
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sang Taek Jung | + 1871 word(s) | 1871 | 2021-01-14 04:16:12 | | | |
| 2 | Camila Xu | Meta information modification | 1871 | 2021-02-05 07:49:01 | | | | |
| 3 | Camila Xu | Meta information modification | 1871 | 2021-02-05 07:50:02 | | |
Video Upload Options
G-protein-coupled receptors (GPCRs), which make up the largest superfamily of human membrane proteins, play pivotal roles in mediating intracellular signaling and inducing cell proliferation, cell growth, and cell motility through the association and subsequent dissociation of G-proteins in response to external stimuli.
1. Introduction
G-protein-coupled receptors (GPCRs), which make up the largest superfamily of human membrane proteins, play pivotal roles in mediating intracellular signaling and inducing cell proliferation, cell growth, and cell motility through the association and subsequent dissociation of G-proteins in response to external stimuli (Figure 1) [1][2]. Many clinical studies have revealed that abnormal functions of GPCRs are highly related to a variety of human diseases and affect the patient survival rate [3][4][5]. Therefore, GPCRs are crucial drug targets to treat patients with various diseases, and their targeting drugs represent more than 30 percent of all US Food and Drug Administration (FDA)-approved drugs [6][7][8]. Annual sales of these drugs have increased to about USD 180 billion in 2018 [9].
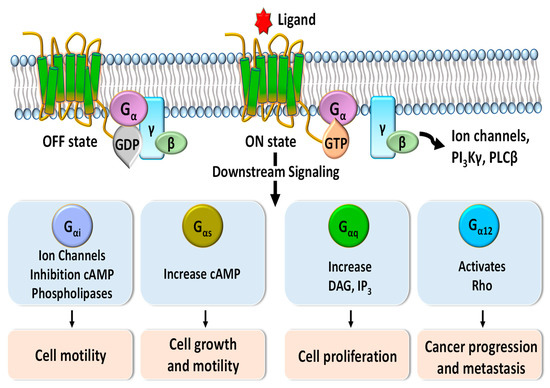
Figure 1. Schematic diagram of G-protein-coupled receptor (GPCR) signaling pathways mediated by Gα protein subunits. Downstream signaling triggered by binding of G proteins changes the concentrations of phospholipase C-beta (PLCβ), phosphoinositide 3-kinases-gamma (PI3Kγ), diacylglycerol (DAG), inositol trisphosphate (IP3), and cyclic adenosine monophosphate (cAMP) and regulates various cellular functions such as cell motility, cell growth, cell proliferation, and cancer progression and metastasis.
Compared to small-molecule chemical drugs and small peptides, therapeutic antibodies have many advantages in terms of higher target specificity, fewer side effects, and superior serum circulating half-life [10]. However, despite clinical and marketing successes of monoclonal antibody products to treat numerous diseases, only two anti-GPCR therapeutic antibody drugs, Amgen’s erenumab (trade name: Aimovig), targeting calcitonin gene-related receptor (CGRPR) to treat migraine (Figure 2a) [11] and Kyowa Kirin’s mogamulizumab (trade name: Poteligeo), targeting chemokine receptor 4 (CCR4) to treat refractory mycosis fungoides and Sézary syndrome (Figure 2b), have been approved to date [12].
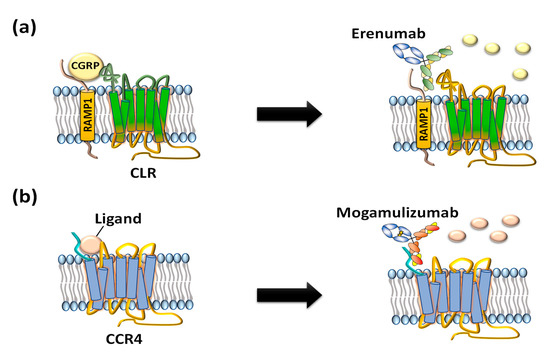
Figure 2. US FDA-approved anti-GPCR antibodies erenumab and mogamulizumab. (a) Erenumab is an antagonistic monoclonal antibody against calcitonin gene-related peptide receptor (CGRPR) consisting of calcitonin receptor-like receptor (CLR) and receptor activity-modifying protein 1 (RAMP1) for treatment of chronic migraine. (b) Mogamulizumab is an antibody against chemokine receptor 4 (CCR4) for treatment of T-cell leukemia by inactivating the GPCR and clearance of target cells by enhanced antibody-dependent cell-mediated cytotoxicity (ADCC).
