
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Seok Ki Choi | + 1073 word(s) | 1073 | 2020-12-09 06:57:23 | | | |
| 2 | Vicky Zhou | Meta information modification | 1073 | 2021-01-15 03:13:52 | | | | |
| 3 | Vicky Zhou | Meta information modification | 1073 | 2021-01-19 04:03:21 | | |
Video Upload Options
A certain class of multifunctional nanomaterials serves as a delivery platform for controlled drug release under image guidance. They have shown significant therapeutic potential and broad applications whereas their design specifics remain a subject of continued interest primarily due to multifunctional factors involved, ranging from nanomaterial properties, imaging modalities, and therapeutic agents to activation strategies.
1. Introduction
Over recent decades, nanoscale therapeutic systems have shown a rapid growth and significant contribution in image-guided delivery [1]. These systems are designed utilizing an integrative approach that combines both imaging and delivery functions in a single nanoscale entity. Their design is composed of three complementary modules that include an imaging probe, a therapeutic component, and a nanomaterial platform. Their imaging function plays a fundamental role in detecting and tracking the nanotherapeutic system following its administration (Figure 1) [2]. This helps to identify whether it is selectively distributed at its targeted site from a pharmacokinetic perspective and thus to determine an optimal timing for its therapeutic activation. Its therapeutic module is achieved with many strategies collectively by drug release [3][4][5][6], photothermal activation [7], photodynamic activation [8][9], or the combination of these. Nanomaterial platforms are more variable in the range of their structure and function as illustrated in dendrimer polymers [10][11][12], upconversion nanocrystals (UCN) [13][14][15], metal-organic frameworks [16], magnetic nanoparticles [17], and hollow nanostructures [18]. This modular approach employed in nanotherapeutic design offers a combinatorial convenience and broad applicability in various therapeutic areas and personalized medicine [1].
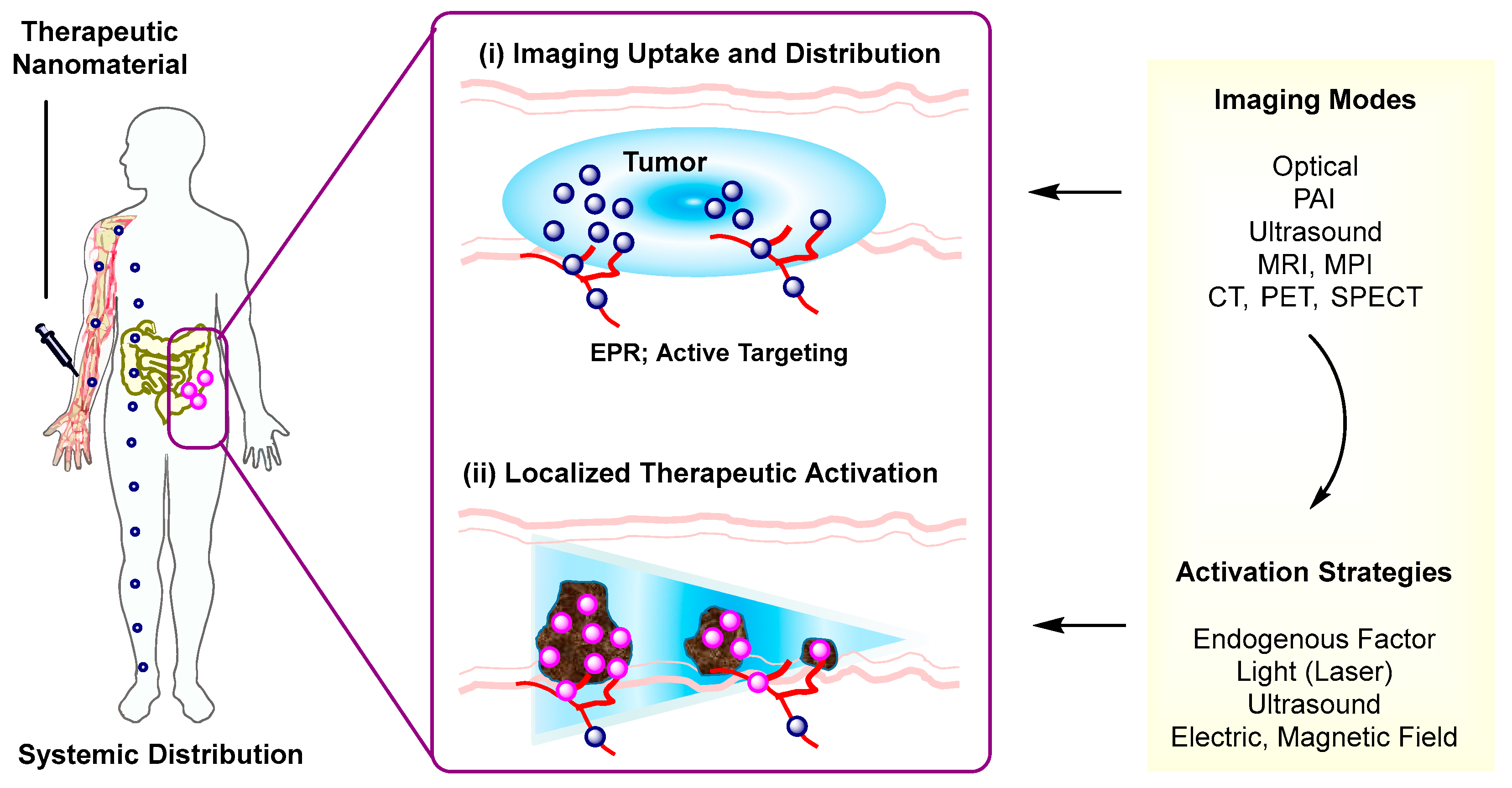
Therapeutic Activation. Effectiveness of therapeutic strategies employed in image-guided delivery is dependent on the types of activation mechanism. Such activation strategies involve stimulations by either endogenous factors (pH, glutathione) [19][20] or external stimuli including light [6][3][4][5], ultrasound (US) [21][22][23], alternating magnetic field [5][17], or electric field [24]. These stimuli provide various mechanisms applicable for therapeutic activation including drug release that occurs through linker cleavage, nanomaterial disassembly, or gate opening. These strategies are described along with design factors involved in the selection and integration of imaging probes, payloads, and linkers to nanomaterials. Of particular attention is their individual role in therapeutic activation that occurs via stimulus-controlled linker cleavage, nanomaterial degradation, or induction of porosity.
