1000/1000
Hot
Most Recent

Biosensors are measurement devices that can sense several biomolecules, and are widely used for the detection of relevant clinical pathogens such as bacteria and viruses, showing outstanding results. Because of the latent existing risk of facing another pandemic like the one we are living through due to COVID-19, researchers are constantly looking forward to developing new technologies for diagnosis and treatment of infections caused by different bacteria and viruses. Regarding that, nanotechnology has improved biosensors' design and performance through the development of materials and nanoparticles that enhance their affinity, selectivity, and efficacy in detecting these pathogens, such as employing nanoparticles, graphene quantum dots, and electrospun nanofibers.
Biosensor's concept was firstly addressed by Clark and Lyons around 1962 when they developed an oxidase enzyme electrode for glucose detection [1]. Since then, nanotechnological development has promoted biosensors evolution and specialization for different purposes [2]. Currently, nanotechnology is at the forefront of science, and its combination with biosensoring applications involves different fields such as medicine, biology, environmental, drug delivery, and food safety [3][4][5][6][7]. However, the detection of pathogens has become one of the most relevant objectives for these devices since bacterial and viral diseases currently represent an important thread for human health [8][9].
Virus and bacteria detection commonly involves the use of several molecular techniques such as the reverse transcription-polymerase chain reaction (RT-PCR), which remains the gold standard for pathogen detection [10]. The classical detection methods for these pathogens usually require isolation, culturing and, biochemical tests [11]. Additionally, serological tests like the Enzyme-Linked Immunosorbent Assay (ELISA) are used for the detection of antibodies and immunoglobulin needed for identification purposes [12]. However, some of these techniques take a long time to obtain results and are usually laborious. Therefore, new approaches based on nanotechnological advances have emerged as suitable and easier options for detecting pathogens in faster and efficient ways [11][13].
On one hand, nanoparticles (NPs) have demonstrated outstanding properties against different pathogens used to develop novel devices and technologies that contribute to this public health issue [14][15]. The interest is not limited to human diseases, but also considers the ones affecting animals since zoonosis is an existent thread. Stringer et al. developed an optical biosensor using gold NPs (AuNPs) and quantum dots (QDs) for the detection of porcine reproductive and respiratory syndrome virus [16].
On the other hand, the international scientific community's interest in using DNA biosensors or sequence-specific DNA detectors for clinical studies is increasingly growing. In 2007, Dell'Atti et al. developed a combined DNA-based piezoelectric biosensor for simultaneous detection and genotyping of high-risk human papilloma virus (HPV) strains [17]. In addition, these biosensors have been employed for DNA damage research and specific gene sequences detection [18][19].
Biosensors and nano-biosensors have been extensively used for the detection of viral and bacterial clinical pathogens. These devices are practical (e.g., enable point-of-care (POC) testing through smartphone-based nano-biosensor), fast, and are considered as innovative technologies that provide an alternative solution to the mentioned disadvantages presented by common detection methods [20][21][22]. These technologies have been employed for studying viruses affecting human health such as Ebola virus, human immunodeficiency virus (HIV), and more recently the newly discovered acute respiratory syndrome coronavirus 2 (SARS-CoV-2), as well as bacteria like Escherichia coli and Salmonella spp. [23][24][25][26][27].
Biosensors can be defined as a measurement system for analyte detection that combines a biological component with a physicochemical detector [28]. The analyte detection depends on the biosensor design and purpose. Some commonly used devices such as smartphones can be employed as a biosensor with the inclusion of simple accessories as published by Soni et al., where they developed a non-invasive smartphone-based biosensor for urea using saliva as sample [29][30]. This allows fast and low-cost preliminary detection [31].
Usually, biosensors detect biomolecules such as nucleic acids, proteins, and cells that are associated with diseases. This is possible because of their three major components: The biologically sensitive element, the detector element, and the reader device [32]. Enzymes, microorganisms, organelles, antibodies, and nucleic acids are used to detect the biomolecules [33]. In addition, researchers must identify the requirements to obtain a functional device according to the intended use. Hence, multidisciplinary studies are fundamental to select the proper material, transducing device, and biological element involved before assembling the biosensor [34].
At a clinical level, biosensors are applied for detecting disease-associated biomolecules [32]. These devices can monitor the biochemical markers of a disease in body fluids, such as saliva, blood, or urine [35][36]. Zhang et al. developed a non-invasive method for glucose testing based on a disposable saliva nano-biosensor to improve patient compliance, reduce complications, and costs derived from diabetes management. In the clinical trials, they obtained outstanding results in terms of accuracy compared to the UV spectrophotometer. Thus, the disposable device can be presented as an alternative for real-time salivary glucose tracking [37].
