pyrazol-1-yl)ethoxymethyl)phenyl)-2,2':6',2"-terpyridine (PZT) contains a terpyridine fragment and a tris(pyrazolyl) fragment, which may allow synthetic differentiation between the tridentate binding sites as a result of the meridional and the facial coordination preferences of these groups around the octahedral metal centres.
This designed bridging ligand may offer the benefits of polydentate ligands and minimisation of isomeric possibilities.
The tris(pyrazolyl)methane fragment is of interest, because the coordination chemistry of such systems has also been studied. It, too, is easy to prepare, by reaction of pyrazole with chloroform, and to derivatise, for example by reaction with formaldehyde, to give a molecule, 2,2,2-tris(1H-pyrazol-1-yl)ethanol, that can be readily joined to the other binding site, a terpyridine containing synthon.
4’-(4-(2,2,2-tris(1H-pyrazol-1-ido)ethoxymethyl)phenyl)-2,2’:6’,2’’-terpyridine (PZT)
Ramin Zibasereshta,b*
a Faculty of Sciences, Maritime University of Imam Khomeini, Noshahr, Iran. Email: rzi12@uclive.ac.nz
b Biomaterials and Medicinal Chemistry Research Centre, Aja University of Medical Sciences, Tehran, Iran. Email: rzi12@uclive.ac.nz
The ligand, 4'-(4-(2,2,2-tris(1H-pyrazol-1-yl)ethoxymethyl)phenyl)-2,2':6',2"-terpyridine (PZT) contains a terpyridine fragment and a tris(pyrazolyl) fragment, which may allow synthetic differentiation between the tridentate binding sites as a result of the meridional and the facial coordination preferences of these groups around the octahedral metal centres.
This designed bridging ligand may offer the benefits of polydentate ligands and minimisation of isomeric possibilities.
The tris(pyrazolyl)methane fragment is of interest, because the coordination chemistry of such systems has also been studied. It, too, is easy to prepare, by reaction of pyrazole with chloroform, and to derivatise, for example by reaction with formaldehyde, to give a molecule, 2,2,2-tris(1H-pyrazol-1-yl)ethanol, that can be readily joined to the other binding site, a terpyridine containing synthon.
The ligand synthesis is relatively straightforward. Syntheses of suitable precursor molecules for both ends of the ditopic ligand are available in the literature. 4'-(4-(bromomethyl)phenyl)-2,2':6',2"-terpyridine is readily prepared by radical bromination of the related alkane, and this was reacted with 2,2,2-tris(1H-pyrazol-1-yl)ethanol, in the presence of sodium hydride.