Imaging Modality. Another important aspect considered here relates to how therapeutic strategies are integrated with imaging modalities. For this purpose, delivery systems need to be designed for their imaging capability, which is achieved with ultrasound (US), magnetic resonance imaging (MRI), magnetic particle imaging (MPI), thermal imaging, photoacoustic imaging (PAI), X-ray computed tomography (CT), γ-ray positron emission tomography (PET), or single photon-emission computerized tomography (SPECT). It should be noted that these imaging methods have been described individually and comprehensively in several review articles [25][26][27][28][29][30][31], including those focused on optical [32], magnetic resonance [33], magnetic particle [34][35], photothermal [36], photoacoustic [37], and ultrasound [38].
Briefly, nanoscale systems are engineered for image-guided delivery through modular assembly. They are variable in modular principles, design characteristics, and activation strategies. Identifying a strategy for most optimal therapeutic efficacy remains an objective of significant interest [39].
2. Critical Aspects in Image-Guided Nanotherapeutic Delivery
As to nanotherapeutic systems developed for image-guided delivery, some of their mechanisms in therapeutic activation are attributed to cellular and pathophysiological factors, primarily low pH conditions [40][33][41] or elevated glutathione (GSH) levels [42]. Other mechanisms involve external stimulations which are more actively controlled via light-triggered linker cleavage [43][44][45][46][12], disassembly [47][48][49][50][51][52][53][54][55], pore gating [56][57][58], photothermal activation [36][59][60], photodynamic activation [61][62][63], US-mediated disassembly [64][65][66], hyperthermia [67], electroporation [68], and magnetic thermal activation [69]. Developing these activation strategies is making a significant impact on advancing knowledge and creating a new capability in nanotherapeutic delivery systems.
It is also worth noting current limitations associated with activation strategies. The degree of spatial resolution conferred by each activation strategy is variable as it is defined by the perimeter of its stimulus. Some strategies characterized by US, electromagnetic field, or thermal stimulation show relatively lower precision in spatiotemporal control compared to light activation. On the other hand, light shows a lower level of tissue penetration compared to US or electromagnetic stimulation [70]. This light limitation is currently addressed using an optical technique that allows tissue bypassing in which laser irradiation is delivered through a catheter inserted in a needle injected in tissue [71].
Another critical aspect which is of broad interest involves how to achieve specific nanomaterial uptake and localization in targeted cells only. Many systems discussed here are designed for tumor uptake via enhanced permeation and retention (EPR) [72], a passive targeting strategy that facilitates particle infiltration through leaky vessels in tumors (Figure 1). However, this passive targeting is not applicable for distinguishing specific tumor biomarkers or targeted binding and uptake at specific biomarker-overexpressing tumor cells. This lack of specificity is achieved otherwise by an active targeting strategy [73][74][4] in which a drug-loaded nanomaterial is functionalized through multivalent conjugation with a target-specific ligand or antibody. This active targeting has been applied to a few tumor biomarkers that include folate receptor (FAR) [60][65][41], αvβ3 integrin [75][76][77][78], IGF1 receptor [79], epidermal growth factor receptor (EGFR) [80], CD105 [81], CCR5 [82], and nucloelin [40]. It would be equally applicable to other promising but less explored biomarkers that include prostate-specific membrane antigen (PSMA) receptors [83], Her2 [84], riboflavin receptors [85][86], and transferrin receptors [87] for brain delivery. In brief, multivalent ligand conjugation serves as an important strategy in the design of actively targeted systems.
Finally, clinical translation of nanotherapeutic agents is associated with significant challenges due to their multifunctional design, difficulty in synthetic scalability, and paucity of efficient clinical devices needed for their optimal activation. Nevertheless, they show a growing potential as evident with numerous types of nanotherapeutic agents either approved or advanced to clinical studies [88]. Of those, release control by endogenous factors is most often engaged as shown in poly(lactic acid-co-glycolic acid) (PLGA) nanoparticles (NPs) encapsulated with leuprolide (Eligard®) [89], albumin NPs bound with paclitaxel (Abraxane®) [90], and liposomes encapsulated with doxorubicin (Doxil®) [91], mifamurtide (Mepact®) [92], vincristine (Marqibo®) [93], or irinotecan (Onivyde®) [94]. Thermal control of drug release is also successfully applied in heat-sensitive liposomes loaded with doxorubicin (ThermoDox®) [95]. Clinical development of photoactivated nanotherapeutics has been relatively slower but was already demonstrated by photodynamic therapy (PDT)-based verteporfin liposome (Visudyne®), which is approved for age-related macular degeneration and is currently being investigated for locally advanced pancreatic cancer [96][97]. This strategy is also applicable in topical and superficial treatments as illustrated with PDT nanoemulsion (BF-200) [98], a topical agent investigated for treating actinic keratosis . In summary, nanotherapeutic activation strategies are currently being evaluated for their clinical translation [88][39].
References
- Tarun Ojha; Larissa Rizzo; Gert Storm; Fabian Kiessling; Twan Lammers; Image-guided drug delivery: preclinical applications and clinical translation. Expert Opinion on Drug Delivery 2015, 12, 1203-1207, 10.1517/17425247.2015.1059420.
- Seok Ki Choi; Activation Strategies in Image-Guided Nanotherapeutic Delivery. Journal of Nanotheranostics 2020, 1, 78-104, 10.3390/jnt1010007.
