
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Nicholas Miwornunyuie | + 2816 word(s) | 2816 | 2021-01-27 07:17:24 | | | |
| 2 | Dean Liu | -1251 word(s) | 1565 | 2021-01-28 08:50:51 | | |
Video Upload Options
Zeolitic imidazolate framework (ZIF-8), a type of MOF used for heavy-metal removal in aqueous solutions, as well as its application, adsorption mechanisms, and the factors that influence its adsorption mechanism.
1. ZIF-8 Synthesis Methods
UPTOHERE It is recognized and established that the parameters and conditions of synthesis have a major influence on the physicochemical properties of ZIF-8[23]. Aside from alternative synthesis routes, such as sonochemical (which uses ultrasound radiations), mechanochemical, and microwave-assisted synthesis, there are two traditional approaches to ZIF-8 synthesis: the solvothermal method and room-temperature synthesis[15][18]. The solvothermal process is a well-recognized method for MOFs. In ZIF-8 synthesis, it involves the reactions of hydrated metal salt (Zn(NO3)2·6H2O) and an imidazole-type ligand (2-Methylimidazole) in an amide solvent such as methanol (CH3OH) or N,N-dimethylformamide (DMF), placed in a closed and tight container at an autogenous pressure (often autoclaved) above the boiling point of the solvent, i.e., at 80–150 °C[18][24]. Under these conditions, amines resulting from the thermal degradation of the solvent causes deprotonation of the connecting imidazole1H (ImH). Usually, when cooled, moderate to high yields (50–90% yield) of ZIF-8 crystals are obtained. In 2006, Yaghi et al. reported the first ZIF-8 synthesis using the solvothermal method[15][19]. Several researchers have used the solvothermal method to produce a pure-phase, water-stable ZIF-8. However, when using this method, essential reaction parameters like concentration temperature, reaction volume, and time are crucial synthesis conditions that need to be controlled. When these parameters are left uncontrolled, it may lead to structural defects and the degradation of the resultant ZIF[24]. The influence of synthesis conditions on the development of ZIF-8 under the microwave-assisted solvothermal method was assessed by a study conducted by Lai et al.[29]. At various temperatures, ranging from 80 °C to 140 °C, they synthesized a series of ZIF-8 particles. Using scanning-electron microscopy (SEM) for a surface-morphology analysis of their resulting ZIF-8 samples, their findings showed that ZIF-8 formation can be largely affected by solvent expansion at higher temperatures and pressure[29]. Therefore, while the solvothermal approach has many advantages, time and temperature have to be strictly monitored. The temperature change, for instance, can affect the morphology of the particles, and the extension of the reaction time can lead to ZIF degradation[18].
However, because of the stringent conditions required and the intense heating of toxic, polar aprotic solvents at high temperature and pressure, which can pose dangerous health risks and other concerns, such as waste generation, high-energy demand, and safety problems, other, greener synthesis alternatives have been explored that are nonsolvothermal or room temperature. The room-temperature synthesis (RTS) of ZIF-8 in an aqueous solution has also been confirmed to be relatively sustainable[26][34]. Cravillon et al.[25] conducted RTS and further characterization of the resultant ZIF-8 by using zinc nitrate hexahydrate (Zn(NO3)2·6H2O), 2-methylimidazole (Hmin), and methanol (CH3OH) in a molar ratio of approximately 1:8:700[25]. The characterization of the resultant ZIF-8 samples yielded a pure, well-shaped, stabilized ZIF-8 nanocrystal without any auxiliary stabilizing agents or activation (conventional heating, microwave, or ultrasound irradiation). This is shown to be more favorable than the solvothermal method in terms of energy usage, reaction time, and safety[14]. A comparative study by Malekmohammadi et al.[23] was carried out for ZIF-8 synthesis in methanolic and aqueous solutions. In their investigation, the influence of synthesis parameters on the crystallinity and textual characteristics of the resulting products was also evaluated. The outcome of their study was confirmed by Cravillon et al.[25], who found that the sort of solvent used, crystallization temperature, and content of the linker (molar ratio) significantly influence the phase purity of the synthesized ZIF. They obtained the highest yield of ZIF-8, at 97%, from a water solution as a solvent and at a room temperature of 25 °C (nonsolvothermal), which was even higher than the solvothermal method at 130 °C[25]. Therefore, for a stable formation of a pure-phase crystalline structure and the textual properties needed for practical application in contaminant removal, synthesis processes and conditions that are easy, economical, and environmentally friendly, with a high percent yield of ZIF-8, are essential to consider[39].
