
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Domenico Di Raimondo | + 4493 word(s) | 4493 | 2021-01-04 03:04:58 | | | |
| 2 | Peter Tang | -96 word(s) | 4397 | 2021-01-19 04:19:43 | | | | |
| 3 | Peter Tang | -12 word(s) | 4385 | 2021-01-20 12:01:32 | | |
Video Upload Options
One of the major obstacles that prevents an effective therapeutic intervention against ischemic stroke is the lack of neuroprotective agents able to reduce neuronal damage; this results in frequent evolution towards a long-term disability with limited alternatives available to aid in recovery. Nevertheless, various treatment options have shown clinical efficacy. Neurotrophins such as brain-derived neurotrophic factor (BDNF), widely produced throughout the brain, but also in distant tissues such as the muscle, have demonstrated regenerative properties with the potential to restore damaged neural tissue. Neurotrophins play a significant role in both protection and recovery of function following neurological diseases such as ischemic stroke or traumatic brain injury. Unfortunately, the efficacy of exogenous administration of these neurotrophins is limited by rapid degradation with subsequent poor half-life and a lack of blood–brain-barrier permeability. Regular exercise seems to be a therapeutic approach able to induce the activation of several pathways related to the neurotrophins release. Exercise, furthermore, reduces the infarct volume in the ischemic brain and ameliorates motor function in animal models increasing astrocyte proliferation, inducing angiogenesis and reducing neuronal apoptosis and oxidative stress. One of the most critical issues is to identify the relationship between neurotrophins and myokines, newly discovered skeletal muscle-derived factors released during and after exercise able to exert several biological functions. Various myokines (e.g., Insulin-Like Growth Factor 1, Irisin) have recently shown their ability to protects against neuronal injury in cerebral ischemia models, suggesting that these substances may influence the degree of neuronal damage in part via inhibiting inflammatory signaling pathways.
1. Introduction
According to the American College of Sports Medicine (ACSM), Physical Activity (PA) can be defined as any bodily movement produced by the contraction of skeletal muscles that results in a substantial increase in caloric requirements over resting energy expenditure [1]. Contrary to usual belief, exercise is not a synonym rather a subcategory of PA, consisting of planned, structured and repetitive bodily movement done to improve and/or maintain one or more components of PA (e.g., swimming) [2]; all exercise involves some combination of isometric (static) and isotonic (dynamic) stress.
Regular PA has multiple benefits. Several recent meta-analyses show robust epidemiological evidence indicating that regular PA is associated with a reduction of all the main adverse cardiovascular outcomes as well as reduced incidence of type 2 diabetes, hypertension, different types of cancers, osteoarticular disorders [3][4][5]. A risk reduction of coronary heart disease and stroke between 20% and 30% both in men and women in response to a high level of leisure time PA and a possible reduction (10% to 20%) of risk of cardiovascular disease in response to moderate level of occupational PA is reported [6][7].
When prescribed for a medical purpose, exercise is commonly divided into the broad subgroups of aerobic/endurance (i.e., running, walking) and anaerobic/resistance (i.e., weight lifting) activity, although many exercise modalities combine both types. The different types of exercise differ substantially from each other: aerobic and anaerobic exercises involve in a dissimilar way the metabolic patterns, the cardiovascular and respiratory response, the types of muscle fibers used [8] More specifically, as defined by ACSM, aerobic exercise is considered as any activity that uses large muscle groups, can be maintained continuously and is rhythmic in nature; anaerobic exercise has been defined as intense PA of very short duration, fueled by the energy sources within the contracting muscles and independent of the use of inhaled oxygen as an energy source [1]. These remarkable discrepancies between the different types of exercise explain the various chronic adaptations in several organs and systems that can be observed in trained subjects after a quite long period of time.
Various studies have been published that prove the advantages of both aerobic and anaerobic exercise resulting in a positive correlation towards improved cardiovascular health [8]. The peculiar characteristics of aerobic training (AT), or endurance training, lead to beneficial effects on various aspects related particularly to cardiovascular wellness. Studies have shown that AT elicits an adaptive, beneficial form of cardiac remodeling that involves cardiomyocyte growth and proliferation, these effects are directly associated with epigenetic adaptations associated with optimization of oxygen use for ATP production within cardiomyocytes [9]. Furthermore, AT improves oxygen delivery to tissues raising the cardiac output and positively influencing all the mechanisms involved [10]. The increased need for oxygen and energetic substrates leads also to an increase in brain flow [11]; for these reasons AT is widely considered the method of choice for stimulating angiogenesis and inducing the associated local and systemic health-related benefits.
Resistance Training (RT), a form of strength training wherein effort is performed against a specific opposing force generated by resistance, mainly enhances muscle strength, endurance and muscle mass, prevents osteoporosis and sarcopenia in elderly [12]. Moreover, RT exert a beneficial influence on the cardiovascular system, by stimulating the synthesis of molecules such as C-type natriuretic peptide (CNP), an endothelium-derived natriuretic peptide (EDNP) which, through its effects in vascular tone and its antifibrotic and antiproliferative properties may be key elements linking anaerobic RT and improved cardiovascular function [13].
Consensus documents released after independent analysis by leading world organization such as the American College of Sports Medicine and the Centers for Disease Control, the National Institutes of Health (NIH), the US Department of Health and Human Services (HHS) and the European Society of Cardiology (ESC) each concluded that habitual moderate-intensity exercise is an effective tool to reduce the overall risk of chronic disease [14][15][16][17][18]. Nowadays, the ACSM recommends for older adults and patients ≥ 50 years with chronic disorders, a program incorporating aerobic, strengthening, flexibility and balance training. Aerobic training should be done ≥5 days/week for 30 min of moderate intensity or ≥3 days per week for 20 min at vigorous intensity [19]. It should be clearly emphasized that the identification of the optimal dose of exercise, more specifically the exercise dose required to produce the higher level of benefit (exercise dose can be defined as the combination of three determinants: duration, frequency, and intensity), varies considerably across key outcomes of clinical relevance (control of risk factors, primary prevention, secondary prevention, mortality). In clinical practice, this means that the prescription of an exercise dose may best be accomplished by identifying a specific therapeutic target for each individual patient. Without prejudice to the above, in the absence of specific recommendations, the availability may be recommended to improve neurological well-being and counteract brain aging.
2. Neuroprotective Effects of Regular Physical Activity
Neurological diseases are multifactorial disorders. Lifestyle together with other factors (genetic profile, inflammation and environment) may have a strong influence on their development and this applies especially to neurodegenerative diseases, such as dementia, becoming more prevalent globally resulting in a constantly increasing cost burden for the global health system [20]. Regular exercise has pleiotropic actions on brain function and plays an important role in the prevention and treatment of many neurological diseases such as vascular dementia, Alzheimer's Disease, Parkinson's disease and epilepsy as extensively discussed in many good reviews about the topic [5][21][22][23]. Figure 1 mainly summarizes the main mechanisms through which PA could influence the management of major neurological diseases.
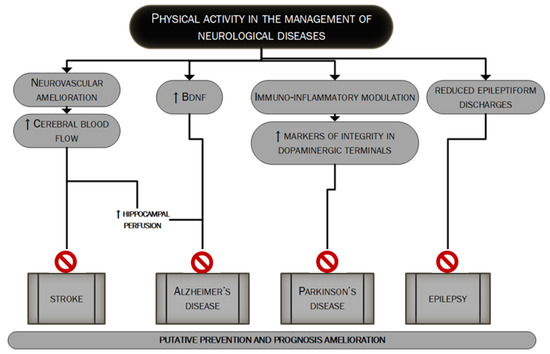
Figure 1. The putative role of PA in the management of major neurological diseases. In this figure, we summarize the main mechanisms through which regular PA could lead to better management of the four major neurological diseases: stroke [24][25][26][27][28], Alzheimer's disease, Parkinson's disease and epilepsy [5][21][22][23]. PA's role could be pivotal in the prevention as well as in the prognosis' improvement.
