1000/1000
Hot
Most Recent

Osteoarthritis (OA) is a common chronic joint disease that is characterized by joint pain and stiffness, and limitation of motion and the major cause of disability, which reduces life quality of patients and brings a large economic burden to the family and society. Current clinical treatment is mostly limited on symptomatic treatment aimed at pain alleviation and functional improvement, rather than suppressing the progression of OA. Nanotechnology is a promising strategy for the treatment of OA. In this review, we summarize the current experimental progress that will focus on technologies such as liposomes, micelles, dendrimers, polymeric nanoparticles (PNPs), exosomes, and inorganic nanoparticles (NPs) for their potential treatment of OA.
Osteoarthritis (OA) is a common chronic joint disease that is characterized by joint pain and stiffness, and limitation of motion and the major cause of disability for adults [1]. The incidence of OA is related to age, gender, obesity, and joint injury etc., [2]. With its high prevalence, OA causes a significant financial burden both on individuals and the society [1]. OA pathophysiology is characterized by progressive and degenerative loss of articular cartilage, osteophyte formation, synovial inflammation, subchondral bone remodeling, and sclerosis. This disease is regarded as a complex disease that involves multiple tissues and processes. The causes are not yet fully understood [3]. In modern concepts, a variety of factors are associated with OA, including genetic susceptibility, biochemics, and biomechanics of the affected joint, and extent of inflammation [4]. Therefore, it has been difficult to identify specific targets for therapy. Current clinical practice is mostly limited on symptomatic treatment aimed at pain alleviation, functional improvement, and even artificial joint replacement, rather than targeting the underlying molecular causes of OA [5].
Articular cartilage is an elastic connective tissue covering the ends of the bones and helps joints to move smoothly. It lacks a vasculature supply and innervation, composed of 1–2% chondroocytes and specialized matrix involving type II collagen, glycosaminoglycans (GAGs, e.g., chondroitin sulfate, hyaluronic acid (HA), and aggrecan), elastin fibrils, and 70% water. The chondrocytes coordinate the synthesis, maintenance, and degradation of extracellular matrix (ECM) through the turnover of matrix proteins [6][7]. A joint cavity is filled with synovial fluid, which is surrounded by articular cartilage and a synovial membrane. The synovial membrane is composed of fibroblasts and macrophages and secrete two important molecules, namely hyaluronan and lubricin. They contribute to the viscosity of synovial fluid and provide boundary lubrication of articular cartilage [8].
An improved understanding of the pathogenesis of OA is very important for providing new theoretical basis and potential targets of OA clinical practice. The three major biological factors including proteolytic enzymes, proinflammatory cytokines, and reactive oxygen species (ROS) cause and exacerbate cartilage degradation in OA. Cartilage homeostasis relies on a balance between chondrocyte anabolic and catabolic activities. It is postulated that there is an imbalance between chondrocyte anabolism by growth factors and catabolism by decomposing enzymes such as matrix metalloproteinases (MMPs, e.g., MMP-3 and MMP-13) and a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS) [9]. These enzymes are generated by chondrocytes. ADAMTS regulate proteoglycan (e.g., aggrecan) degradation and MMPs digest the collagen network (e.g., type II collagen) [10][11]. The decomposition products contribute to further inflammatory responses of the adjacent synovial tissue through Toll-like receptors and integrins and the release of proinflammatory products including cytokines and ROS and lead to a defective cycle between the production of inflammatory factors and the chondrocyte catabolic activity [12][13][14]. Inflammatory cytokines (e.g., IL-1β, IL-6, and TNF-α) secreted by chondrocytes and synovial cells in synovial fluid are critical mediators in OA development [15]. They activate canonical nuclear factor kappa B (NF-κB) signaling, the major pathway mediating the expression of proinflammatory cytokines, involving IL-1β, IL-6, TNF-α, inducible nitric oxide synthase (iNOS), cyclooxygenase 2 (COX-2), and proteolytic enzymes (e.g., MMPs, ADAMTS) [16][17]. Therefore, NF-κB signaling pathway has become one of the potential targets for OA treatment. Recent studies have shown that OA progression is closely related to oxidative stress, which refers to increased levels of intracellular ROS produced from mitochondria and endoplasmic reticulum, resulting in lipids, proteins, and DNA damage [18][19][20]. Cartilage mechanical injury may result in the enhanced release of mitochondrial ROS [21]. ROS can up-regulate proinflammatory cytokine expression in OA [22][23], and the cytokines also induce ROS production [24], thereby accelerating OA development (Figure 1).