Biosensors can be applied for many other clinical diagnostic purposes, such as cholesterol, markers related to cardiovascular diseases, biomarkers of cancer or tumors, allergic responses, disease-causing bacteria, viruses, and fungi infections [38][39][40][41]. Aside from that, biosensors can be employed for bacteria and virus detection in food and water, which are potential sources of diseases [42][43]. Zhao et al. fabricated a low-cost, portable microfluidic chemiresistive biosensor based on monolayer graphene, AuNPs, and streptavidin-antibody system for the rapid in-situ detection of E. coli. In this case, the bacteria are captured on the biosensor's surface and detection is performed through electric readouts [44]. Another approach published by Samanman et al. describes the development of a glutathione-S-transferase tag for white spot binding protein (GST-WBP) immobilized onto a gold electrode through a self-assembled monolayer. This biosensor can detect white spot syndrome virus (WSSV) in shrimp pond water due to binding between WSSV and the immobilized GST-WBP [45].
Over time, many techniques and methods have been developed for detecting pathogens such as viruses and bacteria, including colorimetric methods, fluorescence polarization, and electrochemical analysis [46]. However, those are very expensive and possess limitations related to time-consumption, low precision of the results, poor stability, and short life span [47].
Bacterial and viral outbreaks have caused many issues in biomedical, food, and environmental context, making necessary the development of new strategies that allow faster detection of these pathogens to effectively contain and control their impact on human health [48]. The combination of nanotechnologies and biosensors' characteristics is currently being considered as a potential opportunity for speeding up the development of fast, highly sensitive, and specific devices for genuine bacterial and viral detection. As a consequence, nano-biosensors make use of chemical, electrical, optical, and magnetic properties of materials for detecting biomolecules and pathogens [49][50].
In order to satisfy the previous, nanotechnology has greatly contributed to the development of biosensors due to research in nanomaterials and nanostructures, such as carbon nanotubes, GQDs, metal oxide NPs, metal nanoclusters, plasmonic nanomaterials, polymer nanocomposites, nanogels, among others (Figure 1) [51][52][53][54]. These have been employed for modifying electrode surfaces to improve critical features, such as reproducibility, selectivity, and sensitivity, due to their biocompatible character, structural compatibility, and high adsorption capacity. Therefore, nanomaterials have demonstrated to be suitable for biosensing applications, enhancing the performance with increased sensitivities and lower detection limits [55].
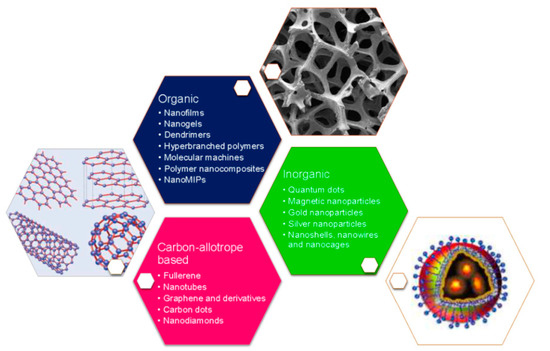
Figure 1. Different nanomaterials and nanostructures used for the development of nano-biosensors. Reprinted with permission from Pirzada, M. et al. Nanomaterials for Healthcare Biosensing Applications. Sensors 19(23): 5311. Copyright (2019) MDPI [56].
Additionally, different nanomaterials have been used to increase the immobilized bioreceptor loadings. However, the strategy for immobilizing the bio-specific entity onto the nanomaterial is considered the biggest challenge for developing a high quality and reliable nano-biosensor. Non-covalent approaches such as electrostatic interactions, polymers entrapment, or van der Waals forces between the nanomaterial and the biomolecule do not alter their specific properties. On the other hand, covalent binding provides more stability and reproducibility of surface functionalization, as well as reducing the risk of unspecific physisorption. Although the previous techniques represent good strategies for binding biological species to surfaces, supramolecular interactions have recently been considered as superior since these are reversible, enabling the regeneration of the transducer element [56][57].
Regarding other uses, nanomaterials can perform as nanocarriers for signaling elements, as well as signal amplification. Depending on the chemical composition, nanomaterials can be subject to direct functionalization during synthesis, or functionalized by coating using functional polymers [58]. Nanomaterials functionalization provides three important advantages: reproducible immobilization of bioreceptor units, increase the biocompatibility, and the development of label-free transduction techniques [57].