Since the first observations that date back to the last years of the second millennium in which the effects of a six-month aerobic exercise training were compared to stretching training, observing an increased performance in tasks that required a high degree of executive control (e.g., response-compatibility and task switching tasks) only after aerobic PA [29] various studies in humans linked lower aerobic fitness in sedentary older individuals with a reduced cerebral blood flow and decreased cognitive performance [30] and aerobic training with the improved cognitive performance [31]. Higher cortical functions, both in healthy subjects and in subjects suffering from one or more neurological diseases, both acute or chronic, are influenced by the subject's level of physical fitness, in a direct or indirect (through the benefits induced by exercise on cardiovascular well-being and the improved control of the main cardiovascular risk factors and systemic inflammation) manner [21]. Specific regions such as the anterior cingulate and hippocampus have been identified as those most involved [32]. PA also influences analgesia, sedation, anxiolysis and sense of well-being perceived during and after exercise [33], through the modulation of the endocannabinoid system neurophysiological pathway [34], although other studies suggest that also the mesolimbic system may be related to exercise-induced analgesia and perceived well-being [35]. There is also evidence of the releasing of endogenous opioids in frontolimbic brain regions after exercise, related to the euphoric state after intense PA, the so-called "runner's high" [36], all these being associated with a positive influence on stress, anxiety and depression [21]. The "antidepressant" effect of exercise can also be attributed to the upregulation of the peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PGC-1α) nuclear mediator in exercised muscles. The overexpression of PGC1α in muscle after regular PA seems able to change the kinurenine metabolism (increasing the conversion of kynurenine to kynurenic acid) thus reducing kinurenine entry into the brain; kinurenine mainly is derived from the catabolism of the essential amino acid tryptophan and high levels of kinurenine are found in people with depression [37].
In view of these valuable epidemiological data supporting the beneficial effects of PA on neurological diseases, some mechanisms have been hypothesized to explain what has been observed. Trying to classify the main, three different mechanisms can be identified: Figure 2 summarizes the main beneficial effects of regular exercise in protecting against major acute and chronic neurological disorders.
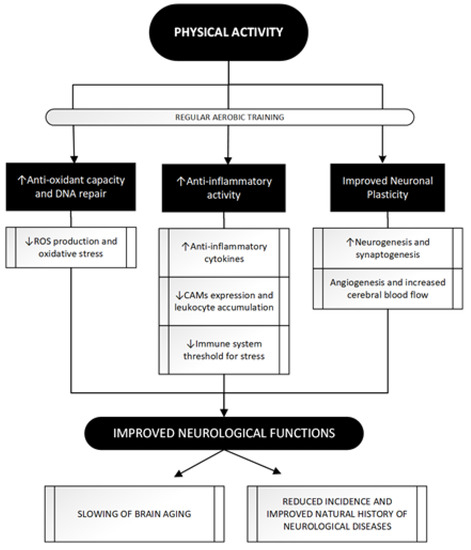
Figure 2. The attempt to improve neurological functions through regular PA: a three paths journey. PA achieves beneficial effects on brain function through its impact in these three main pathways whose modulation eventually leads to a neuroprotective effect. Regular aerobic training as opposed to acute bouts of exercise, seems to be more related to PA positive impact leading to antioxidant [38][39] and anti-inflammatory [40][41][42][43] effects and, through molecular and vascular changes, shapes neuronal and vascular plasticity [44][45][46][47]. See text for further information. ROS, Reactive Oxygen Species; CAMs, Cell Adhesion Molecules; DNA, DeoxyriboNucleic Acid.
3. Immuno-Inflammatory Activation during and after the Acute Phase of an Ischemic Stroke
Stroke is globally the second cause of death after ischaemic heart disease [20]. Many evidence available indicates that almost 90% of cardiovascular disease, including stroke, is caused by potentially modifiable risk factors [48]. In this perspective, improved risk factor control and a better management scheme along with a supporting role of stroke awareness programs could be a key to decrease stroke rate as well as post-stroke residual disability. Evidence shows that exercise can positively influence several physical (e.g., cardiovascular fitness, walking ability, muscle strength) and psychosocial (e.g., depressive symptoms, memory, fatigue) domains after a stroke [49].
Ischemic stroke has been described as a "thrombo-inflammatory disease", since thrombosis and inflammation are highly intertwined processes that interact at multiple points as contributors to brain injury and stroke progression [50] Many chronic systemic inflammatory conditions, such as atherosclerosis, diabetes and obesity are associated with increased risk of stroke, which suggests that systemic inflammation may contribute to the development of stroke in humans [51]. These elements tell us that inflammation is part of ischemic stroke pathobiology as well as part of its pathophysiological background, and thus has an all-around pivotal role. Atrial Fibrillation itself, another pivotal risk factor for ischemic stroke [52], carries a separate systemic inflammatory burden, being associated with an increased "inflammatory cytokines milieu" and endothelial dysfunction [53].
Cerebral ischemia initiates a complex cascade of events at genomic, molecular, and cellular levels, and inflammation is important in this cascade, both in the Central Nervous System and in the periphery [54]. The inflammatory response starts locally in occluded and hypoperfused vessels and in the ischemic brain parenchyma, leading to systemic propagation of in situ–produced inflammatory cytokines and to damage in the blood–brain barrier; this creates a route for entry of immune cells, solutes and water into the brain parenchyma resulting in interstitial inflammation and oedema that damages neuronal tissue [55]. The cerebral ischemic injury induces a series of inflammatory events including the infiltration of circulating immune cells (neutrophils and monocytes) and activation of resident cells (i.e., microglia, astrocytes and endothelial cells) [56]. In this complex scenario, cytokines have a central role modulating local changes and systemic host response to Central Nervous System inflammation, infection and injury [54]. Three major cytokines, tumor necrosis factor (TNF-alpha), interleukin (IL)-1, and IL-6 are produced by cultured brain cells after various stimuli such as ischemia [57]. The source of these and other less detectable cytokines remains unclear although serially measured IL-lβ and IL-6 in patients with ischemic stroke, resulted higher in cerebrospinal fluid than in serum, thus being suggestive of intrathecal production of the cytokines [58].
Functions and actions of ischemia-related cytokines in the brain remain to be elucidated, yet probably include both beneficial and detrimental effects [54], suggesting a "multiphasic role" [59]. Inflammation seems in fact to be more damaging during the early phase of ischemic stroke, while in recovery stages it seems to help clear up debris and support wound healing and this could be the reason why anti-inflammatory drugs (steroidal and non-steroidal agents) failed to demonstrate a benefit in thromboembolic infarction [55].
The level of systemic inflammation in stroke patients strongly depends on the etiopathogenesis of the ischemic episode and the whole clinical profile of the patient (number of comorbidities). This different background on which the acute ischemic brain event occurs, makes it more correct to talk about ischemic strokes rather than ischemic stroke [60], since the different diagnostic subtypes are associated with a distinct level of neurological damage and functional impairment as well as with a diverse degree of local and systemic immunoinflammatory activation [51][54][57][61][62][63][64][65][66]. This is clear if one compares, for example, a small lacunar stroke, often subcentimetric, with a cardioembolic stroke, commonly larger and with multiple synchronous lesions, sustained by a continuously active emboligenous heart disease itself associated with high levels of systemic inflammation [53][67].