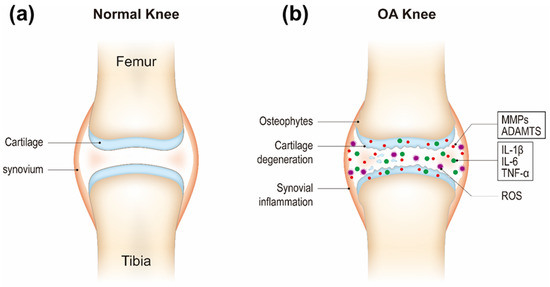
Figure 1. Schematic of healthy (a) and osteoarthritis (OA) knee joints (b).
At present, pharmacological treatments including non-steroidal anti-inflammatory drugs (NSAID), opioids, and glucocorticoids are still the primary approaches. However, systemic administration of drugs can cause severe side effects for long-term use, such as gastrointestinal complications and osteoporosis [25][26]. In addition, the absence of blood supply and the rapid clearance of drugs within synovial joints are main challenges for systemical and local drug delivery, respectively [27].
Nanotechnology is an interdisciplinary discipline including physics, chemistry, biology, electronics, and engineering. Research and development of nanotechnology are actively performed all over the world. Nanotechnology is a field of science for studying and manipulating particles at the atomic, molecular, or macromolecular levels, usually between 1 and 100 nm in size [28]. Due to the particularity of the scale structure, nanoparticles (NPs) have unique properties, including size effects, interfacial phenomena, and quantum effects, etc., thus exhibiting many excellent properties and new functions [28]. The behavior of NPs is more difficult to predict completely than that of microparticles. Therefore, the control and manipulation of nanostructures can eventually exploit novel chemical, physical, and biological characteristics of NPs. Nanotechnology has become very important because of its high ratio of surface area to volume, ideal scale for catalysis, and molecular structures at the nanoscale in the body [28]. Fabrication methods of NPs by nanotechnology usually include top-down approach and bottom-up approach. The top-down approach corresponds to using nanofabrication tools to create nanoscale particles through reducing macro-sized structures. On the other hand, the bottom-up approach utilizes physical and chemical processes to integrate molecular or atomic components into bigger nanoscale particles [29].
Nanotechnology has shown excellent application value in many aspects of our daily lives including sunscreens, cosmetics, textiles, and sports equipment. Nanotechnology is also used in biomedicine, some of which have entered clinical applications including Doxil® for treating ovarian cancer, Ferumoxytol® for treating of iron deficiency anemia, Abraxane® for treating metastatic breast cancer, etc., [30]. However, there is still no current clinical application of nanotechnology for the treatment of OA.
Nanotechnology plays a unique advantage for drug delivery of therapeutics for OA: (1) Improving drug targeting and efficient drug delivery; (2) enhancing drug solubility and stability; (3) preventing drug dispersion and degradation in body fluids and extending drug circulation and retention time in the body; (4) improving drug efficacy and reducing adverse drug reactions [31].
In recent years, the vigorous development of nanotechnology in drug delivery systems has provided new ideas and methods for OA therapy. In this review, we discuss the current developments and novel applications of OA-related NP-based drug delivery including liposomes, micelles, dendrimers, polymeric nanoparticles (PNPs), exosomes, and inorganic NPs. The application of NPs in the treatment of OA is summarized in Table 1 and Table 2. Various NPs used in the treatment of OA are shown in Figure 2. The delivery route and the schematic mechanism of NPs are displayed in Figure 3.