2. ZIF-8 Heavy-Metal Removal Mechanism
There is growing attention to the sustainable removal of heavy metals from aqueous solutions. Several technologies have been employed to ensure that heavy-metal concentrations in wastewater are lowered to meet the standards of the Environmental Protection Agency (EPA)[40]. Recently, the use of MOFs, especially ZIF-8, has caught the attention of most scientists because of its unique characteristics, such as the high surface area, high adsorption capacity, thermochemical stability, hydrophobicity, and sustainable room-temperature synthesis[41]. The primary mechanism underlying ZIF-8 efficiency in heavy-metal removal is its high adsorption capacity. Adsorption in wastewater treatment is a chemical or physical process that involves the accumulation of a compound (contaminant) at the surface of an adsorbent during a natural interaction between the adsorbate and the adsorbents[42]. In heavy-metal removal, the chemical and hydrothermal stability, and adsorption capacity of the adsorbent are crucial for optimal removal. The adsorption capacity and removal efficiency (%) of ZIF-8, like any other adsorbent, is usually determined by:
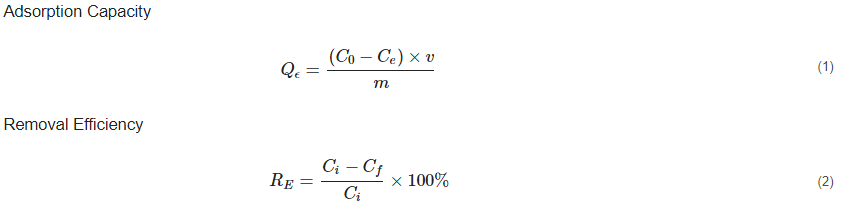
From Equation (1), C0 is the initial concentration, Ce is the equilibrium concentration for the adsorbate in solution, v equals the volume of solution, m is the mass (mg) of the adsorbent, and Qe is the equilibrium adsorption value, used to determine the adsorption capacity of the adsorbent[27]. From equation (2), Ci (mg/l) in the determination of removal efficiency is the initial concentration and Cf (mg/l) is the final concentration of metal ions in the treated effluent[27][38]. The efficiency of the adsorbent is typically described using adsorption-equilibrium isotherms as well as kinetic models. Whereas kinetic models, such as pseudo-first-order and pseudo-second-order kinetics, describe the adsorption rate, adsorption isotherms such as Freundlich and Langmuir isotherms describe the amount of material adsorbed per unit mass of adsorbent as a function of the equilibrium concentration[32][39][43]. In an experiment conducted by Zhang et al. (2016) to assess the ZIF-8 adsorption mechanism and the removal efficiency of copper ions (Cu2+) from aqueous solutions an unexpectedly high adsorption capacity of Cu2+ [800 mg/g] with fast kinetics at an adsorption time of less than 30 min was revealed, obtaining a removal efficiency of 97.2%[2]. The exceptional adsorption capacity of ZIF-8 is underpinned by adsorption mechanisms such as ion-exchange, coordination reaction, surface chemistry, and hydrophobic interactions as diagrammatically expressed in Figure 1 [33][36]. By comparing Fourier Transform Infra-Red (FTIR) spectra and X-Ray Photoelectron Spectroscopy (XPS) before and after adsorption, Zhang et al.[2]attributed the high Cu2+ adsorption of ZIF-8 to the strong coordination reaction and ion exchange between the nitrogen atoms on the Cu2+ surface of the ZIF connectors[2]. Liu et al.