It is of course conceivable that other factors could represent an additional element influencing immuno-inflammatory activation after acute ischemic stroke, infarct size could represent one additional possible factor influencing immuno-inflammatory marker plasma levels although results in this regard are unclear and more evidence is needed [67][68][69][70].
Finally, ischemic stroke-related inflammatory response is clearly variable. This variability depends, as thoroughly discussed, on stroke subtype (and, consequently stroke's metabolic and systemic background) and infarct size but could also depend upon genetic polymorphism of candidate genes of inflammatory cytokines, as proinflammatory genetic genotypes (IL-6 GG, ICAM-1 EE, E-Selectin AA) are significantly more common in subjects with stroke history [51][71]. The implicated genes are both pro-inflammatory and/or anti-inflammatory. IL-10 is, for instance, one of these inflammation-related genes implicated in the pathogenesis of ischemic stroke and is a potent anti-inflammatory cytokine whose action drastically limit the size of brain injury, atherosclerosis and inflammatory response, counteracting the effects of pro-inflammatory molecules such as TNF-α; IL-10 (-1082A/G) has been associated with the risk of Large Vessel Disease, Small Vessel Disease, and other subtypes of ischemic stroke, although further studies should be conducted [72].
Understanding the inflammatory background of subtype-related ischemic stroke could have a strong prognostic value. Interestingly, immune-inflammatory activation of the acute phase of ischemic stroke is associated with the severity of the acute neurological deficit evaluated by the National Institutes of Health Stroke Scale (NIHSS) [73]; higher levels of inflammation seem to correlate with a poorer outcome for the patient.
In this regard, our group [67] found that patients with cardio-embolic strokes, in comparison with other subtypes, showed a higher degree of immuno-inflammatory activation as well as a higher degree of acute neurological deficit whereas patients with lacunar stroke, compared to other subtypes, showed a lower degree of immuno-inflammatory activation with a lower degree of acute neurological deficit.
Figure 3 shows the main features of the immuno-inflammatory activation during an ischemic stroke.
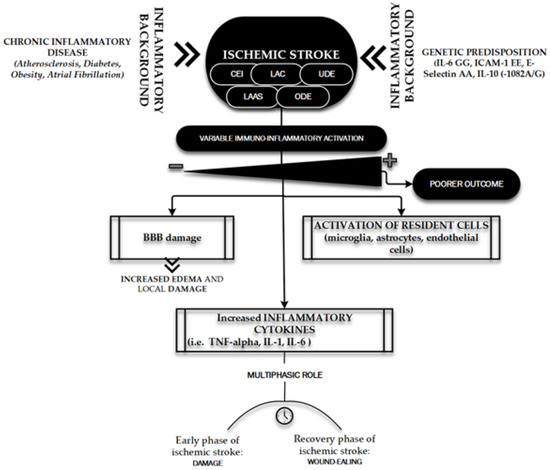
Figure 3. Immuno-inflammatory activation during ischemic stroke. The figure briefly outlines the main determinants and characters involved in the "inflammatory face" of ischemic pathobiology. Chronic inflammatory diseases [51] and genetic predisposition [51][71] represent the key elements playing a leading role in determining the variability of immune-inflammatory activation [51][54][57][60][61][62][63][64][65][66] on which depends the patient's outcome: the inflammatory background elements and the ischemic stroke subtypes (TOAST classification) [74]. CEI, CardioEmbolic Infarct; LAC, LACunar infarct; UDE, a stroke of UnDetermined Etiology; ODE, a stroke of Other Determined Etiology; LAAS, Large Artery AtheroSclerosis; IL, Interleukin; ICAM, Intercellular Cell Adhesion Molecule; TNF, Tumor Necrosis Factor.
4. Anti-Ischemic Effects of Physical Activity
With limited therapeutic opportunities, stroke remains a relevant critical disease with multiple hopefully modifiable pathophysiological mechanisms whose better knowledge could lead to a most successful post-stroke rehabilitation program. An ischemic stroke is often triggered by cardio-embolism or athero-thrombotic arterial obstruction causing a sudden decrease in cerebral blood flow. In view of the peculiar terminal circulation that can be found in the brain, a gradient of decreased blood flow emerges, causing severe neuronal injury and blood flow reduction and resulting in a surrounding penumbra (ischemic penumbra) in which degenerative reactions and blood flow reduction are partly reversible. Neuronal damage due to ischemic stroke is attributable mainly to two mutual pathological processes: oxygen loss and disturbance in glucose supply to affected brain areas, triggering the immune-inflammatory cascade previously briefly discussed. The health of the cerebral vessels is therefore a central element of protection against ischemic stroke [75].
It is well known the role carried out by exercise in mediating the recovery of post-stroke damage. PA can contribute to the functional compensation of surviving brain areas involved in limb function: the possible mechanisms include enhanced activity and axonal growth of the pyramidal and extrapyramidal systems in the ipsi-lesional and contralesional hemispheres [76]. Despite the intriguing pathophysiological context and the numerous experimental data available, only a few studies have attempted to ascertain the vascular and anti-ischemic protection role played by PA before that an ischemic cerebral accident occurs.
PA is a mild stressor for the organism; causing an increased need for oxygen and energetic substrates, stimulates a series of changes aimed to ensure the amelioration of cerebral vascularization both in quantitative (short-term modification) and qualitative (long-term adaptations) way. The quantitative aspect is what acts in the first play. It is in fact well established that acute exercise increases the cardiac output for adaptation to the increased needs mentioned above, thus leading to a prompt increase in the cerebral vascular flow [77][78][79][80]. Regular PA, on the other hand, can induce increased regional cerebral blood flow by promoting qualitative modifications, angiogenesis and collateral vessels recruitment; the mechanism of recruitment of already existing collateral vessels has a protective effect towards the circle of Willis against high-grade extra or intracranial stenosis or occlusion to reduce risk of watershed infarctions, thus making exercise a modulator factor of cerebral autoregulation [24]. Recently-conducted in vivo animal studies showed how treadmill activity promotes angiogenesis in ischemic stroke animal models [25][26][27]. Pianta et al. [27] found that animals that underwent short durations of exercise (30 m and 60 m exercise groups) prior to stroke induction showed reduced infarct volumes compared to non-exercised stroke animals and histologically, qualitative analysis of surviving cells in the peri-infarct area showed a concomitant increase in the number of live cells as the duration of exercise increased. In this study, the amounts of neurotrophins/angiogenetic factors such as Vascular Endothelial Growth Factor (VEGF), VEGFR-2, and Ang-2 were found to be augmented near the site of the brain injury [27]. It's supposed that even an early in life PA confers long-lasting protection against cerebral ischemia, as demonstrated by Serra et al. [28], through an epigenetic stable upregulation of the expression of neurotrophic factors which are linked to increased neuronal growth providing the brain with a greater "neuronal reserve".
Figure 4 summarizes the main anti-ischemic effects of PA.
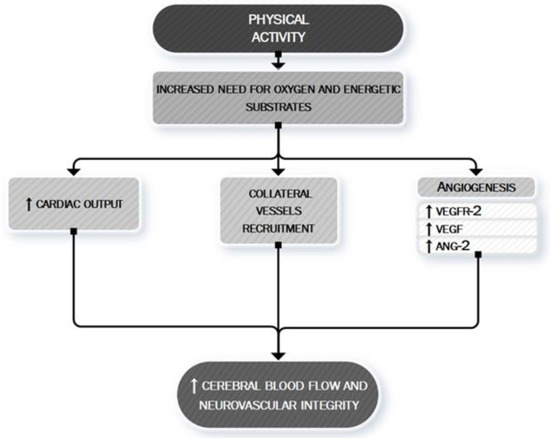
Figure 4. PA: a neurovascular booster. During PA, the increased need for oxygen and energetic substrates leads to the activation of many mechanisms whose ultimate effect is to determine an increased cerebral blood flow and to maintain neurovascular integrity. This aim is exerted both by asserting a boosting of cardiac contractility (quantitative effect) [77][78][79][80] and by a local cerebral modulation of neurovascular dynamic (qualitative effect) through the recruitment of collateral vessels [24] and the stimulation of angiogenic processes [27]. VEGF, vascular endothelial growth factor; VEGFR-2, vascular endothelial growth factor receptor 2; ANG-2, Angiopoietin-2.