- Choi, Seok Ki. Chapter 9 - Photocleavable linkers: design and applications in nanotechnology in Photonanotechnology for Therapeutics and Imaging; Choi, Seok Ki, Eds.; Elsevier: Netherlands, 2020; pp. 243–275.
- Pamela T. Wong; Seok Ki Choi; Mechanisms of Drug Release in Nanotherapeutic Delivery Systems. Chemical Reviews 2015, 115, 3388-3432, 10.1021/cr5004634.
- Yanfei Wang; Daniel S. Kohane; External triggering and triggered targeting strategies for drug delivery. Nature Reviews Materials 2017, 2, 17020, 10.1038/natrevmats.2017.20.
- Yan Zhang; Cheng Xu; Xiangliang Yang; Kanyi Pu; Photoactivatable Protherapeutic Nanomedicine for Cancer. Advanced Materials 2020, 32, e2002661, 10.1002/adma.202002661.
- Pooja M. Tiwari; Komal Vig; Vida Dennis; Shree R. Singh; Functionalized Gold Nanoparticles and Their Biomedical Applications. Nanomaterials 2011, 1, 31-63, 10.3390/nano1010031.
- Rui Yin; Tanupriya Agrawal; Usman Khan; Gaurav K Gupta; Vikrant Rai; Ying-Ying Huang; Michael R. Hamblin; Antimicrobial photodynamic inactivation in nanomedicine: small light strides against bad bugs. Nanomedicine 2015, 10, 2379-2404, 10.2217/nnm.15.67.
- Jie Zhao; Li Duan; Anhe Wang; Jinbo Fei; Junbai Li; Insight into the efficiency of oxygen introduced photodynamic therapy (PDT) and deep PDT against cancers with various assembled nanocarriers. WIREs Nanomedicine and Nanobiotechnology 2019, 12, e1583, 10.1002/wnan.1583.
- Pamela T. Wong; Shengzhuang Tang; Jayme Cannon; Dexin Chen; Rachel Sun; Jennifer Lee; James Phan; Ke Tao; Kang Sun; Biqiong Chen; et al.Jr. James R. BakerSeok Ki Choi Photocontrolled Release of Doxorubicin Conjugated through a Thioacetal Photocage in Folate-Targeted Nanodelivery Systems. Bioconjugate Chemistry 2017, 28, 3016-3028, 10.1021/acs.bioconjchem.7b00614.
- Pamela T. Wong; Shengzhuang Tang; Jhindan Mukherjee; Kenny Tang; Kristina Gam; Danielle Isham; Claire Murat; Rachel Sun; James R. Baker; Seok Ki Choi; et al. Light-controlled active release of photocaged ciprofloxacin for lipopolysaccharide-targeted drug delivery using dendrimer conjugates. Chemical Communications 2015, 52, 10357-10360, 10.1039/c6cc05179k.
- Seok Ki Choi; Thommey P Thomas; Ming-Hsin Li; Alina Kotlyar; Ankur Desai; Jr. James R. Baker; Light-controlled release of caged doxorubicin from folate receptor-targeting PAMAM dendrimer nanoconjugate. Chemical Communications 2010, 46, 2632-2634, 10.1039/b927215c.
- Feiya Xu; Yiming Zhao; Min Hu; Pu Zhang; Ning Kong; Ruiyu Liu; Chengcheng Liu; Seok Ki Choi; Lanthanide-doped core–shell nanoparticles as a multimodality platform for imaging and photodynamic therapy. Chemical Communications 2017, 54, 9525-9528, 10.1039/c8cc05057k.
- Feiya Xu; Min Hu; Chengcheng Liu; Seok Ki Choi; Yolk-structured multifunctional up-conversion nanoparticles for synergistic photodynamic–sonodynamic antibacterial resistance therapy. Biomaterials Science 2016, 5, 678-685, 10.1039/c7bm00030h.
- Pamela T. Wong; Dexin Chen; Shengzhuang Tang; Sean Yanik; Michael Payne; Jhindan Mukherjee; Alexa Coulter; Kenny Tang; Ke Tao; Kang Sun; et al.Jr. James R. BakerSeok Ki Choi Modular Integration of Upconverting Nanocrystal-Dendrimer Composites for Folate Receptor-Specific NIR Imaging and Light-Triggered Drug Release. Small 2015, 11, 6078-6090, 10.1002/smll.201501575.
- Wen Cai; Junqing Wang; Chengchao Chu; Wei Chen; Chunsheng Wu; Gang Liu; Metal-Organic Framework-Based Stimuli-Responsive Systems for Drug Delivery. Advanced Science 2018, 6, 1801526, 10.1002/advs.201801526.
- Jing Huang; Yuancheng Li; Anamaria Orza; Qiong Lu; Peng Guo; Liya Wang; Lily Yang; Hui Mao; Magnetic Nanoparticle Facilitated Drug Delivery for Cancer Therapy with Targeted and Image-Guided Approaches. Advanced Functional Materials 2016, 26, 3818-3836, 10.1002/adfm.201504185.
- Jinping Wang; Nan Li; Functional hollow nanostructures for imaging and phototherapy of tumors. Journal of Materials Chemistry B 2016, 5, 8430-8445, 10.1039/c7tb02381b.
- Emily Gullotti; Yoon Yeo; Extracellularly Activated Nanocarriers: A New Paradigm of Tumor Targeted Drug Delivery. Molecular Pharmaceutics 2009, 6, 1041-1051, 10.1021/mp900090z.
- Helen H. W. Chen; Im-Sook Song; Anwar Hossain; Min-Koo Choi; Yoshiaki Yamane; Zheng D. Liang; Jia Lu; Lily Y.-H. Wu; Zahid H. Siddik; Leo W. J. Klomp; et al.Niramol SavarajMacus Tien KuoKuo Macus Tien Elevated Glutathione Levels Confer Cellular Sensitization to Cisplatin Toxicity by Up-Regulation of Copper Transporter hCtr1. Molecular Pharmacology 2008, 74, 697-704, 10.1124/mol.108.047969.