[20] also conducted a study using ZIF-8 for the removal of arsenite (As(III)) and arsenate (As(V)) in aqueous systems. They obtained a maximum adsorption capacity (Qmax) of 60 mg/g and 49 mg/g for As(III) and As(V), respectively, which was significantly higher than other adsorbents like iron chitosan flakes, iron-coated zeolite, CuO nanoparticles, etc. compared in their study[40][41]. Zhao et al. [32] also conducted an experiment assessing the removal of four binary heavy metal ions (Cu2+, Ni2+, Co2+, and Cd2+) from aqueous solutions by adsorption onto ZIF-8 nanocrystals. In their study, they used ZIF-8 both as bare nanocrystals and in the formation of a nanocomposite membrane to remove binary heavy metals. In both experiments, although ZIF-8 showed a distinctive selectivity towards Cu2+, the adsorption capacity of ZIF-8 in the removal of these binary heavy metals was exceptionally high[32]. Begum et al. [38]also used ZIF-8 as a nanocomposite membrane with magnesium hydroxide (Mg(OH)2), graphene oxide, and amine groups for Cr(VI). They obtained a maximum removal efficiency of 98% of Cr(VI) at an adsorption equilibrium of 4.88 mg g−1[38].
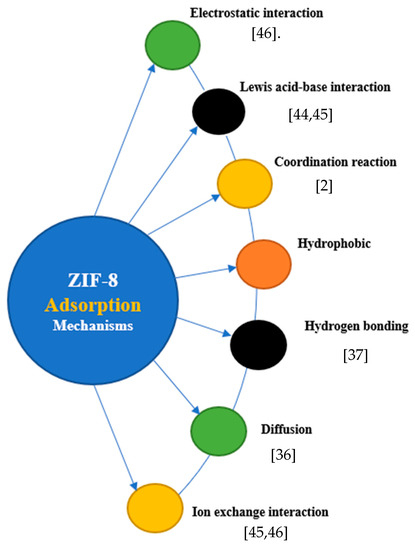
Figure 1. ZIF-8 adsorption mechanisms for heavy-metal removal. Electrostatic interaction; Lewis acid-base interaction[45]; Coordination reaction[2]; Hydrogen boding[37]; Diffusion[36] Ion exchange interraction[45][46]
In many cases, there will be more than one mechanism responsible for the high adsorptive capabilities of ZIF, such as diffusion, hydrogen bonding, and electrostatic interactions[2][44][45][46]. In a study by Begum et al.[38], the surface of ZIF-8 was positively charged because =N−, −NH− and −NH2 groups of imidazolate ligands were protonated in an aqueous system, which provided an electrostatic interaction for Cr(VI) for adsorption. The bonding of hydroxyl groups to zinc and the presence of GO and Mg (OH)2 also strengthened interactions with Cr(VI), further reducing it to Cr(III), partially. In addition, Mg2+, which was positively charged, coordinated with oxyanion, triggering chemisorption, and provided a platform for species adsorption[38]. Zhou et al. (2019) also investigated the use of ZIF-8 for simultaneous removal of mixed contaminants, e.g., copper and norfloxacin from an aqueous solution. They obtained a high removal efficiency of 95.4% and 80.3% for copper and norfloxacin, respectively. Nevertheless, they noticed that the adsorption mechanism for the mixed contaminant was mainly due to the ion exchange for Cu (II) adsorption, whereas electrostatic interactions, π-π stacking between the imidazole ring of ZIF-8, and the benzene ring in norfloxacin were responsible for norfloxacin adsorption[46]. Hence, depending on conditions and constituent of wastewater, surface functional groups, surface charge, and coordination strength, one or more mechanisms may be found to interplay in the removal mechanism. In an experiment by Jian et al.[36], the ZIF-8 adsorption of arsenic from aqueous solutions was mainly controlled by pore diffusion (intraparticle diffusion), similar to the results of Huang et al.[34]. However, it is important to state that the effectiveness of these mechanisms is controlled by critical operating conditions such as pH, temperature, contact time, and adsorbent features[46].