A neuroradiological study demonstrates that physically active older adults have been found to display a higher number of small cerebral vessels than physically less active older adults [81]. A very recent study conducted in humans has found that a single session of aerobic exercise is adequate to produce transient measurable changes in cerebral perfusion [82] although is unclear if this represents the earliest stages of neural and/or vascular plasticity or a distinct transient mechanism.
Finally, exercise's anti-ischemic effect may be also linked to the mechanism of preconditioning [83]. Preconditioning is defined as the exposure of a system or an organ to a conditioning stimulus to induce tolerance or resistance to a subsequent injury [84] by triggering cells and organisms to express intrinsic protective factors, thus helping them acquire tolerance and self-defense against later possible damage [85]. Several studies have demonstrated that preconditioning exercise (i.e., prophylactic exercise prior to stroke) provides significant neuroprotection against acute stroke through the promotion of angiogenesis, mediation of inflammatory responses, and inhibition of neuronal apoptosis [83]. In the 2,3,5-triphenyltetrazorlium chloride (TTC) study, the tissue infarction in rats with preconditioning exercise was decreased compared with the brain of rats without preconditioning [83]. Preconditioning with different exercise protocols such as moderate continuous training (MCT) and high-intensity interval training (HIIT) confers similar protection against neurological deficits and tissue injury following an acute stroke [86] although the benefit seems to be directly proportional to the intensity of training [87] and to a longer period of exercising before the ischemic injury [88][89].
The mechanisms through which preconditioning exercise could protect the brain are various. Preconditioning exercise activates astrocytes and improves angiogenesis in the penumbra areas following brain ischemia [90] exerting regulation of multiple factors significant for neurovascular integrity—i.e., endotelin-1, VEGF, IGF-1 and midkine (MK) a heparin-binding growth factor promoting angiogenesis [90][91][92][93][94][95].
Moreover, preconditioning exercise improves different structural and functional components of the blood–brain barrier (BBB) [83] whose dysfunction is one of the key pathobiological mechanisms leading to local and systemic immuno-inflammatory activation and to increase local injury via cerebral edema.
The complex interaction between preconditioning and brain protection towards an ischemic event is shown in Figure 5.
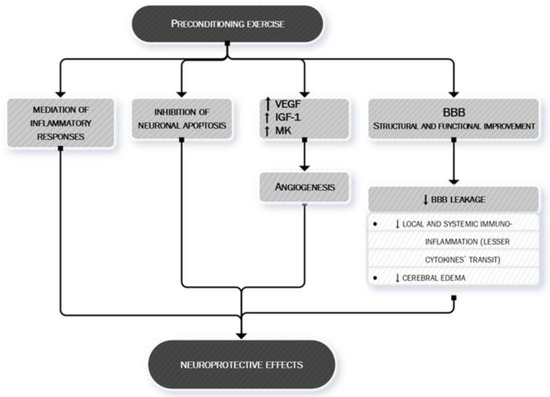
Figure 5. Preconditioning exercise: brain's eustress. PA is a mild stressor for the organism. In particular, preconditioning exercise (see text for more information) can be defined as an "eustress" because it triggers cells to express multiple factors, thus helping the organism to acquire tolerance and self-defense against later possible damage, such as ischemic stroke. Preconditioning exercise triggers the brain to activate vascular and inflammatory mechanisms (seen in the figure) [83][90][91][92][93][94][95] whose production remodulates cerebral neurovascular structure so that later pathologic stimuli are less likely to act or less likely to exert important damage. Preconditioning exercise improves the function and structure of blood–brain barrier (BBB) [83] thus limiting the leakage of both fluids (edema) and proteins (cytokines) who play an important role in ischemic stroke pathobiology. VEGF, vascular endothelial growth factor; IGF-1, Insulin-like Growth factor 1; MK, midkine; BBB, blood–brain barrier.
5. Is There Any Role for Myokines and Neurotrophins?
A myokine is a peptide or a protein secreted by skeletal muscle cells, either locally (in skeletal muscle interstitium without entering systemic circulation) or in the bloodstream during but also independently of muscle contraction. Since during or after PA various peptides may also be secreted by other organs/tissues, increased circulating levels of a peptide upon muscle activity is not a condition sufficient to establish whether the molecule behaves as a myokine [96]. The skeletal muscle secretome might mediate the effects of exercise on metabolic and cardiovascular health and seems to be involved in the protection against several types of cancer [5]. The overall concept of crosstalk between contracting muscle and distant organs appears to be also likely to be suitable for the brain. The hypothesis is, therefore, that the muscle secretome might be involved in mediating the beneficial effects of exercise on brain health. The proteins with a mainly targeted mechanism of action towards the neural network released in response to exercise are named "neurotrophins". This is a family of molecules with various neurotrophic activities (stimulation of neuronal survival, growth and/or differentiation) [97], whose levels increase after exercise in several experimental models. In consideration of this general premise, and before addressing in detail the description of what is known today about the role played by muscle myokines and more generically by neurotrophins on the facilitation of neuronal recovery after ischemic damage, we want to establish the three fundamental questions that we will try to answer individually during the discussion of each substance in order to define as clearly as possible the role played by them:
-
(1) If it is true that both in animal models and in human experiments it has been demonstrated that after PA there is an increase in the concentration of different neurotrophins within different structures of the nervous system, is it possible to definitely identify the cells that produce and release them?
-
(2) Are the cells that produce and release neurotrophins located within the nervous system or is there evidence that the muscle itself during contraction or after contraction produces and releases neurotrophins into circulation that would then reach their site of action through the bloodstream?
-
(3) The increased concentration of neurotrophins in the nervous system after exercise can be actually related to their protective role for a good neurological function, thus demonstrating that regular PA through myokines and/or neurotrophins acts in protecting the central nervous system?
Figure 6 shows some of the myokines most closely monitored to date and their potential mechanisms of action in the context of neuroprotection.
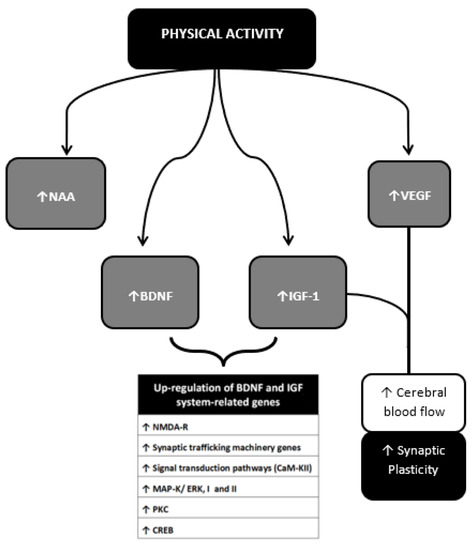
Figure 6. Neurotrophins, Myokines and other muscle-derived factors involved in neuroprotection. Neuroprotective effects of PA result from multiple molecular changes operating directly in the cerebral tissue and indirectly via vascular modulation. These molecular targets interplay with each other achieving different outcomes. The BDNF-IGF1 network and the VEGF-IGF1 network represent two main examples. BDNF-IGF1 network mediates the upregulation of genes mainly related to neuronal viability [46][47]. See text for further information. NAA, N-acetylaspartate; BDNF brain-Derived Neurotrophic Factor; IGF-1, Insulin-Like Growth Factor 1; VEGF, Vascular Endothelial Growth Factor; GABA, Gamma-AminoButyric Acid; NO, Nitric Oxyde; NMDA-R, N-methyl-d-aspartate receptor; CaM-KII, calcium/calmodulin protein kinase II; MAP-K/ERK I and II, mitogen-activated protein kinase; PK-C, protein kinase C; CREB, cAMP response element-binding protein.