- Xu Zhu; Jun Guo; Cancan He; Huaxiao Geng; Gengsheng Yu; Jinqing Li; Hairong Zheng; Xiao-Juan Ji; Fei Yan; Ultrasound triggered image-guided drug delivery to inhibit vascular reconstruction via paclitaxel-loaded microbubbles. Scientific Reports 2016, 6, 21683, 10.1038/srep21683.
- Siyu Wang; Xixi Guo; Weijun Xiu; Yang Liu; Lili Ren; Huaxin Xiao; Fang Yang; Lianhui Wang; Chenjie Xu; Lianhui Wang; et al. Accelerating thrombolysis using a precision and clot-penetrating drug delivery strategy by nanoparticle-shelled microbubbles. Science Advances 2020, 6, eaaz8204, 10.1126/sciadv.aaz8204.
- H Holger Grüll; S Sander Langereis; Hyperthermia-triggered drug delivery from temperature-sensitive liposomes using MRI-guided high intensity focused ultrasound. Journal of Controlled Release 2012, 161, 317-327, 10.1016/j.jconrel.2012.04.041.
- Fangyuan Li; Yu Qin; Jiyoung Lee; Hongwei Liao; Nan Wang; Thomas P. Davis; Ruirui Qiao; Daishun Ling; Stimuli-responsive nano-assemblies for remotely controlled drug delivery. Journal of Controlled Release 2020, 322, 566-592, 10.1016/j.jconrel.2020.03.051.
- Arash Hatefi; Tamara Minko; Advances in image-guided drug delivery. Drug Delivery and Translational Research 2012, 2, 1-2, 10.1007/s13346-011-0057-8.
- Luis Solorio; Ravi B. Patel; Hanping Wu; Tianyi Krupka; Agata A. Exner; Advances in image-guided intratumoral drug delivery techniques. Therapeutic Delivery 2010, 1, 307-322, 10.4155/tde.10.20.
- Rubel Chakravarty; Hao Hong; Weibo Cai; Positron Emission Tomography Image-Guided Drug Delivery: Current Status and Future Perspectives. Molecular Pharmaceutics 2014, 11, 3777-3797, 10.1021/mp500173s.
- Asahi Tomitaka; Hamed Arami; Arash Ahmadivand; Nezih Pala; Anthony J. McGoron; Yasushi Takemura; Marcelo Febo; Madhavan P. N. Nair; Magneto-plasmonic nanostars for image-guided and NIR-triggered drug delivery. Scientific Reports 2020, 10, 1-10, 10.1038/s41598-020-66706-2.
- D. Gao; X. Guo; Xingcai Zhang; S. Chen; Ying Wangb; T. Chen; G. Huang; Yanzheng Gao; Zhe Yang; Zhe Yang; et al. Multifunctional phototheranostic nanomedicine for cancer imaging and treatment. Materials Today Bio 2019, 5, 100035, 10.1016/j.mtbio.2019.100035.
- Monty Liong; Jie Lu; Michael Kovochich; Tian Xia; Stefan G. Ruehm; Andre E. Nel; Fuyuhiko Tamanoi; Jeffrey I. Zink; Multifunctional Inorganic Nanoparticles for Imaging, Targeting, and Drug Delivery. ACS Nano 2008, 2, 889-896, 10.1021/nn800072t.
- Nicholas E. Wojtynek; Aaron M. Mohs; Image‐guided tumor surgery: The emerging role of nanotechnology. WIREs Nanomedicine and Nanobiotechnology 2020, 12, e1624, 10.1002/wnan.1624.
- Shan Jiang; Muthu Kumara Gnanasammandhan; Yong Zhang; Optical imaging-guided cancer therapy with fluorescent nanoparticles. Journal of The Royal Society Interface 2009, 7, 3-18, 10.1098/rsif.2009.0243.
- Yu Zou; Du Li; Yue Wang; Zhijun Ouyang; Yucheng Peng; Helena Tomás; Jindong Xia; João Rodrigues; Mingwu Shen; X Shi; et al. Polyethylenimine Nanogels Incorporated with Ultrasmall Iron Oxide Nanoparticles and Doxorubicin for MR Imaging-Guided Chemotherapy of Tumors. Bioconjugate Chemistry 2020, 31, 907-915, 10.1021/acs.bioconjchem.0c00036.
- Michele H. Pablico-Lansigan; Shu F. Situ; Anna Cristina S. Samia; Magnetic particle imaging: advancements and perspectives for real-time in vivo monitoring and image-guided therapy. Nanoscale 2012, 5, 4040-4055, 10.1039/c3nr00544e.
- Kai Wu; Diqing Su; Renata Saha; Jinming Liu; Vinit Kumar Chugh; Jian-Ping Wang; Magnetic Particle Spectroscopy: A Short Review of Applications Using Magnetic Nanoparticles. ACS Applied Nano Materials 2020, 3, 4972-4989, 10.1021/acsanm.0c00890.
- Meng Qiu; Dou Wang; Weiyuan Liang; Liping Liu; Yin Zhang; Xing Chenyang; David Kipkemoi Sang; Chenyang Xing; Zhongjun Li; Biqin Dong; et al.Feng XingDianyuan FanShiyun BaoHan ZhangYihai Cao Novel concept of the smart NIR-light–controlled drug release of black phosphorus nanostructure for cancer therapy. Proceedings of the National Academy of Sciences 2018, 115, 501-506, 10.1073/pnas.1714421115.
- Katheryne E Wilson; Kimberly Homan; Stanislav Y Emelianov; Biomedical photoacoustics beyond thermal expansion using triggered nanodroplet vaporization for contrast-enhanced imaging. Nature Communications 2012, 3, 618, 10.1038/ncomms1627.