Generally, amenable techniques to isolate therapeutic human antibodies include (1) humanization of candidate antibodies followed by the selection of hybridoma cells derived from immunized animals; (2) screening of the human naïve antibody library displayed on the surface of bacteriophages, bacteria, or yeast, which take advantage of a physical linkage between genotype and phenotype; and (3) hybridoma selection after immunizing an antigen into humanized transgenic animals, referred to as XenoMouseTM, which contains the genes for variable regions of the heavy (VH) and light (VL) chains of the human antibody repertoire [13].
Regardless of the antibody isolation technique, preparing pure GPCR antigens with the native conformation of the human in vivo condition is essential for successful isolation of therapeutic functional human anti-GPCR antibodies. In particular, GPCRs containing seven transmembrane α-helices are usually expressed at very low levels in heterologous expression systems; therefore, it is very hard to purify the antigen with a native conformation as a soluble form. Furthermore, the limited surface area of the extracellular region of GPCRs in the whole GPCR structure makes it very difficult to prepare the GPCR antigen as a target for therapeutic anti-GPCR antibodies. Even though antigen preparation is one of the most difficult steps in development of therapeutic anti-GPCR antibodies, two anti-GPCR antibodies have overcome the challenges and have been recently approved. In addition, dozens of anti-GPCR antibodies are under clinical development or are waiting for clinical evaluations of therapeutic efficacy and toxicity.
2. GPCR Extracellular Region Fusion Proteins as GPCR Antigens
Interaction between the extracellular region of GPCRs and their ligands triggers conformational changes in the intracellular region of the protein, resulting in the association of G-proteins and the transmission of extracellular signals into cells [14]. GPCRs consist of seven transmembrane α-helical bundles, four extracellular regions: N-terminal, extracellular loop 1 (ECL1), extracellular loop 2 (ECL2), and extracellular loop 3 (ECL3), and four intracellular regions: intracellular loop 1 (ICL1), intracellular loop 2 (ICL2), intracellular loop 3 (ICL3), and C-terminal. The unique three-dimensional structure of each GPCR determines its ligand specificity to elicit its characteristic cellular responses. To isolate antibodies that recognize the extracellular region of a GPCR, a simple strategy is to chemically conjugate or genetically fuse a designed peptide comprising the part of the GPCR extracellular region with a carrier protein (Figure 3a). The prepared antigen can be used to immunize animals or screen antibodies from a human naïve antibody library [15]. Although the extracellular region peptide prepared with a carrier protein is unable to perfectly mimic the extracellular peptide conformation of a native GPCR, some human GPCR extracellular region polypeptides containing post-translational modifications such as glycosylation can be expressed in mammalian cells [16]. A mimic GPCR extracellular loop was fused with a carrier protein and employed to isolate erenumab, targeting calcitonin gene-related peptide receptor (CGRPR) [17], mogamulizumab, targeting chemokine receptor 4 (CCR4) [18], and vantictumab, targeting Frizzled-7 (FZD7) [19].