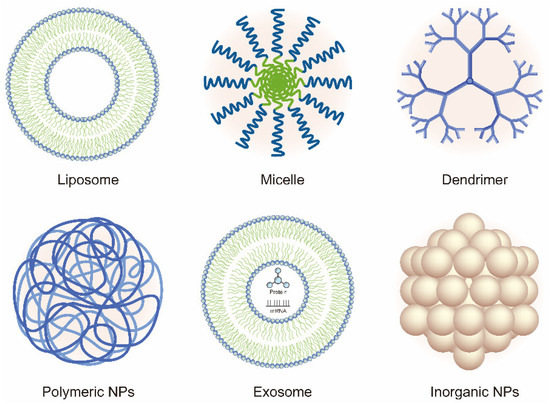
Figure 2. Schematic of the various nanoparticles (NPs) used in the treatment of OA.
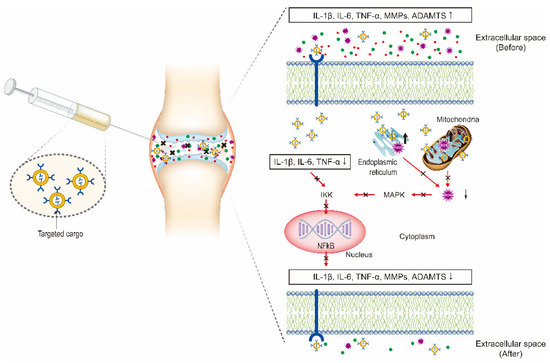
Figure 3. Intra-articular (IA) delivery of cargo-loaded nanoparticles for the treatment of OA. The therapeutic effects are obtained by the NPs targeting the three major factors including inflammatory factors, proteolytic enzymes, and reactive oxygen species (ROS) to inhibit NF-κB signaling pathway.
Table 1. Intra-articular drug delivery nanoparticles for the treatment of OA.
| Type of NPs | Formulation | OA Model/Route of Delivery | Outcome |
|---|---|---|---|
| Liposomes | Adenosine; CGS21680 |
Bbesity-induced (mice)/i.a. Post-traumatic (rats)/i.a. |
Favorable histology and prevent OA progression |
| Rapamycin | Spontaneous (guinea pigs)/i.a. | IL-6 ↓; MMP-13 ↓; collagen II ↑; OARSI score ↓; favorable histology | |
| FP/GNP/DPPC | Collagenase (rats)/i.a. | Anti-apoptosis ↑; pro-inflammatory cytokines ↓; GSH/ SOD/catalase ↑; NFκB ↓ | |
| Clodronate | Post-traumatic (mice)/i.a. | M1 macrophages ↓; collagen X ↓; favorable histology | |
| Micelles | MRC-PPL/Psoralidin | Papain (mice)/i.a. | MMP-13 ↓TNF-α ↓; NFκB ↓; favorable histology |
| PAE/Curcumin | MIA (mice)/i.a. | IL-1β ↓; TNF-α ↓; favorable histology | |
| HA-PEG/KGN | Post-traumatic (rats)/i.a. | OARSI score ↓; favorable histology and prevent OA progression | |
| Dendrimers | PAMAM/IGF-1 | Post-traumatic (rats)/i.a. | Synovial inflammation scores ↓; area of degenerated cartilage ↓ favorable histology and μCT |
| dPGS | Post-traumatic (rats)/s.c. | Mankin score ↓; Glasson score ↓; favorable histology | |
| PNPs | p66shc si-PLGA | MIA (rats)/i.a. | IL-1β ↓; TNF-α ↓; COX2 ↓ favorable histology and μCT |
| p47phox si-PLGA | MIA (rats)/i.a. | ROS ↓; favorable histology | |
| Etoricoxib/PLGA-PEG-PLGA | Post-traumatic (rats)/i.a. | COX2 ↓; iNOS ↓; MMP-13 ↓; ADAMTS-5 ↓; OARSI score ↓; favorable histology and μCT | |
| PLA-PEG-adenosine | Post-traumatic (rats)/i.a. | NFκB ↓; OARSI score ↓; favorable histology | |
| Polyurethane-KGN | Post-traumatic (rats)/i.a. | OARSI score ↓; favorable histology | |
| KGN-PLA | Post-traumatic (mice)/i.a. | OARSI score↓; favorable histology | |
| HABP-PEG-COLBP | Post-traumatic (mice)/i.a. | IL-1β ↓; IL-6 ↓; MMP-13 ↓; OARSI score↓; favorable histology | |
| BBR-CNPs | Post-traumatic (rats)/i.a. | Bcl-2 ↑; bax ↓; caspase-3 ↓; favorable histology | |
| Curcuminoid-HA-CNPs | Post-traumatic (rats)/i.a. | NFκB ↓; MMP-1 ↓; MMP-13 ↓; collagen II ↑; favorable histology | |
| CrmA-HA-CNP | Post-traumatic (rats)/i.a. | IL-1β ↓; MMP-3 ↓; MMP-13 ↓; OARSI score↓; favorable histology |