- Delaney G. Fisher; Richard J. Price; Recent Advances in the Use of Focused Ultrasound for Magnetic Resonance Image-Guided Therapeutic Nanoparticle Delivery to the Central Nervous System. Frontiers in Pharmacology 2019, 10, 1348, 10.3389/fphar.2019.01348.
- Seok Ki Choi; Photoactivation Strategies for Therapeutic Release in Nanodelivery Systems. Advanced Therapeutics 2020, 3, 2000117, 10.1002/adtp.202000117.
- Daiqin Chen; Dongzhi Yang; Casey A. Dougherty; Weifei Lu; Hongwei Wu; Xianran He; Ting Cai; Marcian E. Van Dort; Brian D. Ross; Hao Hong; et al. In Vivo Targeting and Positron Emission Tomography Imaging of Tumor with Intrinsically Radioactive Metal–Organic Frameworks Nanomaterials. ACS Nano 2017, 11, 4315-4327, 10.1021/acsnano.7b01530.
- Zhenhua Li; Kai Dong; Sa Huang; Enguo Ju; Zhen Liu; Meili Yin; Jinsong Ren; Xiaogang Qu; A Smart Nanoassembly for Multistage Targeted Drug Delivery and Magnetic Resonance Imaging. Advanced Functional Materials 2014, 24, 3612-3620, 10.1002/adfm.201303662.
- Xingang Liu; Min Wu; Qinglian Hu; Hongzhen Bai; Shuoqing Zhang; Youqing Shen; Guping Tang; Yuan Ping; Redox-Activated Light-Up Nanomicelle for Precise Imaging-Guided Cancer Therapy and Real-Time Pharmacokinetic Monitoring. ACS Nano 2016, 10, 11385-11396, 10.1021/acsnano.6b06688.
- Sarit S. Agasti; Apiwat Chompoosor; Chang-Cheng You; Partha Ghosh; Chae Kyu Kim; Vincent M. Rotello; Photoregulated Release of Caged Anticancer Drugs from Gold Nanoparticles. Journal of the American Chemical Society 2009, 131, 5728-5729, 10.1021/ja900591t.
- Gang Han; Chang-Cheng You; Byoung-Jin Kim; Rosemary S. Turingan; Neil S. Forbes; Craig T. Martin; Vincent Rotello; Light-Regulated Release of DNA and Its Delivery to Nuclei by Means of Photolabile Gold Nanoparticles. Angewandte Chemie International Edition 2006, 45, 3165-3169, 10.1002/anie.200600214.
- Seok Ki Choi; Manisha Verma; Justin Silpe; Ryan E. Moody; Kenny Tang; Jeffrey J. Hanson; Jr. James R. Baker; A photochemical approach for controlled drug release in targeted drug delivery. Bioorganic & Medicinal Chemistry 2012, 20, 1281-1290, 10.1016/j.bmc.2011.12.020.
- Seok Ki Choi; Thommey P. Thomas; Ming-Hsin Li; Ankur Desai; Alina Kotlyar; Jr. James R. Baker; Photochemical release of methotrexate from folate receptor-targeting PAMAM dendrimer nanoconjugate. Photochemical & Photobiological Sciences 2012, 11, 653-660, 10.1039/c2pp05355a.
- Bin Yan; John-Christopher Boyer; Neil R. Branda; Yue Zhao; Near-Infrared Light-Triggered Dissociation of Block Copolymer Micelles Using Upconverting Nanoparticles. Journal of the American Chemical Society 2011, 133, 19714-19717, 10.1021/ja209793b.
- Yeye Zhang; Guangzhao Lu; Yuan Yu; He Zhang; Jie Gao; Zhiguo Sun; Ying Lu; Hao Zou; NIR-Responsive Copolymer Upconversion Nanocomposites for Triggered Drug Release in Vitro and in Vivo. ACS Applied Bio Materials 2018, 2, 495-503, 10.1021/acsabm.8b00681.
- Hui Zhao; Wenbo Hu; Hengheng Ma; Rongcui Jiang; Yufu Tang; Yu Ji; Xiaomei Lu; Bing Hou; Weixing Deng; Wei Huang; et al.Chao Yin Photo-Induced Charge-Variable Conjugated Polyelectrolyte Brushes Encapsulating Upconversion Nanoparticles for Promoted siRNA Release and Collaborative Photodynamic Therapy under NIR Light Irradiation. Advanced Functional Materials 2017, 27, 1702592, 10.1002/adfm.201702592.
- Yinan Zhong; Chao Wang; Liang Cheng; Fenghua Meng; Zhiyuan Zhong; Zhuang Liu; Gold Nanorod-Cored Biodegradable Micelles as a Robust and Remotely Controllable Doxorubicin Release System for Potent Inhibition of Drug-Sensitive and -Resistant Cancer Cells. Biomacromolecules 2013, 14, 2411-2419, 10.1021/bm400530d.
- Zhenjiang Zhang; Jing Wang; Xin Nie; Tao Wen; Yinglu Ji; Xiaochun Wu; Yuliang Zhao; Chunying Chen; Near Infrared Laser-Induced Targeted Cancer Therapy Using Thermoresponsive Polymer Encapsulated Gold Nanorods. Journal of the American Chemical Society 2014, 136, 7317-7326, 10.1021/ja412735p.
- Ben Shi; Ning Ren; Luyan Gu; Ge Xu; Rongchen Wang; Tianli Zhu; Ying Zhu; Chunhai Fan; Chunchang Zhao; He Tian; et al. Theranostic Nanoplatform with Hydrogen Sulfide Activatable NIR Responsiveness for Imaging‐Guided On‐Demand Drug Release. Angewandte Chemie International Edition 2019, 58, 16826-16830, 10.1002/anie.201909883.
- Gurusamy Saravanakumar; Junseok Lee; Jihoon Kim; Won Jong Kim; Visible light-induced singlet oxygen-mediated intracellular disassembly of polymeric micelles co-loaded with a photosensitizer and an anticancer drug for enhanced photodynamic therapy. Chemical Communications 2014, 51, 9995-9998, 10.1039/c5cc01937k.