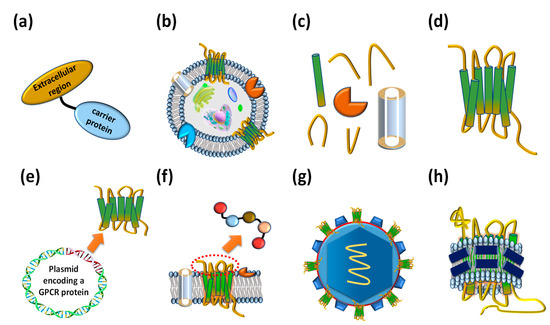
Figure 3. Various types of antigens that have been employed to isolate anti-GPCR antibodies. (a) Extracellular region fused proteins. (b) Whole cells expressing GPCRs on their cellular membrane. (c) Membrane factions expressing GPCRs. (d) Purified whole GPCRs. (e) DNA molecules encoding GPCRs. (f) Extracellular region peptides. (g) Virus-like particles (VLPs) displaying GPCRs on the surface. (h) Reconstituted GPCR–lipid–belt protein (GLB) complexes.
Erenumab is an antibody developed to treat patients with chronic migraine. It regulates the function of calcitonin receptor-like receptor (CLR), interacting with the receptor activity-modifying protein (RAMP) family. The single transmembrane domain has selectivity for three types of ligands: calcitonin gene-related peptide (CGRP), adrenomedullin 1, and adrenomedullin 2 [20]. The calcitonin gene-related peptide receptor (CGRPR), comprising CLR and RAMP1, is mainly distributed in the peripheral and central nervous systems.
Moreover, it is closely related to migraine through vasodilation following Gαs protein release and the activation of adenylyl cyclase [21][22]. Based on the finding that the CGRP binding site spans the extracellular regions of CLR and RAMP1 [23], a heterodimeric Fc (fragment crystallizable) fusion protein consisting of an ectodomain of RAMP and an N-terminal extracellular region of CLR was designed as an antigen to isolate CGRPR antagonistic antibodies (Figure 4a). In addition, the prepared heterodimeric Fc fusion was immunized into XenoMouse to generate erenumab, followed by hybridoma screening [24][25] (Figure 4b). Erenumab showed a high binding affinity to CGRPR (KD = 56 pM) and excellent inhibition of cAMP production (IC50 = 2.3 nM) [26]. Additionally, structural analysis of the CGRPR-CGRP complex confirmed that erenumab directly blocks the conformation of CGRP into CGRPR [27]. In the phase III clinical trial, patients treated with 70 mg and 140 mg of erenumab once a month for at least 6 months showed 43.3% and 50% reductions of number of days of migraine [28], respectively, and these efficacy results enabled the antibody to be the first anti-GPCR antibody approved by the US FDA in May 2018 and by the European Medicines Agency (EMA) in July 2018.
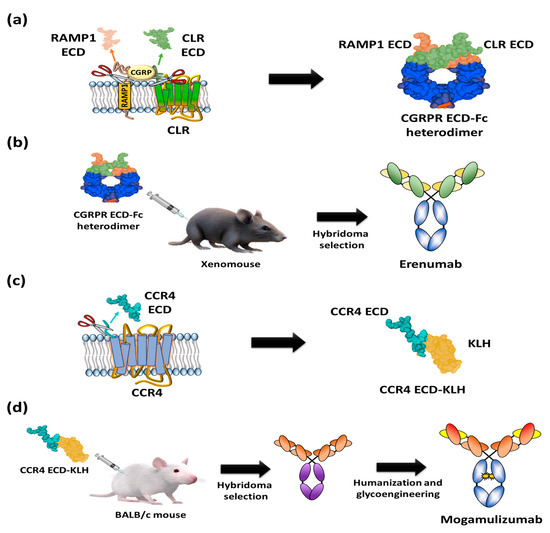
Figure 4. Schematic diagrams of antigen preparation and overall procedure for discovery of US FDA-approved anti-GPCR antibody. (a) A heterodimer CGRPR Fc protein prepared by Fc fusion constructs of N-terminal extracellular regions of CLR and ectodomain of RAMP1. (b) Immunization of heterodimeric CGRPR Fc proteins into XenoMouse hybridoma selection to isolate erenumab. (c) The N-terminal region of CCR4 (28 amino acids: N2-C29) fused with keyhole limpet hemocyanin (KLH). (d) Injection of the N-terminal region of CCR4-KLH into BALB/c mice and hybridoma selection, humanization, and defucosylation of N-inked glycans of Fc to generate mogamulizumab.