Moreover, nano-biosensor materials' high surface area is considered a major advantage compared to conventional devices, and plays an important role in the sensitivity and fast response of the devices [59][60]. Therefore, these are conceived as excellent tools used for the detection, function, and interaction of proteins and nucleic acids, which improve the quality and performance of diagnosis for bacterial and viral diseases [61]. The following sections present an overview of some promising nanotechnological features in biosensors.
Conventional clinical analyses including an antibody or nucleic acid-based, biochemical, and enzymatic methods, are very reliable but take a long time to obtain a result. Health disciplines demand the acquisition of faster outcomes to speed up the appropriate treatment [62][63]. In this sense, biosensors and nano-biosensors are useful tools that offer an accurate response in shorter times due to their ability to provide real-time and faster clinical results [64]. Currently, there is an increasing interest in their use to detect pathogens in the human body (Table 1) [63].
Table 1. Developed biosensors for detecting bacterial and viral pathogens in the human body.
|
Device |
Target Pathogen |
LOD |
Response Time |
Reference |
|
|
Long-period fiber grating using bacteriophage T4 covalently immobilized on optical fiber surface. |
E. coli |
103 CFU/mL |
20 min |
[65] |
|
|
Label free polyaniline based impedimetric. |
E. coli O157:H7 |
102 CFU/mL |
- |
[66] |
|
|
Electrochemical biosensor using antibody-modified NPs (polymer-coated magnetic NPs and carbohydrate-capped AuNPs). |
E. coli O157:H7 |
101 CFU/mL |
45 min |
[67] |
|
|
Graphene-based potentiometric. |
S. aureus |
1 CFU/mL |
10–15 min |
[68] |
|
|
Aptamer based biosensor and dual florescence resonance energy transfer from QDs to carbon NPs. |
Vibrio parahaemolyticus and Salmonella typhimurium |
25 CFU/mL and 35 CFU/mL, respectively |
80 min |
[69] |
|
|
Impedimetric biosensor based on site specifically attached engineered antimicrobial peptides. |
Pseudomona aeruginosa |
102 CFU/mL |
30 min |
[70] |
|
|
Electrochemical DNA biosensor based on flower-like ZnO nanostructures. |
Neisseria meningitides |
5 ng/μL |
- |
[71] |
|
|
Graphene-enabled biosensor with a highly specific immobilized monoclonal antibody. |
Zika virus |
0.45 nM |
4–8 min |
[72] |
|
|
Giant magnetoresistance biosensor. |
Influenza A virus |
1.5 × 102 TCID50/mL |
- |
[73] |
|
|
Electrochemical biosensor based on DNA hybridization. |
Hepatitis A virus |
6.94 fg/μL |
15 min |
[74] |
|
|
Impedimetric electrochemical DNA biosensor for label free detection. |
Zika virus |
25 nM |
1.5 h |
[75] |
|
|
Two-dimensional molybdenum disulphide nanosheets based disposable biosensor. |
Chikungunya virus |
3.4 nM |
3 h |
[76] |
|
|
Electrochemical DNA biosensor using gold nanorods. |
Hepatitis B virus |
2.0 × 10−12 mol/L |
5 h |
[77] |
|
|
Intensity-modulated surface plasmon resonance (IM-SPR) biosensor |
Avian influenza A H7N9 virus |
144 copies/mL |
10 min |
[78] |
|
|
Silicon nanowire biosensor. |
Dengue virus |
2.0 fM |
- |
[79] |
|
AuNPs: gold nanoparticles; E. coli: Escherichia coli; IM-SPR: Intensity-modulated Surface Plasmon Resonance; LOD: limit of detection; NPs: nanoparticles; QDs: quantum dots; S. aureus: Staphylococcus aureus; SPR: Surface Plasmon Resonance.
Molecular determination demands to improve the analytical performance of biosensors, which have enhanced their unique features to develop POC devices in order to run a rapid and cost-effective analysis of complex biological matrices [80]. Commercial versions of these devices are available to detect pathogens such as E. coli, Helicobacter pylori, influenza A and B viruses, HIV, tuberculosis, and malaria [81]. Advantages such as small samples and low energy required to avoid complications in terms of transportation and processing, make them suitable for easy and fast use in the identification of bacterial and viral pathogens [64]. Needless to say, nanomaterials advances have benefited biosensor performance to achieve the task [82].