- Valentina Brega; Federica Scaletti; Xianzhi Zhang; Li-Sheng Wang; Prudence Li; Qiaobing Xu; Vincent M. Rotello; Iii Samuel W. Thomas; Polymer Amphiphiles for Photoregulated Anticancer Drug Delivery. ACS Applied Materials & Interfaces 2018, 11, 2814-2820, 10.1021/acsami.8b18099.
- Yang Li; Shujuan Wang; Yulan Huang; Yuwen Chen; Wenbi Wu; Yu Liu; Jing Zhang; Yue Feng; Xian Jiang; MaLing Gou; et al. Light-activated drug release from prodrug nanoassemblies by structure destruction. Chemical Communications 2018, 55, 13128-13131, 10.1039/c9cc06673j.
- Shuqing He; Kristina Krippes; Sandra Ritz; Zhijun Chen; Andreas Best; Hans-Jürgen Butt; Volker Mailänder; Si Wu; Ultralow-intensity near-infrared light induces drug delivery by upconverting nanoparticles. Chemical Communications 2014, 51, 431-434, 10.1039/c4cc07489k.
- Jianan Liu; Wenbo Bu; Limin Pan; Xiangzhi Cui; NIR-Triggered Anticancer Drug Delivery by Upconverting Nanoparticles with Integrated Azobenzene-Modified Mesoporous Silica. Angewandte Chemie International Edition 2013, 52, 4375-4379, 10.1002/anie.201300183.
- Ruichan Lv; Piaoping Yang; Fei He; Shili Gai; Chunxia Li; Yunlu Dai; Guixin Yang; Jun Lin; A Yolk-like Multifunctional Platform for Multimodal Imaging and Synergistic Therapy Triggered by a Single Near-Infrared Light. ACS Nano 2015, 9, 1630-1647, 10.1021/nn5063613.
- Shan Fang; Jing Linab; Chunxiao Li; Peng Huang; Wenxiu Hou; Chunlei Zhang; Jingjing Liu; Sisi Huang; Yongxiang Luo; Wenpei Fan; et al.Daxiang CuiYunsheng XuZhiming Li Dual-Stimuli Responsive Nanotheranostics for Multimodal Imaging Guided Trimodal Synergistic Therapy. Small 2016, 13, 1602580, 10.1002/smll.201602580.
- Junping Zhong; Sihua Yang; Liewei Wen; Da Xing; Imaging-guided photoacoustic drug release and synergistic chemo-photoacoustic therapy with paclitaxel-containing nanoparticles. Journal of Controlled Release 2016, 226, 77-87, 10.1016/j.jconrel.2016.02.010.
- Junseok Lee; Juhee Park; Kaushik Singha; Won Jong Kim; Mesoporous silica nanoparticle facilitated drug release through cascade photosensitizer activation and cleavage of singlet oxygen sensitive linker. Chemical Communications 2012, 49, 1545-1547, 10.1039/c2cc38510d.
- Xuesong Wang; Nana Tian; Chao Li; Yuanjun Hou; Xuesong Wang; Qianxiong Zhou; Incorporation of 7-dehydrocholesterol into liposomes as a simple, universal and efficient way to enhance anticancer activity by combining PDT and photoactivated chemotherapy. Chemical Communications 2018, 55, 14081-14084, 10.1039/c9cc05691b.
- Qing Pei; Xiuli Hu; Xiaohua Zheng; Shi Liu; Yawei Li; Xiabin Jing; Zhigang Xie; Light-Activatable Red Blood Cell Membrane-Camouflaged Dimeric Prodrug Nanoparticles for Synergistic Photodynamic/Chemotherapy. ACS Nano 2018, 12, 1630-1641, 10.1021/acsnano.7b08219.
- Janna N. Sloand; Theodore T. Nguyen; Scott A. Zinck; Erik C. Cook; Tawanda J. Zimudzi; Scott A. Showalter; Adam B. Glick; Julianna C. Simon; Scott H. Medina; Ultrasound-Guided Cytosolic Protein Delivery via Transient Fluorous Masks. ACS Nano 2020, 14, 4061-4073, 10.1021/acsnano.9b08745.
- Tianliang Li; Jia Zhou; Chunlei Zhang; Xiao Zhi; Jiaqi Niu; Hualin Fu; Jie Song; Daxiang Cui; Surface-engineered nanobubbles with pH-/light-responsive drug release and charge-switchable behaviors for active NIR/MR/US imaging-guided tumor therapy. NPG Asia Materials 2018, 10, 1046-1060, 10.1038/s41427-018-0094-6.
- Jun Chen; Sithira Ratnayaka; Aaron Alford; Veronika Kozlovskaya; Fei Liu; Bing Xue; Kenneth Hoyt; Eugenia Kharlampieva; Theranostic Multilayer Capsules for Ultrasound Imaging and Guided Drug Delivery. ACS Nano 2017, 11, 3135-3146, 10.1021/acsnano.7b00151.
- M Mariska De Smet; E Edwin Heijman; Sander Langereis; Nicole M. Hijnen; Holger Grüll; Magnetic resonance imaging of high intensity focused ultrasound mediated drug delivery from temperature-sensitive liposomes: An in vivo proof-of-concept study. Journal of Controlled Release 2011, 150, 102-110, 10.1016/j.jconrel.2010.10.036.
- Samdeep K. Mouli; Patrick Tyler; Joseph L. McDevitt; Aaron C. Eifler; Yang Guo; Jodi Nicolai; Robert .J. Lewandowski; Weiguo Li; Daniel Procissi; Robert K. Ryu; et al.Y. Andrew WangRiad SalemAndrew C. LarsonReed A. Omary Image-Guided Local Delivery Strategies Enhance Therapeutic Nanoparticle Uptake in Solid Tumors. ACS Nano 2013, 7, 7724-7733, 10.1021/nn4023119.