Mogamulizumab is a humanized monoclonal antibody targeting chemokine receptor 4 (CCR4) with a glyco-engineered Fc region to enhance the antibody-dependent cellular cytotoxicity (ADCC) for the clearance of adult t-cell leukemia (ATL). The protein CCR4 is overexpressed on the surface of FOXP3+ regulatory T (Treg) cells of ATL patients and is involved in the evasion of immune surveillance against tumors [29][30]. To isolate anti-CCR4 antibodies, extracellular partial N-terminal peptide (28 amino acids: N2-C29) was fused to a carrier protein, keyhole limpet hemocyanin (KLH) (Figure 4c), and injected into mice for the screening of antigen-specific antibodies (Figure 4d) [31]. After humanization of the candidate antibodies, glycol engineering was performed to defucosylate the N-linked glycan of Fc to enhance FcγIIIa binding and Natural Killer (NK) cell-mediated ADCC activity [32][33][34][35]. The resulting mogamulizumab (KW-0761) exhibited significant efficacy in 50% of ATL patients treated in clinical trials, leading to successful commercialization in 2012 in Japan and in 2019 in the US for the treatment of mycosis fungoides (MF) and Sézary syndrome (SS) [12][36].
Uncontrolled cell signaling in the Wnt/β-catenin pathway affects various types of tumors [37]. Vantictumab (anti-FZD7), a Wnt/β-catenin pathway-blocking antibody, was isolated by screening the human naïve Fab antibody library displayed on bacteriophages using the Fc-fused extracellular N-terminal domain of the GPCR as an antigen. The resulting antibody (vantictumab) could inhibit Wnt pathway signaling through specific binding to five kinds of Frizzled receptors: FZD1, FZD2, FZD5, FZD7, and FZD8 [19]. In human phase 1 clinical examination, vantictumab significantly inhibited the growth of pancreatic, colon, and breast cancer cells in combination with other chemotherapeutic agents [38].
3. GPCR-Expressing Cells or Membrane Fractions as GPCR Antigens
The use of GPCR-expressing cells (Figure 3b) or their membrane fractions as antigens containing integral and associated proteins (Figure 3c) has been limited due to difficulty in overexpressing a target GPCR on the cell surface due to the presence of numerous other membrane components. In addition, a very advanced handling technique is necessary to apply the fragile whole cells as antigens for repeated rounds of antibody screening. Alternatively, the membrane fraction from cells displaying a target GPCR has been used as a type of antigen. The biggest advantage of using GPCR-expressing cells or membrane fractions is their native conformation that allows for the isolation of a desired anti-GPCR antibody capable of recognizing the native structure of GPCR compared to using other GPCR mimetic antigens.
Representative examples of therapeutic anti-GPCR antibodies that have been isolated using animal immunization with GPCR-overexpressing cells or their membranes fraction as antigens are glutazumab, targeting glucagon-like peptide-1 receptor (GLP1R) [39][40], volagidemab, targeting glucagon receptor (GCGR) [41], plozalizumab, targeting chemokine receptor type 2 (CCR2) [42], leronlimab (Pro 140), targeting C-C chemokine receptor type 5 (CCR5) [43], ulocuplumab (BMS-936564), targeting C-X-C chemokine receptor type 4 (CXCR4) [44], and avdoralimab (IPH5401), targeting C5a receptor (C5aR) [45].
Glutazumab is an agonist antibody targeting GLP1R for the treatment of type 2 diabetes resulting from an abnormal cellular response to insulin. It was developed by hybridoma selection from mice that were immunized with GLP1R-expressing mammalian cells, humanization, and genetic fusion of the GLP1 (29 amino acids: H7-G35), a ligand of GLP1R, at the N-terminus of the variable light chain of IgG [39][46]. Glutazumab was efficacious in suppressing the interaction between GLP-1 and GLP1R and showed significant efficacy in suppressing glucagon secretion in a human phase II clinical trial in Australia and New Zealand .