References
- American College of Sports Medicine. ACM’s Guidelines for Exercise Testing and Prescription, 10th ed.; Riebe, D., Ehrman, J.K., Liguori, G., Magal, M., Eds.; Wolters Kluwer: Philadelphia, PA, USA, 2018; ISBN 9781496339065.
- Chodzko-Zajko, W.J.; Proctor, D.N.; Fiatarone Singh, M.A.; Minson, C.T.; Nigg, C.R.; Salem, G.J.; Skinner, J.S. Exercise and physical activity for older adults. Med. Sci. Sports Exerc. 2009, 41, 1510–1530, doi:10.1249/MSS.0b013e3181a0c95c.
- Wahid, A.; Manek, N.; Nichols, M.; Kelly, P.; Foster, C.; Webster, P.; Kaur, A.; Friedemann Smith, C.; Wilkins, E.; Rayner, M.; et al. Quantifying the Association Between Physical Activity and Cardiovascular Disease and Diabetes: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2016, 5, e002495, doi:10.1161/JAHA.115.002495.
- Sanchis-Gomar, F.; Fiuza-Luces, C.; Lucia, A. Exercise as the master polypill of the 21st century for the prevention of cardiovascular disease. Int. J. Cardiol. 2015, 181, 360–361, doi:10.1016/j.ijcard.2014.12.048.
- Di Raimondo, D.; Musiari, G.; Miceli, G.; Arnao, V.; Pinto, A. Preventive and Therapeutic Role of Muscle Contraction against Chronic Diseases. Curr. Pharm. Des. 2016, 22, 4686–4699, doi:10.2174/1381612822666160510125011.
- Li, J.; Siegrist, J. Physical activity and risk of cardiovascular disease--a meta-analysis of prospective cohort studies. Int. J. Environ. Res. Public Health 2012, 9, 391–407, doi:10.3390/ijerph9020391.
- Di Raimondo, D.; Miceli, G.; Musiari, G.; Tuttolomondo, A.; Pinto, A. New insights about the putative role of myokines in the context of cardiac rehabilitation and secondary cardiovascular prevention. Ann. Transl. Med. 2017, 5, 300, doi:10.21037/atm.2017.07.30.
- Patel, H.; Alkhawam, H.; Madanieh, R.; Shah, N.; Kosmas, C.E.; Vittorio, T.J. Aerobic vs anaerobic exercise training effects on the cardiovascular system. World J. Cardiol. 2017, 9, 134–138, doi:10.4330/wjc.v9.i2.134.
- Fulghum, K.; Hill, B.G. Metabolic Mechanisms of Exercise-Induced Cardiac Remodeling. Front. Cardiovasc. Med. 2018, 5, 127, doi:10.3389/fcvm.2018.00127.
- Leuchtmann, A.B.; Mueller, S.M.; Aguayo, D.; Petersen, J.A.; Ligon-Auer, M.; Flück, M.; Jung, H.H.; Toigo, M. Resistance training preserves high-intensity interval training induced improvements in skeletal muscle capillarization of healthy old men: A randomized controlled trial. Sci. Rep. 2020, 10, 6578, doi:10.1038/s41598-020-63490-x.
- Rivera-Brown, A.M.; Frontera, W.R. Principles of exercise physiology: Responses to acute exercise and long-term adaptation to training. PM R 2012, 4, 797–804, doi:10.1016/j.pmrj.2012.10.007.
- Meka, N.; Katragadda, S.; Cherian, B.; Arora, R.R. Endurance exercise and resistance training in cardiovascular disease. Ther. Adv. Cardiovasc. Dis. 2008, 2, 115–121, doi:10.1177/1753944708089701.
- Akseki Temür, H.; Vardar, S.A.; Demir, M.; Palabıyık, O.; Karaca, A.; Guksu, Z.; Ortanca, A.; Süt, N. The alteration of NTproCNP plasma levels following anaerobic exercise in physically active young men. Anatol. J. Cardiol. 2015, 15, 97–102, doi:10.5152/akd.2014.5204.
- Pate, R.R.; Pratt, M.; Blair, S.N.; Haskell, W.L.; Macera, C.A.; Bouchard, C.; Buchner, D.; Ettinger, W.; Heath, G.W.; King, A.C. Physical activity and public health: A recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. JAMA 1995, 273, 402–407, doi:10.1001/jama.273.5.402.
- Physical Activity and Cardiovascular Health: NIH Consensus Development Panel on Physical Activity and Cardiovascular Health. JAMA 1996, 276, 241–246, doi:10.1001/jama.1996.03540030075036.
- U.S. Department of Health and Human Services. Physical Activity and Health: A Report of the Surgeon General; Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion: Atlanta, GA, USA, 1996.
- World Health Organization. Global Recommendations on Physical Activity for Health; World Health Organization: Geneva, Switzerland, 2010.
- Pelliccia, A.; Sharma, S.; Gati, S.; Bäck, M.; Börjesson, M.; Caselli, S.; Collet, J.P.; Corrado, D.; Drezner, J.A.; Halle, M.; et al. 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur. Heart J. 2020, ehaa605, doi:10.1093/eurheartj/ehaa605.
- Wasfy, M.M.; Baggish, A.L. Exercise Dose in Clinical Practice. Circulation 2016, 133, 2297–2313, doi:10.1161/CIRCULATIONAHA.116.018093.
- GBD 2015 Neurological Disorders Collaborator Group. Global, regional, and national burden of neurological disorders during 1990-2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol. 2017, 16, 877–897, doi:10.1016/S1474-4422(17)30299-5.
- Arnao, V.; Di Raimondo, D.; Tuttolomondo, A.; Pinto, A. Neurotrophic and Neuroprotective Effects of Muscle Contraction. Curr. Pharm. Des. 2016, 22, 3749–3763, doi:10.2174/1381612822666160428111234.
- Batouli, S.A.H.; Saba, V. At least eighty percent of brain grey matter is modifiable by physical activity: A review study. Behav. Brain Res. 2017, 332, 204–217, doi:10.1016/j.bbr.2017.06.002.
- Hötting, K.; Röder, B. Beneficial effects of physical exercise on neuroplasticity and cognition. Neurosci. Biobehav. Rev. 2013, 37, 2243–2257, doi:10.1016/j.neubiorev.2013.04.005.
- Schmidt, W.; Endres, M.; Dimeo, F.; Jungehulsing, G.J. Train the vessel, gain the brain: Physical activity and vessel function and the impact on stroke prevention and outcome in cerebrovascular disease. Cerebrovasc. Dis. 2013, 35, 303–312, doi:10.1159/000347061.
- Gao, Y.; Zhao, Y.; Pan, J.; Yang, L.; Huang, T.; Feng, X.; Li, C.; Liang, S.; Zhou, D.; Liu, C.; et al. Treadmill exercise promotes angiogenesis in the ischemic penumbra of rat brains through caveolin-1/VEGF signaling pathways. Brain Res. 2014, 1585, 83–90, doi:10.1016/j.brainres.2014.08.032.