Abbreviations: BBR: Berberine chloride; BDMC: bisdemethoxycurcumin; CNPs: Chitosan nanoparticles; COLBP: Collagen binding peptide; dPGS: Dendritic polyglycerol sulfates; DPPC: dipalmitoyl phosphatidylcholine; FP: Fish oil protein; GNPs: Gold nanoparticles; GSH: Glutathione reductase; HA: Hyaluronic acid; HABP: Hyaluronic acid-binding peptide; HA/CS-CrmA: Hyaluronic acid-chitosan nanoparticles containing plasmid DNA encoding CrmA; i.a.: intra-articular; IGF-1: Insulin-like growth factor 1; KGN: Kartogenin; MIA: Monoidoacetic acid; MRC: MMP-13 responsive/Coll-II α1 chain-binding peptide–CollB; OARSI: Osteoarthritis Research Society International; PAE: Poly(β-amino ester); PAMAM: polyamidoamine; PEG: poly (ethylene glycol); PNPs: Polymeric nanoparticles; PPL: Poly (2-ethyl-2-oxazoline)-poly (ε-caprolactone); s.c.: subcutaneous; SOD: Superoxide dismutase.
Table 2. The therapeutic effects of exosomes derived from different sources for OA.
| Source | Cargo | OA Model/Route of Delivery | Outcome |
|---|---|---|---|
| Human chondrocytes | miR-140 | Post-traumatic (rats)/i.a. | OARSI score ↓; favorable histology |
| Rat BMSCs | miR-9-5p | Post-traumatic (rats)/i.a. | IL-1 ↓; IL-6 ↓; TNF-α ↓; MMP-13 ↓; COMP ↓; SDC1 ↓; favorable histology |
| Human IPFP MSCs | miR-100-5p | Post-traumatic (mice)/i.a. | Collagen II ↑; MMP-13 ↓ ADAMTS-5 ↓; mTOR ↓; favorable histology |
| Rat BMSCs | miR-135b | Post-traumatic (rats)/i.a. | OARSI score ↓; Sp1 ↓ |
| SM-MSCs | miR-140-5p | Post-traumatic (rats)/i.a. | RalA ↑; OARSI score ↓ favorable histology |
| Human BMSCs | lncRNA KLF3-AS1 | Collagenase II (rats)/i.a. | MMP-13 ↓; Mankin score ↓; favorable histology |
| Human BMSCs | lncRNA-KLF3-AS1 | Collagenase II (mice)/i.a. | MMP-13 ↓; GIT1 ↑ |
| Rat BMSCs | Not mentioned | MIA (rats)/i.a. | IL-1β↓; IL-6↓; TNF-α↓; MMP-13↓; pain↓; favorable histology |
| Mouse BMSCs | Not mentioned | Collagenase VII (mice)/i.a. | TNF-α ↓; MMP-13 ↓; favorable histology and μCT |
| human ESC-MSCs | Not mentioned | Post-traumatic (mice)/i.a. | ADAMTS-5 ↓; OARSI score ↓; favorable histology |
| SM-MSCs; iPSC-MSCs | Not mentioned | Collagenase (mice)/i.a. | ICRS ↓; OARSI score ↓ favorable histology |
Abbreviations: AFSC: Amniotic fluid stem cells; BMSCs: Bone marrow mesenchymal stem cells; ESC: Embryonic stem cell; GIT1: G-proteincoupled receptor kinase interacting protein-1; i.a.: intra-articular; ICRS: International Cartilage Research Society; IPFP: Infrapatellar fat pad; iPSCs: Induced pluripotent stem cells; MIA: Monoiodoacetate; mTOR: Mammalian target of rapamycin; SDC1: Syndecan-1; SM: Synovial membrane; Sp1: Specificity Protein 1; TGFβ: Transforming growth factor β.