Leronlimab (Pro 140) is a humanized monoclonal antibody targeting CCR5, which is expressed on the surface of T lymphocytes and is essential for the fusion of HIV with immune cells. Anti-CCR5 antibodies were isolated by immunizing mice with CCR5-expressing mammalian cells, and humanization of the resulting antibodies enabled the development of leronlimab (Pro 140) [43]. The antibody inhibits HIV infection pathways by selectively binding to the N-terminus and extracellular loop 2 of CCR5, and a human phase III clinical trial is ongoing [47].
References
- Wu, J.; Xie, N.; Zhao, X.; Nice, E.C.; Huang, C. Dissection of aberrant GPCR signaling in tumorigenesis—A systems biology approach. Cancer Genom Proteom. 2012, 9, 37–50.
- New, D.C.; Wong, Y.H. Molecular mechanisms mediating the G protein-coupled receptor regulation of cell cycle progression. J. Mol. Signal. 2007, 2, 2–2.
- Zougman, A.; Hutchins, G.G.; Cairns, D.A.; Verghese, E.; Perry, S.L.; Jayne, D.G.; Selby, P.J.; Banks, R.E. Retinoic acid-induced protein 3: Identification and characterisation of a novel prognostic colon cancer biomarker. Eur. J. Cancer. 2013, 49, 531–539.
- Sriram, K.; Moyung, K.; Corriden, R.; Carter, H.; Insel, P.A. GPCRs show widespread differential mRNA expression and frequent mutation and copy number variation in solid tumors. PLoS Biol. 2019, 17, e3000434.
- Feigin, M.E.; Xue, B.; Hammell, M.C.; Muthuswamy, S.K. G-protein–coupled receptor GPR161 is overexpressed in breast cancer and is a promoter of cell proliferation and invasion. Proc. Natl. Acad. Sci. USA 2014, 111, 4191–4196.
- Santos, R.; Ursu, O.; Gaulton, A.; Bento, A.P.; Donadi, R.S.; Bologa, C.G.; Karlsson, A.; Al-Lazikani, B.; Hersey, A.; Oprea, T.I.; et al. A comprehensive map of molecular drug targets. Nat. Rev. Drug Discov. 2017, 16, 19–34.
- Rask-Andersen, M.; Masuram, S.; Schioth, H.B. The druggable genome: Evaluation of drug targets in clinical trials suggests major shifts in molecular class and indication. Annu. Rev. Pharmacol. Toxicol. 2014, 54, 9–26.
- Hauser, A.S.; Attwood, M.M.; Rask-Andersen, M.; Schioth, H.B.; Gloriam, D.E. Trends in GPCR drug discovery: New agents, targets and indications. Nat. Rev. Drug Discov. 2017, 16, 829–842.
- Hauser, A.S.; Chavali, S.; Masuho, I.; Jahn, L.J.; Martemyanov, K.A.; Gloriam, D.E.; Babu, M.M. Pharmacogenomics of GPCR Drug Targets. Cell 2018, 172, 41–54.
- Igawa, T.; Tsunoda, H.; Kuramochi, T.; Sampei, Z.; Ishii, S.; Hattori, K. Engineering the variable region of therapeutic IgG antibodies. MAbs 2011, 3, 243–252.
- Dolgin, E. First GPCR-directed antibody passes approval milestone. Nat. Rev. Drug Discov. 2018, 17, 457–459.
- Kasamon, Y.L.; Chen, H.; de Claro, R.A.; Nie, L.; Ye, J.; Blumenthal, G.M.; Farrell, A.T.; Pazdur, R. FDA Approval Summary: Mogamulizumab-kpkc for Mycosis Fungoides and Sezary Syndrome. Clin. Cancer Res. 2019, 25, 7275–7280.
- Hoogenboom, H.R. Selecting and screening recombinant antibody libraries. Nat. Biotechnol. 2005, 23, 1105–1116.