- Tang, Y.; Zhang, Y.; Zheng, M.; Chen, J.; Chen, H.; Liu, N. Effects of treadmill exercise on cerebral angiogenesis and MT1-MMP expression after cerebral ischemia in rats. Brain Behav. 2018, 8, e01079, doi:10.1002/brb3.1079.
- Pianta, S.; Lee, J.Y.; Tuazon, J.P.; Castelli, V.; Mantohac, L.M.; Tajiri, N.; Borlongan, C.V. A Short Bout of Exercise Prior to Stroke Improves Functional Outcomes by Enhancing Angiogenesis. Neuromol. Med. 2019, 21, 517–528, doi:10.1007/s12017-019-08533-x.
- Serra, F.T.; Carvalho, A.D.; Araujo, B.H.S.; Torres, L.B.; Cardoso, F.D.S.; Henrique, J.S.; Placencia, E.V.D.; Lent, R.; Gomez-Pinilla, F.; Arida, R.M.; et al. Early exercise induces long-lasting morphological changes in cortical and hippocampal neurons throughout of a sedentary period of rats. Sci. Rep. 2019, 9, 13684, doi:10.1038/s41598-019-50218-9.
- Kramer, A.F.; Hahn, S.; Cohen, N.J.; Banich, M.T.; McAuley, E.; Harrison, C.R.; Chason, J.; Vakil, E.; Bardell, L.; Boileau, R.A.; Colcombe, A. Ageing, fitness and neurocognitive function. Nature 1999, 400, 418–419, doi:10.1038/22682.
- Tarumi, T.; Zhang, R. Cerebral blood flow in normal aging adults: Cardiovascular determinants, clinical implications, and aerobic fitness. J. Neurochem. 2017, 144, 595–608, doi:10.1111/jnc.14234.
- Northey, J.M.; Cherbuin, N.; Pumpa, K.L.; Smee, D.J.; Rattray, B. Exercise interventions for cognitive function in adults older than 50: A systematic review with meta-analysis. Br. J. Sports Med. 2017, 52, 154–160, doi:10.1136/bjsports-2016-096587.
- Kleinloog, J.P.D.; Mensink, R.P.; Ivanov, D.; Adam, J.J.; Uludağ, K.; Joris, P.J. Aerobic Exercise Training Improves Cerebral Blood Flow and Executive Function: A Randomized, Controlled Cross-Over Trial in Sedentary Older Men. Front. Aging Neurosci. 2019, 11, 333, doi:10.3389/fnagi.2019.00333.
- Sparling, P.B.; Giuffrida, A.; Piomelli, D.; Rosskopf, L.; Dietrich, A. Exercise activates the endocannabinoid system. Neuroreport 2003, 14, 2209–2211, doi:10.1097/00001756-200312020-00015.
- Dietrich, A.; McDaniel, W.F. Endocannabinoids and exercise. Br. J. Sports Med. 2004, 38, 536–541, doi:10.1136/bjsm.2004.011718.
- Boekhoudt, L.; Omrani, A.; Luijendijk, M.C.; Wolterink-Donselaar, I.G.; Wijbrans, E.C.; van der Plasse, G.; Adan, R.A. Chemogenetic activation of dopamine neurons in the ventral tegmental area, but not substantia nigra, induces hyperactivity in rats. Eur. Neuropsychopharmacol. 2016, 26, 1784–1793, doi:10.1016/j.euroneuro.2016.09.003.
- Boecker, H.; Sprenger, T.; Spilker, M.E.; Henriksen, G.; Koppenhoefer, M.; Wagner, K.J.; Valet, M.; Berthele, A.; Tolle, T.R. The runner’s high: Opioidergic mechanisms in the human brain. Cereb. Cortex 2008, 18, 2523–2531, doi:10.1093/cercor/bhn013.
- Agudelo, L.Z.; Femenía, T.; Orhan, F.; Porsmyr-Palmertz, M.; Goiny, M.; Martinez-Redondo, V.; Correia, J.C.; Izadi, M.; Bhat, M.; Schuppe-Koistinen, I.; et al. Skeletal muscle PGC-1α1 modulates kynurenine metabolism and mediates resilience to stress-induced depression. Cell 2014, 159, 33–45, doi:10.1016/j.cell.2014.07.051.
- Safdar, A.; DeBeer, J.; Tarnopolsky, M.A. Dysfunctional Nrf2-Keap1 redox signaling in skeletal muscle of the sedentary old. Free Radic. Biol. Med. 2010, 49, 1487–1493, doi:10.1016/j.freeradbiomed.2010.08.010.
- Dékány, M.; Nemeskéri, V.; Györe, I.; Harbula, I.; Malomsoki, J.; Pucsok, J. Antioxidant status of interval-trained athletes in various sports. Int. J. Sports Med. 2006, 27, 112–116, doi:10.1055/s-2005-865634.
- Flynn, M.G.; McFarlin, B.K.; Markofski, M.M. The Anti-Inflammatory Actions of Exercise Training. Am. J. Lifestyle Med. 2007, 1, 220–235, doi:10.1177/1559827607300283.
- Zaldivar, F.; Wang-Rodriguez, J.; Nemet, D.; Schwindt, C.; Galassetti, P.; Mills, P.J.; Wilson, L.D.; Cooper, D.M. Constitutive pro- and anti-inflammatory cytokine and growth factor response to exercise in leukocytes. J. Appl. Physiol. 2006, 100, 1124–1133, doi:10.1152/japplphysiol.00562.2005.
- Di Raimondo, D.; Tuttolomondo, A.; Musiari, G.; Schimmenti, C.; D’Angelo, A.; Pinto, A. Are the Myokines the Mediators of Physical Activity-Induced Health Benefits? Curr. Pharm. Des. 2016, 22, 3622–3647, doi:10.2174/1381612822666160429121934.
- Koh, Y.; Park, J. Cell adhesion molecules and exercise. J. Inflamm. Res. 2018, 24, 11, 297-306, doi:10.2147/JIR.S170262.
- Gligoroska, J.P.; Manchevska, S. The effect of physical activity on cognition—Physiological mechanisms. Mater. Sociomed. 2012, 24, 198–202, doi:10.5455/msm.2012.24.198-202.
- Melo, R.T.R.; Damázio, L.C.M.; Lima, M.C.; Pereira, V.G.; Okano, B.S.; Monteiro, B.S.; Natali, A.J.; Carlo, R.J.D.; Maldonado, I.R.S.C. Effects of physical exercise on skeletal muscles of rats with cerebral ischemia. Braz. J. Med. Biol. Res. 2019, 52, e8576, doi:10.1590/1414-431X20198576.
- Vaynman, S.; Ying, Z.; Gomez-Pinilla, F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur. J. Neurosci. 2004, 20, 2580–2590, doi:10.1111/j.1460-9568.2004.03720.x.
- Farmer, J.; Zhao, X.; van Praag, H.; Wodtke, K.; Gage, F.H.; Christie, B.R. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neuroscience 2004, 124, 71–79, doi:10.1016/j.neuroscience.2003.09.029.
- Avan, A.; Digaleh, H.; Di Napoli, M.; Stranges, S.; Behrouz, R.; Shojaeianbabaei, G.; Amiri, A.; Tabrizi, R.; Mokhber, N.; Spence, J.D.; et al. Socioeconomic status and stroke incidence, prevalence, mortality, and worldwide burden: An ecological analysis from the Global Burden of Disease Study 2017. BMC Med. 2019, 17, 191, doi:10.1186/s12916-019-1397-3.
- Han, P.; Zhang, W.; Kang, L.; Ma, Y.; Fu, L.; Jia, L.; Yu, H.; Chen, X.; Hou, L.; Wang, L.; et al. Clinical Evidence of Exercise Benefits for Stroke. Adv. Exp. Med. Biol. 2017, 1000, 131–151, doi:10.1007/978-981-10-4304-8_9.