- Zhi Wei Tay; Prashant Chandrasekharan; Andreina Chiu-Lam; Daniel W. Hensley; Rohan Dhavalikar; Xinyi Y. Zhou; Elaine Y. Yu; Patrick W. Goodwill; Bo Zheng; Carlos Rinaldi; et al.Steven M. Conolly Magnetic Particle Imaging-Guided Heating in Vivo Using Gradient Fields for Arbitrary Localization of Magnetic Hyperthermia Therapy. ACS Nano 2018, 12, 3699-3713, 10.1021/acsnano.8b00893.
- Choi, Seok. Ki. Light sources for photonanotechnology in Photonanotechnology for Therapeutics and Imaging; Choi, Seok. Ki, Eds.; Elsevier: Netherlands, 2020; pp. 1-21.
- Abdul K. Parchur; Gayatri Sharma; Jaidip M. Jagtap; Venkateswara Rao Gogineni; Peter S. LaViolette; Michael J. Flister; Sarah B White; Amit Joshi; Vascular Interventional Radiology-Guided Photothermal Therapy of Colorectal Cancer Liver Metastasis with Theranostic Gold Nanorods. ACS Nano 2018, 12, 6597-6611, 10.1021/acsnano.8b01424.
- Hiroshi Maeda; The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Advances in Enzyme Regulation 2001, 41, 189-207, 10.1016/s0065-2571(00)00013-3.
- Philip S. Low; Walter A. Henne; Derek D. Doorneweerd; Discovery and Development of Folic-Acid-Based Receptor Targeting for Imaging and Therapy of Cancer and Inflammatory Diseases. Accounts of Chemical Research 2007, 41, 120-129, 10.1021/ar7000815.
- Nazila Kamaly; Zeyu Xiao; Pedro M. Valencia; Aleksandar F. Radovic-Moreno; Omid C. Farokhzad; Targeted polymeric therapeutic nanoparticles: design, development and clinical translation. Chemical Society Reviews 2012, 41, 2971-3010, 10.1039/c2cs15344k.
- Anita Gianella; Peter A. Jarzyna; Venkatesh Mani; Sarayu Ramachandran; Claudia Calcagno; Jun Tang; Benjamin H. Kann; Wouter J. R. Dijk; Victor L. Thijssen; Arjan W. Griffioen; et al.Gert StormZahi A. FayadWillem J. M. Mulder Multifunctional Nanoemulsion Platform for Imaging Guided Therapy Evaluated in Experimental Cancer. ACS Nano 2011, 5, 4422-4433, 10.1021/nn103336a.
- Min Li; Yang Gao; Youyong Yuan; Yuzhe Wu; Zifang Song; Ben Zhong Tang; Bin Liu; Qi Chang Zheng; One-Step Formulation of Targeted Aggregation-Induced Emission Dots for Image-Guided Photodynamic Therapy of Cholangiocarcinoma. ACS Nano 2017, 11, 3922-3932, 10.1021/acsnano.7b00312.
- Sijumon Kunjachan; Robert Pola; Felix Gremse; Benjamin Theek; Josef Ludger Anton Ehling; Diana Moeckel; Benita Hermanns-Sachweh; Michal Pechar; Karel Ulbrich; Wim E. Hennink; et al.Gert StormWiltrud LederleFabian KiesslingTwan Lammers Passive versus Active Tumor Targeting Using RGD- and NGR-Modified Polymeric Nanomedicines. Nano Letters 2014, 14, 972-981, 10.1021/nl404391r.
- Xiaolian Sun; Xinglu Huang; Xuefeng Yan; Yu Wang; Jinxia Guo; Orit Jacobson; Dingbin Liu; Lawrence P. Szajek; Wenlei Zhu; Gang Niu; et al.Dale O. KiesewetterShouheng SunXiaoyuan Chen Chelator-Free 64Cu-Integrated Gold Nanomaterials for Positron Emission Tomography Imaging Guided Photothermal Cancer Therapy. ACS Nano 2014, 8, 8438-8446, 10.1021/nn502950t.
- Hongyu Zhou; Weiping Qian; Fatih M. Uckun; Liya Wang; Y. Andrew Wang; Hongyu Chen; David Kooby; Qian Yu; Malgorzata Lipowska; Charles A. Staley; et al.Hui MaoLily Yang IGF1 Receptor Targeted Theranostic Nanoparticles for Targeted and Image-Guided Therapy of Pancreatic Cancer. ACS Nano 2015, 9, 7976-7991, 10.1021/acsnano.5b01288.
- Marites Pasuelo Melancon; Min Zhou; Rui Zhang; Chiyi Xiong; Peter Allen; Xiaoxia Wen; Qian Huang; Michael Wallace; Jeffrey N. Myers; R. Jason Stafford; et al.Dong LiangAndrew D. EllingtonChun Li Selective Uptake and Imaging of Aptamer- and Antibody-Conjugated Hollow Nanospheres Targeted to Epidermal Growth Factor Receptors Overexpressed in Head and Neck Cancer. ACS Nano 2014, 8, 4530-4538, 10.1021/nn406632u.
- Feng Chen; Hao Hong; Yin Zhang; Hector F. Valdovinos; Sixiang Shi; Glen S. Kwon; Charles P. Theuer; Todd E. Barnhart; Weibo Cai; In Vivo Tumor Targeting and Image-Guided Drug Delivery with Antibody-Conjugated, Radiolabeled Mesoporous Silica Nanoparticles. ACS Nano 2013, 7, 9027-9039, 10.1021/nn403617j.
- Bo Pang; Yongfeng Zhao; Hannah Luehmann; Xuan Yang; Lisa Detering; Xia Younan; Chao Zhang; Lei Zhang; Detering Lisa; Qiushi Ren; et al.Yongjian LiuYounan Xia 64Cu-Doped PdCu@Au Tripods: A Multifunctional Nanomaterial for Positron Emission Tomography and Image-Guided Photothermal Cancer Treatment. ACS Nano 2016, 10, 3121-3131, 10.1021/acsnano.5b07968.