- Lefkowitz, R.J.; Shenoy, S.K. Transduction of receptor signals by beta-arrestins. Science 2005, 308, 512–517.
- Webb, D.R.; Handel, T.M.; Kretz-Rommel, A.; Stevens, R.C. Opportunities for functional selectivity in GPCR antibodies. Biochem. Pharmacol. 2013, 85, 147–152.
- Soto, A.G.; Smith, T.H.; Chen, B.; Bhattacharya, S.; Cordova, I.C.; Kenakin, T.; Vaidehi, N.; Trejo, J. N-linked glycosylation of protease-activated receptor-1 at extracellular loop 2 regulates G-protein signaling bias. Proc. Natl. Acad. Sci. USA 2015, 112, E3600–E3608.
- Markham, A. Erenumab: First Global Approval. Drugs 2018, 78, 1157–1161.
- Subramaniam, J.M.; Whiteside, G.; McKeage, K.; Croxtall, J.C. Mogamulizumab: First global approval. Drugs 2012, 72, 1293–1298.
- Smith, D.C.; Rosen, L.S.; Chugh, R.; Goldman, J.W.; Xu, L.; Kapoun, A.; Brachmann, R.K.; Dupont, J.; Stagg, R.J.; Tolcher, A.W.; et al. First-in-human evaluation of the human monoclonal antibody vantictumab (OMP-18R5; anti-Frizzled) targeting the WNT pathway in a phase I study for patients with advanced solid tumors. J. Clin. Oncol. 2013, 31, 2540.
- Brain, S.D.; Grant, A.D. Vascular actions of calcitonin gene-related peptide and adrenomedullin. Physiol. Rev. 2004, 84, 903–934.
- Hay, D.L.; Pioszak, A.A. Receptor Activity-Modifying Proteins (RAMPs): New Insights and Roles. Annu. Rev. Pharmacol. Toxicol. 2016, 56, 469–487.
- Kee, Z.; Kodji, X.; Brain, S.D. The Role of Calcitonin Gene Related Peptide (CGRP) in Neurogenic Vasodilation and Its Cardioprotective Effects. Front. Physiol. 2018, 9, 1249.
- Liang, Y.L.; Khoshouei, M.; Deganutti, G.; Glukhova, A.; Koole, C.; Peat, T.S.; Radjainia, M.; Plitzko, J.M.; Baumeister, W.; Miller, L.J.; et al. Cryo-EM structure of the active, Gs-protein complexed, human CGRP receptor. Nature 2018, 561, 492–497.
- King, C.T.; Gegg, C.V.; Hu, S.N.-Y.; Sen Lu, H.; Chan, B.M.; Berry, K.A.; Brankow, D.W.; Boone, T.J.; Kezunovic, N.; Kelley, M.R.; et al. Discovery of the Migraine Prevention Therapeutic Aimovig (Erenumab), the First FDA-Approved Antibody against a G-Protein-Coupled Receptor. ACS Pharmacol. Transl. Sci. 2019, 2, 485–490.
- Booe, J.M.; Walker, C.S.; Barwell, J.; Kuteyi, G.; Simms, J.; Jamaluddin, M.A.; Warner, M.L.; Bill, R.M.; Harris, P.W.; Brimble, M.A.; et al. Structural Basis for Receptor Activity-Modifying Protein-Dependent Selective Peptide Recognition by a G Protein-Coupled Receptor. Mol. Cell 2015, 58, 1040–1052.
- Shi, L.; Lehto, S.G.; Zhu, D.X.; Sun, H.; Zhang, J.; Smith, B.P.; Immke, D.C.; Wild, K.D.; Xu, C. Pharmacologic Characterization of AMG 334, a Potent and Selective Human Monoclonal Antibody against the Calcitonin Gene-Related Peptide Receptor. J. Pharmacol. Exp. Ther. 2016, 356, 223–231.
- Garces, F.; Mohr, C.; Zhang, L.; Huang, C.S.; Chen, Q.; King, C.; Xu, C.; Wang, Z.L. Molecular Insight into Recognition of the CGRPR Complex by Migraine Prevention Therapy Aimovig (Erenumab). Cell Rep. 2020, 30, 1714–1723.