- De Meyer, S.F.; Denorme, F.; Langhauser, F.; Geuss, E.; Fluri, F.; Kleinschnitz, C. Thromboinflammation in Stroke Brain Damage. Stroke 2016, 47, 1165–1172, doi:10.1161/STROKEAHA.115.011238.
- Tuttolomondo, A.; Di Raimondo, D.; Pecoraro, R.; Arnao, V.; Pinto, A.; Licata, G. Inflammation in ischemic stroke subtypes. Curr. Pharm. Des. 2012, 18, 4289–4310, doi:10.2174/138161212802481200.
- Kirchhof, P.; Benussi, S.; Kotecha, D.; Ahlsson, A.; Atar, D.; Casadei, B.; Castella, M.; Diener, H.C.; Heidbuchel, H.; Hendriks, J.; et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur. Heart J. 2016, 37, 2893–2962, doi:10.1093/eurheartj/ehw210.
- Maida, C.D.; Vasto, S.; Di Raimondo, D.; Casuccio, A.; Vassallo, V.; Daidone, M.; Del Cuore, A.; Pacinella, G.; Cirrincione, A.; Simonetta, I.; et al. Inflammatory activation and endothelial dysfunction markers in patients with permanent atrial fibrillation: A cross-sectional study. Aging 2020, 12, 8423–8433, doi:10.18632/aging.103149.
- Tuttolomondo, A.; Di Raimondo, D.; Di Sciacca, R.; Pinto, A.; Licata, G. Inflammatory cytokines in acute ischemic stroke. Curr. Pharm. Des. 2008, 14, 3574–3589, doi:10.2174/138161208786848739.
- Shekhar, S.; Cunningham, M.W.; Pabbidi, M.R.; Wang, S.; Booz, G.W.; Fan, F. Targeting vascular inflammation in ischemic stroke: Recent developments on novel immunomodulatory approaches. Eur. J. Pharmacol. 2018, 833, 531–544, doi:10.1016/j.ejphar.2018.06.028.
- Wang, Q.; Tang, X.N.; Yenari, M.A. The inflammatory response in stroke. J. Neuroimmunol. 2007, 184, 53–468, doi:10.1016/j.jneuroim.2006.11.014.
- Tuttolomondo, A.; Di Sciacca, R.; Di Raimondo, D.; Renda, C.; Pinto, A.; Licata, G. Inflammation as a therapeutic target in acute ischemic stroke treatment. Curr. Top. Med. Chem. 2009, 9, 1240–1260, doi:10.2174/156802609789869619.
- Tarkowski, E.; Rosengren, L.; Blomstrand, C.; Wikkelsö, C.; Jensen, C.; Ekholm, S.; Tarkowski, A. Early intrathecal production of interleukin-6 predicts the size of brain lesion in stroke. Stroke 1995, 26, 1393–1398, doi:10.1161/01.str.26.8.1393.
- Jayaraj, R.L.; Azimullah, S.; Beiram, R.; Jalal, F.Y.; Rosenberg, G.A. Neuroinflammation: Friend and foe for ischemic stroke. J. Neuroinflamm. 2019, 16, 142, doi:10.1186/s12974-019-1516-2.
- Pinto, A.; Tuttolomondo, A.; Di Raimondo, D.; Fernandez, P.; Licata, G. Cerebrovascular risk factors and clinical classification of strokes. Semin. Vasc. Med. 2004, 4, 287–303, doi:10.1055/s-2004-861497.
- Tuttolomondo, A.; Casuccio, A.; Della Corte, V.; Maida, C.; Pecoraro, R.; Di Raimondo, D.; Vassallo, V.; Simonetta, I.; Arnao, V.; Pinto, A. Endothelial function and arterial stiffness indexes in subjects with acute ischemic stroke: Relationship with TOAST subtype. Atherosclerosis 2017, 256, 94–99, doi:10.1016/j.atherosclerosis.2016.10.044.
- Della Corte, V.; Tuttolomondo, A.; Pecoraro, R.; Di Raimondo, D.; Vassallo, V.; Pinto, A. Inflammation, Endothelial Dysfunction and Arterial Stiffness as Therapeutic Targets in Cardiovascular Medicine. Curr. Pharm. Des. 2016, 22, 4658–4668, doi:10.2174/1381612822666160510124801.
- Tuttolomondo, A.; Pecoraro, R.; Casuccio, A.; Di Raimondo, D.; Buttà, C.; Clemente, G.; Della Corte, V.; Guggino, G.; Arnao, V.; Maida, C.; et al. Peripheral frequency of CD4+ CD28-cells in acute ischemic stroke: Relationship with stroke subtype and severity markers. Medicine 2015, 94, e813, doi:10.1097/MD.0000000000000813.
- Tuttolomondo, A.; Pecoraro, R.; Di Raimondo, D.; Di Sciacca, R.; Canino, B.; Arnao, V.; Buttà, C.; Della Corte, V.; Maida, C.; Licata, G.; et al. Immune-inflammatory markers and arterial stiffness indexes in subjects with acute ischemic stroke with and without metabolic syndrome. Diabetol. Metab. Syndr. 2014, 6, 28, doi:10.1186/1758-5996-6-28.
- Tuttolomondo, A.; Pecoraro, R.; Di Raimondo, D.; Arnao, V.; Clemente, G.; Della Corte, V.; Maida, C.; Simonetta, I.; Licata, G.; Pinto, A. Stroke subtypes and their possible implication in stroke prevention drug strategies. Curr. Vasc. Pharmacol. 2013, 11, 824–837, doi:10.2174/157016111106140128113705.
- Tuttolomondo, A.; Pedone, C.; Pinto, A.; Di Raimondo, D.; Fernandez, P.; Di Sciacca, R.; Licata, G. Predictors of outcome in acute ischemic cerebrovascular syndromes: The GIFA study. Int. J. Cardiol. 2008, 125, 391–396, doi:10.1016/j.ijcard.2007.03.109.
- Licata, G.; Tuttolomondo, A.; Di Raimondo, D.; Corrao, S.; Di Sciacca, R.; Pinto, A. Immuno-inflammatory activation in acute cardio-embolic strokes in comparison with other subtypes of ischaemic stroke. Thromb. Haemost. 2009, 101, 929–937.
- Aukrust, P.; Halvorsen, B.; Yndestad, A.; Ueland, T.; Øie, E.; Otterdal, K.; Gullestad, L.; Damås, J.K. Chemokines and cardiovascular risk. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1909–1919, doi:10.1161/ATVBAHA.107.161240.
- Nakase, T.; Yamazaki, T.; Ogura, N.; Suzuki, A.; Nagata, K. The impact of inflammation on the pathogenesis and prognosis of ischemic stroke. J. Neurol. Sci. 2008, 15, 271, 104-109, doi:10.1016/j.jns.2008.03.020.
- Smith, C.J.; Emsley, H.C.; Gavin, C.M.; Georgiou, R.F.; Vail, A.; Barberan, E.M.; del Zoppo, G.J.; Hallenbeck, J.M.; Rothwell, N.J.; Hopkins, S.J.; et al. Peak plasma interleukin-6 and other peripheral markers of inflammation in the first week of ischaemic stroke correlate with brain infarct volume, stroke severity and long-term outcome. BMC Neurol. 2004, 4, 2, doi:10.1186/1471-2377-4-2.
- Flex, A.; Gaetani, E.; Papaleo, P.; Straface, G.; Proia, A.S.; Pecorini, G.; Tondi, P.; Pola, P.; Pola, R. Proinflammatory genetic profiles in subjects with history of ischemic stroke. Stroke 2004, 35, 2270–2275, doi:10.1161/01.STR.0000140740.19421.fe.