The solution of 2,2,2-tris(1H-pyrazol-1-yl)ethanol (tpe) (0.368 g, 1.505 mmol) and 4'-(p-bromomethylphenyl)-2,2':6',2"-terpyridine (btp) (0.605 g, 1.505 mmol) in dry THF (30 mL) was added dropwise to a suspension of NaH (0.15 g) in dry THF (20 mL) under argon over a period of 3 h. The mixture was stirred at reflux under argon for 24 h and then allowed to cool at room temperature. To this yellow solution was added enough water dropwise carefully to consume the excess NaH. The mixture was extracted from diethyl ether (4 × 50 mL), and the combined organic extracts were washed with 50 mL saturated NaHCO3 solution, then with 50 mL saturated NaCl solution, and finally with 50 mL water. To the aqueous solution was added diethyl ether. The organic layer was extracted. After drying the combined extracts with anhydrous MgSO4, filtered and the solvent was removed under reduced pressure to yield a pale yellow powder; mp 183-184˚. Purification was achieved by recrystallisation of the crude material from CH2Cl2-hexanes (1:5) to afford (0.7190 g, 84%) as a pale yellow microcrystalline material. Crystals suitable for X-ray diffraction were obtained by layering a dichloromethane solution of the ligand with hexanes. 1H NMR (500 MHz; solvent CDCl3): δ 8.734 (2H, d, H6, H6"), 8.727 (2H, s, H3', H5'), 8.67 (2H, d, H3, H3"), 7.89 (2H, m, H4, H4"), 7.86 (2H, d, H2"', H6"'), 7.68 (3H, d, H03, H03', H03"), 7.46 (3H, d, H05, H05', H05"), 7.36 (2H, m, H5, H5"), 7.30 (2H, d, H3"', H5"'), 6.36 (3H, m, H04, H04', H04"), 5.20 (2H, s, H01), 4.61 (2H, s, H7). 13C NMR (75 MHz; solvent CDCl3): δ 156.12, 155.86, 149.81 (C3, C3"), 149.06, 141.35 (C03, C03', C03"), 138.07, 138.04 (C4, C4"), 136.94, 130.90 (C05, C05', C05"), 128.13 (C3"', C5"'), 127.38 (C2"', C6"'), 123.86 (C5, C5"), 121.38 (C6, C6"), 118.81 (C3', C5'), 106.55 (C04, C04', C04"), 89.84 (C02), 73.80 (C7), 73.63 (C01). 1H NMR (500 MHz; solvent dmso-d6, see Figure above for labeling): δ 8.86 (2H, d, H3, H3"), 8.80 (2H, s, H3', H5'), 8.77 (2H, d, H6, H6"), 8.14 (2H, dd, H4, H4"), 7.99 (2H, d, H2"', H6"'), 7.79 (3H, d, H03, H03', H03"), 7.63 (5H, m, H05, H05', H05", H5, H5"), 7.48 (2H, d, H3"', H5"'), 6.53 (3H, m, H04, H04', H04"), 5.17 (2H, s, H01), 4.74 (2H, s, H7).13C NMR (75 MHz; solvent dmso-d6) δ155.86, 155.07, 149.53 (C3, C3"), 149.33, 141.07 (C03, C03', C03"), 139.00, 137.71 (C4, C4"), 136.99, 131.23 (C05, C05', C05"), 128.56 (C3"', C5"'), 127.07 (C2"', C6"'), 124.77 (C5, C5"), 121.15 (C6, C6"), 118.07 (C3', C5'), 106.65 (C04, C04', C04"), 89.42 (C02), 72.80 (C01), 72.46 (C7). IR (KBr): /cm-1 = 3140 m, 3055 m, 3017 m, 2939 m, 2885 w, 1983 w, 1929 w, 1790 w, 1728 w, 1697 w, 1582 m, 1520 m, 1466 m, 1389 s sh, 1312 s, 1258 m, 1196 m, 1126 s, 1096 s, 1042 w, 988 m, 949 w, 872 s sh, 895 m, 764 s, 656 m, 617 m, 525 m sh. ESI-MS: m/z 566.2931 ([M+H]+, 100%). Anal. Calc. for C33H27N9O.0.5H2O (574.65): C 68.98, H 4.91, N 21.94%; found: C 69.32, H 4.79, N 21.77%.