- Goadsby, P.J.; Reuter, U.; Hallstrom, Y.; Broessner, G.; Bonner, J.H.; Zhang, F.; Sapra, S.; Picard, H.; Mikol, D.D.; Lenz, R.A. A Controlled Trial of Erenumab for Episodic Migraine. N. Engl. J. Med. 2017, 377, 2123–2132.
- Ishida, T.; Ueda, R. CCR4 as a novel molecular target for immunotherapy of cancer. Cancer Sci. 2006, 97, 1139–1146.
- Sugiyama, D.; Nishikawa, H.; Maeda, Y.; Nishioka, M.; Tanemura, A.; Katayama, I.; Ezoe, S.; Kanakura, Y.; Sato, E.; Fukumori, Y.; et al. Anti-CCR4 mAb selectively depletes effector-type FoxP3+CD4+ regulatory T cells, evoking antitumor immune responses in humans. Proc. Natl. Acad. Sci. USA 2013, 110, 17945–17950.
- Shitara, K.; Hanai, N.; Shoji, E.; Sakurada, M.; Furuya, A.; Nakamura, K.; Niwa, R.; Shibata, K.; Yamasaki, M. Method for producing recombinant antibody and antibody fragment thereof. U.S. Patent 8,632,996, 21 January. 2014.
- Niwa, R.; Shoji-Hosaka, E.; Sakurada, M.; Shinkawa, T.; Uchida, K.; Nakamura, K.; Matsushima, K.; Ueda, R.; Hanai, N.; Shitara, K. Defucosylated chimeric anti-CC chemokine receptor 4 IgG1 with enhanced antibody-dependent cellular cytotoxicity shows potent therapeutic activity to T-cell leukemia and lymphoma. Cancer Res. 2004, 64, 2127–2133.
- Niwa, R.; Sakurada, M.; Kobayashi, Y.; Uehara, A.; Matsushima, K.; Ueda, R.; Nakamura, K.; Shitara, K. Enhanced natural killer cell binding and activation by low-fucose IgG1 antibody results in potent antibody-dependent cellular cytotoxicity induction at lower antigen density. Clin. Cancer Res. 2005, 11, 2327–2336.
- Yano, H.; Ishida, T.; Inagaki, A.; Ishii, T.; Ding, J.; Kusumoto, S.; Komatsu, H.; Iida, S.; Inagaki, H.; Ueda, R. Defucosylated anti CC chemokine receptor 4 monoclonal antibody combined with immunomodulatory cytokines: A novel immunotherapy for aggressive/refractory Mycosis fungoides and Sezary syndrome. Clin. Cancer Res. 2007, 13, 6494–6500.
- Yoshie, O.; Matsushima, K. CCR4 and its ligands: From bench to bedside. Int. Immunol. 2014, 27, 11–20.
- Ishida, T.; Joh, T.; Uike, N.; Yamamoto, K.; Utsunomiya, A.; Yoshida, S.; Saburi, Y.; Miyamoto, T.; Takemoto, S.; Suzushima, H.; et al. Defucosylated anti-CCR4 monoclonal antibody (KW-0761) for relapsed adult T-cell leukemia-lymphoma: A multicenter phase II study. J. Clin. Oncol. 2012, 30, 837–842.
- Clevers, H.; Nusse, R. Wnt/beta-catenin signaling and disease. Cell 2012, 149, 1192–1205.
- Gurney, A.; Axelrod, F.; Bond, C.J.; Cain, J.; Chartier, C.; Donigan, L.; Fischer, M.; Chaudhari, A.; Ji, M.; Kapoun, A.M.; et al. Wnt pathway inhibition via the targeting of Frizzled receptors results in decreased growth and tumorigenicity of human tumors. Proc. Natl. Acad. Sci. USA 2012, 109, 11717–11722.