References
- Pena, M.B.; Bulka, C.M.; Swett, K.; Perreira, K.; Kansal, M.; Loop, M.; Daviglus, M.; Rodriguez, C. Occupational environmental exposures and cardiac structure and function: The echocardiographic study of latinos (echo-sol). J. Am. Coll. Cardiol. 2018, 71, A1666.
- Zhang, Y.; Xie, Z.; Wang, Z.; Feng, X.; Wang, Y.; Wu, A. Unveiling the adsorption mechanism of zeolitic imidazolate framework-8 with high efficiency for removal of copper ions from aqueous solutions. Dalton Trans. 2016, 45, 12653–12660.
- Gavrilescu, M. Removal of Heavy Metals from the Environment by Biosorption. Eng. Life Sci. 2004, 4, 219–232.
- Arthanareeswaran, G.; Balaguru, S.; Arthanareeswaran, G.; Das, D.B. Removal of hazardous material from wastewater by using metal organic framework (MOF) embedded polymeric membranes. Sep. Sci. Technol. 2018, 54, 434–446.
- Kim, H.S.; Kim, Y.J.; Seo, Y.R. An Overview of Carcinogenic Heavy Metal: Molecular Toxicity Mechanism and Prevention. J. Cancer Prev. 2015, 20, 232–240.
- Wright, S.L.; Kelly, F.J. Plastic and Human Health: A Micro Issue? Environ. Sci. Technol. 2017, 51, 6634–6647.
- El-Said, M. Scientists Reveal New Method to Remove Heavy Metals from Water. Available online: https://dailynewsegypt.com/2018/03/14/scientists-reveal-new-method-remove-heavy-metals-water/ (accessed on 2 December 2020).
- United Nations. The Sustainable Development Goals Report 2019. Available online: https://undocs.org/E/2019/68 (accessed on 2 December 2020).
- Sun, D.T.; Peng, L.; Reeder, W.S.; Moosavi, S.M.; Tiana, D.; Britt, D.K.; Oveisi, E.; Queen, W.L. Rapid, Selective Heavy Metal Removal from Water by a Metal–Organic Framework/Polydopamine Composite. ACS Central Sci. 2018, 4, 349–356.
- Ping, Q.; Cohen, B.; Dosoretz, C.; He, Z. Long-term investigation of fouling of cation and anion exchange membranes in microbial desalination cells. Desalination 2013, 325, 48–55.
- Dias, E.M.; Petit, C. Towards the use of metal–organic frameworks for water reuse: A review of the recent advances in the field of organic pollutants removal and degradation and the next steps in the field. J. Mater. Chem. A 2015, 3, 22484–22506.
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444.
- Yu, J.; Mu, C.; Yan, B.; Qin, X.; Shen, C.; Xue, H.; Panga, H. Nanoparticle/MOF composites: Preparations and applications. Mater. Horizons 2017, 4, 557–569.
- Milburn, K. Synthesis and Characterization of ZIF-8 and ZIF-8/Polymer Composites. Available online: https://livrepository.liverpool.ac.uk/2006519/1/MilburnKat_Oct2014_2003880.pdf (accessed on 2 December 2020).
- Paseta, L.; Antorán, D.; Coronas, J.; Téllez, C. 110th Anniversary: Polyamide/Metal–Organic Framework Bilayered Thin Film Composite Membranes for the Removal of Pharmaceutical Compounds from Water. Ind. Eng. Chem. Res. 2019, 58, 4222–4230.
- Echaide-Górriz, C.; Sorribas, S.; Téllez, C.; Coronas, J. MOF nanoparticles of MIL-68(Al), MIL-101(Cr) and ZIF-11 for thin film nanocomposite organic solvent nanofiltration membranes. RSC Adv. 2016, 6, 90417–90426.