- Kumar, P.; Yadav, A.K.; Misra, S.; Kumar, A.; Chakravarty, K.; Prasad, K. Role of Interleukin-10 (-1082A/G) gene polymorphism with the risk of ischemic stroke: A meta-analysis. Neurol. Res. 2016, 38, 823–830, doi:10.1080/01616412.2016.1202395.
- Tuttolomondo, A.; Di Raimondo, D.; Pecoraro, R.; Serio, A.; D’Aguanno, G.; Pinto, A.; Licata, G. Immune-inflammatory markers and arterial stiffness indexes in subjects with acute ischemic stroke. Atherosclerosis 2010, 213, 311–318, doi:10.1016/j.atherosclerosis.2010.08.065.
- Adams, H.P., Jr.; Bendixen, B.H.; Kappelle, L.J.; Biller, J.; Love, B.B.; Gordon, D.L.; Marsh, E.E., 3rd. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993, 24, 35–41, doi:10.1161/01.str.24.1.35.
- Zhang, H.; Lee, J.Y.; Borlongan, C.V.; Tajiri, N. A brief physical activity protects against ischemic stroke. Brain Circ. 2019, 5, 112–118, doi:10.4103/bc.bc_32_19.
- Xing, Y.; Bai, Y. A Review of Exercise-Induced Neuroplasticity in Ischemic Stroke: Pathology and Mechanisms. Mol. Neurobiol. 2020, 57, 4218–4231, doi:10.1007/s12035-020-02021-1.
- Chapman, S.B.; Aslan, S.; Spence, J.S.; Defina, L.F.; Keebler, M.W.; Didehbani, N.; Lu, H. Shorter term aerobic exercise improves brain, cognition, and cardiovascular fitness in aging. Front. Aging Neurosci. 2013, 5, 75, doi:10.3389/fnagi.2013.00075.
- Smith, K.J.; Ainslie, P.N. Regulation of cerebral blood flow and metabolism during exercise. Exp. Physiol. 2017, 102, 1356–1371, doi:10.1113/EP086249.
- Alfini, A.J.; Weiss, L.R.; Nielson, K.A.; Verber, M.D.; Smith, J.C. Resting Cerebral Blood Flow After Exercise Training in Mild Cognitive Impairment. J. Alzheimers Dis. 2019, 67, 671–684, doi:10.3233/JAD-180728.
- Guadagni, V.; Drogos, L.L.; Tyndall, A.V.; Davenport, M.H.; Anderson, T.J.; Eskes, G.A.; Longman, R.S.; Hill, M.D.; Hogan, D.B.; Poulin, M.J. Aerobic exercise improves cognition and cerebrovascular regulation in older adults. Neurology 2020, 94, e2245–e2257, doi:10.1212/WNL.0000000000009478.
- Bullitt, E.; Rahman, F.N.; Smith, J.K.; Kim, E.; Zeng, D.; Katz, L.M.; Marks, B.L. The effect of exercise on the cerebral vasculature of healthy aged subjects as visualized by MR angiography. Am. J. Neuroradiol. 2009, 30, 1857–1863, doi:10.3174/ajnr.A1695.
- Steventon, J.J.; Foster, C.; Furby, H.; Helme, D.; Wise, R.G.; Murphy, K. Hippocampal Blood Flow Is Increased After 20 min of Moderate-Intensity Exercise. Cereb. Cortex 2020, 30, 525–533, doi:10.1093/cercor/bhz104.
- Sakakima, H. Endogenous neuroprotective potential due to preconditioning exercise in stroke. Phys. Ther. Res. 2019, 22, 45–52, doi:10.1298/ptr.R0006.
- Baillieul, S.; Chacaroun, S.; Doutreleau, S.; Detante, O.; Pépin, J.L.; Verges, S. Hypoxic conditioning and the central nervous system: A new therapeutic opportunity for brain and spinal cord injuries? Exp. Biol. Med. 2017, 242, 1198–1206, doi:10.1177/1535370217712691.
- Dong, Y.; Zhao, R.; Chen, X.Q.; Yu, A.C. 14-3-3gamma and neuroglobin are new intrinsic protective factors for cerebral ischemia. Mol. Neurobiol. 2010, 41, 218–231, doi:10.1007/s12035-010-8142-4.
- Rezaei, R.; Nasoohi, S.; Haghparast, A.; Khodagholi, F.; Bigdeli, M.R.; Nourshahi, M. High intensity exercise preconditioning provides differential protection against brain injury following experimental stroke. Life Sci. 2018, 207, 30–35, doi:10.1016/j.lfs.2018.03.007.
- Islam, M.R.; Young, M.F.; Wrann, C.D. Neuroprotective potential of exercise preconditioning in stroke. Cond. Med. 2017, 1, 27–34.
- Deplanque, D.; Masse, I.; Lefebvre, C.; Libersa, C.; Leys, D.; Bordet, R. Prior TIA, lipid-lowering drug use, and physical activity decrease ischemic stroke severity. Neurology 2006, 67, 1403–1410, doi:10.1212/01.wnl.0000240057.71766.71.
- Deplanque, D.; Masse, I.; Libersa, C.; Leys, D.; Bordet, R. Previous leisure-time physical activity dose dependently decreases ischemic stroke severity. Stroke Res. Treat. 2012, 2012, 614925, doi:10.1155/2012/614925.
- Otsuka, S.; Sakakima, H.; Sumizono, M.; Takada, S.; Terashi, T.; Yoshida, Y. The neuroprotective effects of preconditioning exercise on brain damage and neurotrophic factors after focal brain ischemia in rats. Behav. Brain Res. 2016, 303, 9–18, doi:10.1016/j.bbr.2016.01.049.
- Zhang, Q.; Zhang, L.; Yang, X.; Wan, Y.; Jia, J. The effects of exercise preconditioning on cerebral blood flow change and endothelin-1 expression after cerebral ischemia in rats. J. Stroke Cerebrovasc. Dis. 2014, 23, 1696–1702, doi:10.1016/j.jstrokecerebrovasdis.2014.01.016.
- Carro, E.; Nuñez, A.; Busiguina, S.; Torres-Aleman, I. Circulating insulin-like growth factor I mediates effects of exercise on the brain. J. Neurosci. 2000, 20, 2926–2933, doi:10.1523/JNEUROSCI.20-08-02926.2000.
- Taylor, J.M.; Montgomery, M.H.; Gregory, E.J.; Berman, N.E. Exercise preconditioning improves traumatic brain injury outcomes. Brain Res. 2015, 1622, 414–429, doi:10.1016/j.brainres.2015.07.009.
- Cotman, C.W.; Berchtold, N.C.; Christie, L.A. Exercise builds brain health: Key roles of growth factor cascades and inflammation. Trends Neurosci. 2007, 30, 464–472, doi:10.1016/j.tins.2007.06.011.
- Muramatsu, T. Midkine and pleiotrophin: Two related proteins involved in development, survival, inflammation and tumorigenesis. J. Biochem. 2002, 132, 359–371, doi:10.1093/oxfordjournals.jbchem.a003231.
- Aguer, C.; Loro, E.; Di Raimondo, D. Editorial: The Role of the Muscle Secretome in Health and Disease. Front. Physiol. 2020, 11, 1101, doi:10.3389/fphys.2020.01101.
- Pasarica, D.; Gheorghiu, M.; Topârceanu, F.; Bleotu, C.; Ichim, L.; Trandafir, T. Neurotrophin-3, TNF-alpha and IL-6 relations in serum and cerebrospinal fluid of ischemic stroke patients. Roum. Arch. Microbiol. Immunol. 2005, 64, 27–33.