- Li, C.; Yang, M.; Wang, X.; Zhang, H.; Yao, C.; Sun, S.; Liu, Q.; Pan, H.; Liu, S.; Huan, Y.; et al. Glutazumab, a novel long-lasting GLP-1/anti-GLP-1R antibody fusion protein, exerts anti-diabetic effects through targeting dual receptor binding sites. Biochem. Pharmacol. 2018, 150, 46–53.
- Yan, H.; Gu, W.; Yang, J.; Bi, V.; Shen, Y.; Lee, E.; Winters, K.A.; Komorowski, R.; Zhang, C.; Patel, J.J.; et al. Fully human monoclonal antibodies antagonizing the glucagon receptor improve glucose homeostasis in mice and monkeys. J. Pharmacol. Exp. Ther. 2009, 329, 102–111.
- Pettus, J.; Reeds, D.; Cavaiola, T.S.; Boeder, S.; Levin, M.; Tobin, G.; Cava, E.; Thai, D.; Shi, J.; Yan, H.; et al. Effect of a glucagon receptor antibody (REMD-477) in type 1 diabetes: A randomized controlled trial. Diabetes Obes. Metab. 2018, 20, 1302–1305.
- Gilbert, J.; Lekstrom-Himes, J.; Donaldson, D.; Lee, Y.; Hu, M.; Xu, J.; Wyant, T.; Davidson, M.; MLN1202 Study Group. Effect of CC chemokine receptor 2 CCR2 blockade on serum C-reactive protein in individuals at atherosclerotic risk and with a single nucleotide polymorphism of the monocyte chemoattractant protein-1 promoter region. Am. J. Cardiol. 2011, 107, 906–911.
- Olson, W.C.; Rabut, G.E.; Nagashima, K.A.; Tran, D.N.; Anselma, D.J.; Monard, S.P.; Segal, J.P.; Thompson, D.A.; Kajumo, F.; Guo, Y.; et al. Differential inhibition of human immunodeficiency virus type 1 fusion, gp120 binding, and CC-chemokine activity by monoclonal antibodies to CCR5. J. Virol. 1999, 73, 4145–4155.
- Kuhne, M.R.; Mulvey, T.; Belanger, B.; Chen, S.; Pan, C.; Chong, C.; Cao, F.; Niekro, W.; Kempe, T.; Henning, K.A.; et al. BMS-936564/MDX-1338: A fully human anti-CXCR4 antibody induces apoptosis in vitro and shows antitumor activity in vivo in hematologic malignancies. Clin. Cancer Res. 2013, 19, 357–366.
- Massard, C.; Cassier, P.; Bendell, J.C.; Marie, D.B.; Blery, M.; Morehouse, C.; Ascierto, M.; Zerbib, R.; Mitry, E.; Tolcher, A.W. 1203P—Preliminary results of STELLAR-001, a dose escalation phase I study of the anti-C5aR, IPH5401, in combination with durvalumab in advanced solid tumours. Ann. Oncol. 2019, 30, v492.
- Zhang, C.; Jing, S.; Zhang, H.; Wang, X.; Chenjiang, Y.A.O. Antibody Specifically Binding to GLP-1 R and Fusion Protein thereof with GLP-1 Patent. U.S. Patent 10,059,773, 28 August 2018.
- Trkola, A.; Ketas, T.J.; Nagashima, K.A.; Zhao, L.; Cilliers, T.; Morris, L.; Moore, J.P.; Maddon, P.J.; Olson, W.C. Potent, broad-spectrum inhibition of human immunodeficiency virus type 1 by the CCR5 monoclonal antibody PRO 140. J. Virol. 2001, 75, 579–588.
- Trkola, A.; Ketas, T.J.; Nagashima, K.A.; Zhao, L.; Cilliers, T.; Morris, L.; Moore, J.P.; Maddon, P.J.; Olson, W.C. Potent, broad-spectrum inhibition of human immunodeficiency virus type 1 by the CCR5 monoclonal antibody PRO 140. J. Virol. 2001, 75, 579–588.