- Ying Chen; Catherine A. Foss; Youngjoo Byun; Sridhar Nimmagadda; Mrudula Pullambhatla; James J. Fox; Mark Castanares; Shawn E. Lupold; John W. Babich; Ronnie C. Mease; et al.Martin G. Pomper Radiohalogenated Prostate-Specific Membrane Antigen (PSMA)-Based Ureas as Imaging Agents for Prostate Cancer. Journal of Medicinal Chemistry 2008, 51, 7933-7943, 10.1021/jm801055h.
- Rameshwer Shukla; Thommey P. Thomas; Ankur M. Desai; Alina Kotlyar; Steve J. Park; James R Baker Jr; James R. Baker; HER2 specific delivery of methotrexate by dendrimer conjugated anti-HER2 mAb. Nanotechnology 2008, 19, 295102-295102, 10.1088/0957-4484/19/29/295102.
- Thommey P. Thomas; Seok Ki Choi; Ming-Hsin Li; Alina Kotlyar; Jr. James R. Baker; Design of riboflavin-presenting PAMAM dendrimers as a new nanoplatform for cancer-targeted delivery. Bioorganic & Medicinal Chemistry Letters 2010, 20, 5191-5194, 10.1016/j.bmcl.2010.07.005.
- Anna Plantinga; Amanda Witte; Ming-Hsin Li; Andrew Harmon; Seok Ki Choi; Mark M. Banaszak Holl; Bradford G. Orr; Jr. James R. Baker; Kumar Sinniah; Bioanalytical Screening of Riboflavin Antagonists for Targeted Drug Delivery—A Thermodynamic and Kinetic Study. ACS Medicinal Chemistry Letters 2011, 2, 363-367, 10.1021/ml100296z.
- Zhong Ming Qian; Targeted Drug Delivery via the Transferrin Receptor-Mediated Endocytosis Pathway. Pharmacological Reviews 2002, 54, 561-587, 10.1124/pr.54.4.561.
- Hongliang He; Lisha Liu; Emily E. Morin; Min Liu; Anna Schwendeman; Survey of Clinical Translation of Cancer Nanomedicines—Lessons Learned from Successes and Failures. Accounts of Chemical Research 2019, 52, 2445-2461, 10.1021/acs.accounts.9b00228.
- Oliver Sartor; Eligard: leuprolide acetate in a novel sustained-release delivery system.. Urology 2003, 61, 25-31, 10.1016/s0090-4295(02)02396-8.
- Alex Sparreboom; Charity D. Scripture; Vuong Trieu; Paul J. Williams; Tapas De; Andrew Yang; Bridget Beals; William D Figg; Michael Hawkins; Neil Desai; et al. Comparative Preclinical and Clinical Pharmacokinetics of a Cremophor-Free, Nanoparticle Albumin-Bound Paclitaxel (ABI-007) and Paclitaxel Formulated in Cremophor (Taxol). Clinical Cancer Research 2005, 11, 4136-4143, 10.1158/1078-0432.ccr-04-2291.
- Olga Lyass; Beatrice Uziely; Rami Ben-Yosef; Dinah Tzemach; Norman I. Heshing; Michal Lotem; George Brufman; Alberto A. Gabizon; Correlation of toxicity with pharmacokinetics of pegylated liposomal doxorubicin (Doxil) in metastatic breast carcinoma. Cancer 2000, 89, 1037-1047, 10.1002/1097-0142(20000901)89:5<1037::aid-cncr13>3.0.co;2-z.
- Karthik Venkatakrishnan; Yi Liu; Dennis Noe; Jaime Mertz; Michael Bargfrede; Thomas Marbury; Kambiz Farbakhsh; Cristina Oliva; Ashley Milton; Pharmacokinetics and pharmacodynamics of liposomal mifamurtide in adult volunteers with mild or moderate hepatic impairment. British Journal of Clinical Pharmacology 2014, 77, 998-1010, 10.1111/bcp.12261.
- Jeffrey A. Silverman; Steven R. Deitcher; Marqibo® (vincristine sulfate liposome injection) improves the pharmacokinetics and pharmacodynamics of vincristine. Cancer Chemotherapy and Pharmacology 2012, 71, 555-564, 10.1007/s00280-012-2042-4.
- Haijun Zhang; Onivyde for the therapy of multiple solid tumors. OncoTargets and Therapy 2016, 9, 3001-3007, 10.2147/ott.s105587.
- Michael Dunne; Brittany Epp-Ducharme; Alexandros Marios Sofias; Maximilian Regenold; David N. Dubins; Christine Allen; Heat-activated drug delivery increases tumor accumulation of synergistic chemotherapies. Journal of Controlled Release 2019, 308, 197-208, 10.1016/j.jconrel.2019.06.012.
- M T Huggett; M Jermyn; A Gillams; R Illing; S Mosse; M Novelli; E Kent; S G Bown; T Hasan; B W Pogue; et al.S.P. Pereira Phase I/II study of verteporfin photodynamic therapy in locally advanced pancreatic cancer. British Journal of Cancer 2014, 110, 1698-1704, 10.1038/bjc.2014.95.
- Girgis Obaid; Mans Broekgaarden; Anne-Laure Bulin; Huang-Chiao Huang; Jerrin Kuriakose; Joyce Liu; Tayyaba Hasan; Photonanomedicine: a convergence of photodynamic therapy and nanotechnology. Nanoscale 2016, 8, 12471-12503, 10.1039/c5nr08691d.
- Tim Maisch; Francesco Santarelli; Stephan Schreml; Philipp Babilas; Rolf-Markus Szeimies; Fluorescence induction of protoporphyrin IX by a new 5-aminolevulinic acid nanoemulsion used for photodynamic therapy in a full-thickness ex vivo skin model. Experimental Dermatology 2009, 19, e302-e305, 10.1111/j.1600-0625.2009.01001.x.