- Sun, X.; Cheng, N.; Sun, X.; Sun, X. Recent Progress on MOF-Derived Nanomaterials as Advanced Electrocatalysts in Fuel Cells. Catalysts 2016, 6, 116.
- Li, J.; Wang, H.; Yuan, X.-Z.; Zhang, J.; Chew, J.W. Metal-organic framework membranes for wastewater treatment and water regeneration. Co-ord. Chem. Rev. 2020, 404, 213116.
- Park, K.S.; Ni, Z.; Côté, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191.
- Zou, D.; Liu, D.; Zhang, J. From Zeolitic Imidazolate Framework-8 to Metal-Organic Frameworks (MOFs): Representative Substance for the General Study of Pioneering MOF Applications. Energy Environ. Mater. 2018, 1, 209–220.
- Chen, B.; Yang, Z.; Zhu, Y.; Xia, Y. Zeolitic imidazolate framework materials: Recent progress in synthesis and applications. J. Mater. Chem. A 2014, 2, 16811–16831.
- Jang, E.; Kim, E.; Kim, H.; Lee, T.; Yeom, H.-J.; Kim, Y.-W.; Choi, J. Formation of ZIF-8 membranes inside porous supports for improving both their H2/CO2 separation performance and thermal/mechanical stability. J. Membr. Sci. 2017, 540, 430–439.
- Malekmohammadi, M.; Fatemi, S.; Razavian, M.; Nouralishahi, A. A comparative study on ZIF-8 synthesis in aqueous and methanolic solutions: Effect of temperature and ligand content. Solid State Sci. 2019, 91, 108–112.
- Phan, A.; Doonan, C.J.; Uribe-Romo, F.J.; Knobler, C.B.; O’Keeffe, M.; Yaghi, O.M. Synthesis, Structure, and Carbon Dioxide Capture Properties of Zeolitic Imidazolate Frameworks. Accounts Chem. Res. 2010, 43, 58–67.
- Cravillon, J.; Münzer, S.; Lohmeier, S.-J.; Feldhoff, A.; Huber, K.; Wiebcke, M. Rapid Room-Temperature Synthesis and Characterization of Nanocrystals of a Prototypical Zeolitic Imidazolate Framework. Chem. Mater. 2009, 21, 1410–1412.
- Nordin, N.A.H.M.; Ismail, A.; Mustafa, A.; Goh, P.S.; Rana, D.; Matsuura, T. Aqueous room temperature synthesis of zeolitic imidazole framework 8 (ZIF-8) with various concentrations of triethylamine. RSC Adv. 2014, 4, 33292–33300.
- Abdi, J.; Abedini, H. MOF-based polymeric nanocomposite beads as an efficient adsorbent for wastewater treatment in batch and continuous systems: Modelling and experiment. Chem. Eng. J. 2020, 400, 125862.
- Tatarko, J.L., Jr. ThinkIR: The University of Louisville ’ s Institutional Repository The production, properties and applications of the zinc. Electron. theses Diss. 2015, 5, 168.
- Lai, L.S.; Yeong, Y.F.; Lau, K.K.; Shariff, A.M. Effect of Synthesis Parameters on the Formation of ZIF-8 Under Microwave-assisted Solvothermal. Procedia Eng. 2016, 148, 35–42.
- Wu, Y.-N.; Zhou, M.; Zhang, B.; Wu, B.; Li, J.; Qiao, J.; Guan, X.; Li, F. Amino acid assisted templating synthesis of hierarchical zeolitic imidazolate framework-8 for efficient arsenate removal. Nanoscale 2014, 6, 1105–1112.
- Li, J.; Wu, Z.; Duan, Q.; Alsaedi, A.; Hayat, T.; Chen, C. Decoration of ZIF-8 on polypyrrole nanotubes for highly efficient and selective capture of U(VI). J. Clean. Prod. 2018, 204, 896–905.