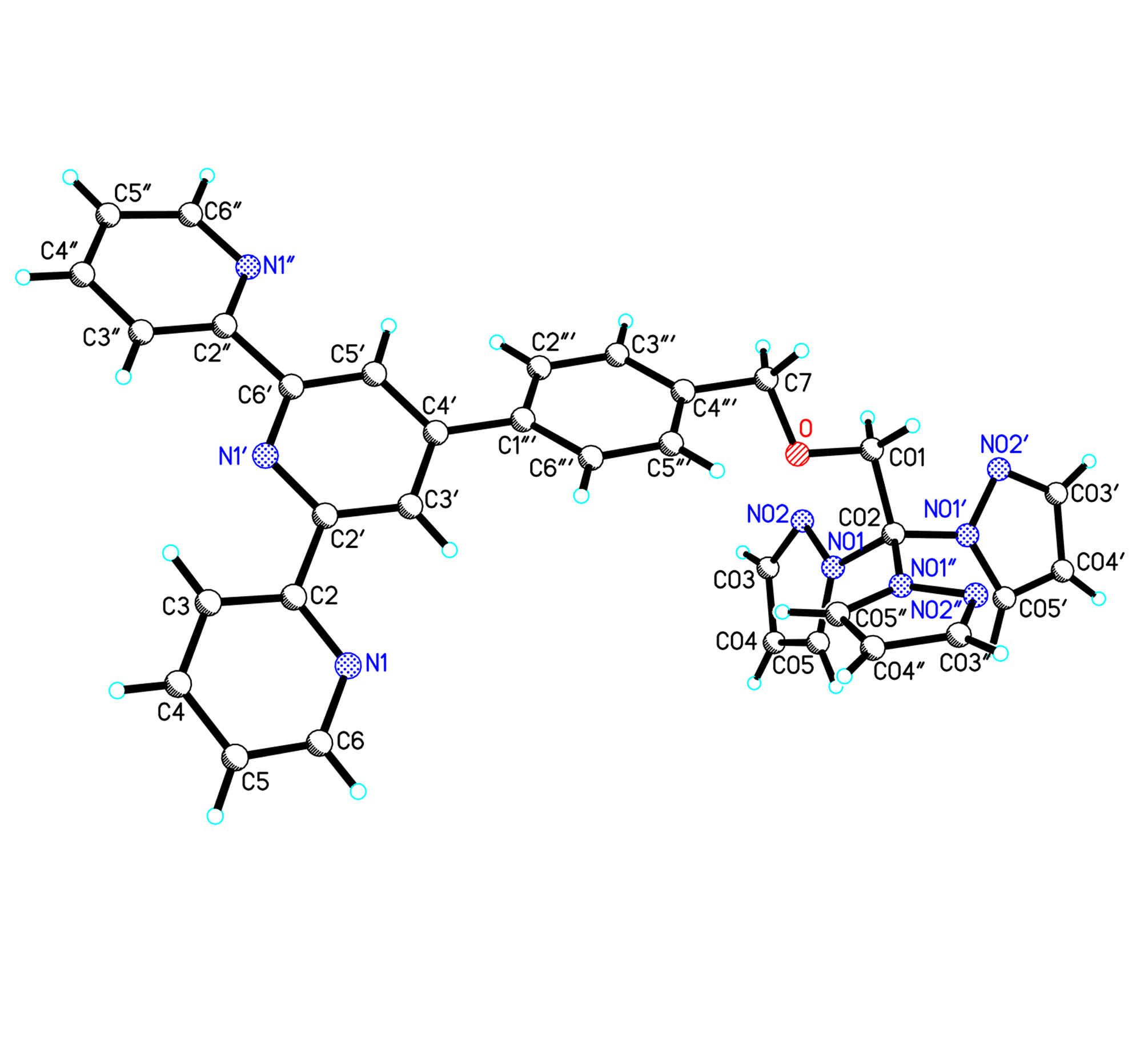
Crystals suitable for X-ray diffraction were obtained by layering a dichloromethane solution of the ligand with hexanes. The ligand was crystallised in orthorhombic Pbca space group (R1 = 0.06). As shown in Figure 4.22, the three pyridyl rings are approximately coplanar, making an interannular angle with the central pyridine ring of 11.5o, with a transoid arrangement about each interannular C-C bond. This is in accord with the transoid configuration observed in the crystal structures of 2,2'-bipyridine , 2,2':6',2":6",2"'-quaterpyridine and 4'-phenyl-2,2':6',2"-terpyridine. The C-C and C-N distances within the phenyl and the pyridine rings are normal (average 1.307 and 1.351 Ǻ, respectively). The interannular C-C bonds also closely resemble those seen in 2,2'-bipyridine, 2,2':6',2":6",2"'-quaterpyridine, and 4'-phenyl-2,2':6',2"-terpyridine with lengths of 1.498 Ǻ.
The phenyl ring is not coplanar with the terpyridyl fragment, but is twisted about the interannular bond such that the plane of the phenyl ring makes an angle of 24.5o with the plane of the central pyridine ring. This represents a compromise between a coplanar arrangement in which π-conjugation and non-bonded H-H contacts between the ortho protons of the phenyl ring and H2 H4 are maximized. This angle is larger than that of 4'-phenyl-2,2':6',2"-terpyridine structure (10.9o). One of the pyrazole rings of the pyrazolyl site is approximately in the plane of C7-O-C01 linker with a slight twist of 10.6o. The angles between the remaining pyrazole rings and the plane of the etheric chain linker are 76.3o and 82.1o, respectively. The free nitrogen atoms of the pyrazole rings are all pointed out from each other in such a way that the interactions between the hydrogen atoms of the pyrazolyl rings and the free nitrogen atoms at the adjacent rings are small.
Figure 1. Molecular structure of ligand pzt. Selected bond lengths [Å] and angles [°]: N(1)-C(6) 1.340(4), N(1)-C(2) 1.358(4), N(1')-C(6') 1.348(4), N(1')-C(2') 1.361(4), N(1")-C(2") 1.338(4), N(1")-C(6") 1.358(4), N(01)-N(02) 1.369(4), N(01)-C(05) 1.392(4), N(01)-C(02) 1.490(4), N(02)-C(03) 1.349(5), N(01')-N(02') 1.354(4), N(01')-C(05') 1.370(4), N(01')-C(02) 1.447(4), N(01")-C(05") 1.369(4), N(01")-N(02")1.379(4), N(01")-C(02) 1.457(4), N(02")-C(03") 1.320(4), O-C(01) 1.414(4), O-C(7) 1.415(4), C(01)-O-C(7) 113.0(2), O-C(7)-C(4"') 107.6(3), O-C(01)-C(02) 105.5(3).