- Zhao, Y.; Pan, Y.; Liu, W.; Zhanga, L. Removal of Heavy Metal Ions from Aqueous Solutions by Adsorption onto ZIF-8 Nanocrystals. Chem. Lett. 2015, 44, 758–760.
- Kobielska, P.A.; Howarth, A.J.; Farha, O.K.; Nayak, S. Metal–organic frameworks for heavy metal removal from water. Co-ord. Chem. Rev. 2018, 358, 92–107.
- Huang, Y.; Zeng, X.; Guo, L.; Lan, J.; Zhang, L.; Cao, D. Heavy metal ion removal of wastewater by zeolite-imidazolate frameworks. Sep. Purif. Technol. 2018, 194, 462–469.
- Shen, B.; Wang, B.; Zhu, L.; Jiang, L. Properties of cobalt-and nickel-doped zif-8 framework materials and their application in heavy-metal removal from wastewater. Nanomaterials 2020, 10, 1636.
- Jian, M.; Liu, B.; Zhang, G.; Liu, R.; Zhang, X. Adsorptive removal of arsenic from aqueous solution by zeolitic imidazolate framework-8 (ZIF-8) nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2015, 465, 67–76.
- Liu, B.; Jian, M.; Wang, H.; Zhang, G.; Liu, R.; Zhang, X.; Qu, J. Comparing adsorption of arsenic and antimony from single-solute and bi-solute aqueous systems onto ZIF-8. Colloids Surf. A Physicochem. Eng. Asp. 2018, 538, 164–172.
- Begum, J.; Hussain, Z.; Noor, T. Adsorption and kinetic study of Cr(VI) on ZIF-8 based composites. Mater. Res. Express 2020, 7, 015083.
- Khan, I.U.; Jaafar, J.; Jilani, A.; Ismail, A.; Hashim, H.; Jaafar, J.; Rahman, M.A.; Rehman, G.U. Economical, environmental friendly synthesis, characterization for the production of zeolitic imidazolate framework-8 (ZIF-8) nanoparticles with enhanced CO2 adsorption. Arab. J. Chem. 2018, 11, 1072–1083.
- Yahya, A.; Shomar, B.H. Potential use of treated wastewater and sludge in the agricultural sector of the Gaza Strip. Clean Technol. Environ. Policy 2004, 6, 128–137.
- Abbasi, Z.; Cseri, L.; Zhang, X.; Ladewig, B.P.; Wang, H. Metal–Organic Frameworks (MOFs) and MOF-Derived Porous Carbon Materials for Sustainable Adsorptive Wastewater Treatment; Elsevier: Amsterdam, The Netherlands, 2020; pp. 163–194.
- Wanga, X.; Wang, X.; Zhao, G.; Chen, C.; Chai, Z.; Alsaedi, A.; Hayat, T.; Wanga, X. Metal–organic framework-based materials: Superior adsorbents for the capture of toxic and radioactive metal ions. Chem. Soc. Rev. 2018, 47, 2322–2356.
- Simonin, J.-P. On the comparison of pseudo-first order and pseudo-second order rate laws in the modeling of adsorption kinetics. Chem. Eng. J. 2016, 300, 254–263.
- Manousi, N.; Giannakoudakis, D.A.; Rosenberg, E.; Zachariadis, G.A. Extraction of Metal Ions with Metal–Organic Frameworks. Molecules 2019, 24, 4605.
- Zhou, L.; Li, N.; Owens, G.; Chena, Z. Simultaneous removal of mixed contaminants, copper and norfloxacin, from aqueous solution by ZIF-8. Chem. Eng. J. 2019, 362, 628–637.
- Jung, B.K.; Jun, J.W.; Hasan, Z.; Jhung, S.H. Adsorptive removal of p-arsanilic acid from water using mesoporous zeolitic imidazolate framework-8. Chem. Eng. J. 2015, 267, 9–15.




