| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Katherine E. Odegaard | + 6326 word(s) | 6326 | 2021-01-13 03:04:55 | | | |
| 2 | Gurudutt Pendyala | Meta information modification | 6326 | 2021-01-13 03:25:47 | | | | |
| 3 | Dean Liu | Meta information modification | 6326 | 2021-01-13 04:57:07 | | | | |
| 4 | Vicky Zhou | -4660 word(s) | 1666 | 2022-04-13 11:37:40 | | |
Video Upload Options
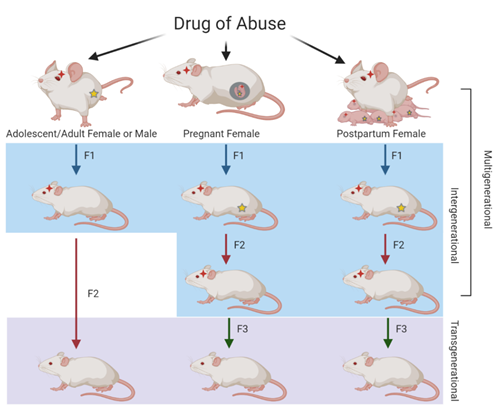
The inheritance of substance abuse, including opioid abuse, may be influenced by genetic and non-genetic factors related to the environment, such as stress and socioeconomic status. These non-genetic influences on the heritability of a trait can be attributed to epigenetics. Epigenetic inheritance can result from modifications passed down from the mother, father, or both, resulting in either maternal, paternal, or parental epigenetic inheritance, respectively. These epigenetic modifications can be passed to the offspring to result in multigenerational, intergenerational, or transgenerational inheritance. Human and animal models of opioid exposure have shown generational effects that result in molecular, developmental, and behavioral alterations in future generations.
Generational Inheritance
Opioids
Morphine
Heroin
Oxycodone
References
- Ostling, P.S.; Davidson, K.S.; Anyama, B.O.; Helander, E.M.; Wyche, M.Q.; Kaye, A.D. America’s Opioid Epidemic: A Comprehensive Review and Look into the Rising Crisis. Curr. Pain Headache Rep. 2018, 22, 32.
- Badreldin, N.; Grobman, W.A.; Chang, K.T.; Yee, L.M. Opioid prescribing patterns among postpartum women. Am. J. Obstet. Gynecol. 2018, 219.
- Bateman, B.T.; Franklin, J.M.; Bykov, K.; Avorn, J.; Shrank, W.H.; Brennan, T.A.; Landon, J.E.; Rathmell, J.P.; Huybrechts, K.F.; Fischer, M.A.; et al. Persistent opioid use following cesarean delivery: Patterns and predictors among opioid-naïve women. Am. J. Obstet. Gynecol. 2016, 215.
- Brook, D.W.; Brook, J.S.; Zhang, C.; Cohen, P.; Whiteman, M. Drug use and the risk of major depressive disorder, alcohol dependence, and substance use disorders. Arch. Gen. Psychiatry 2002, 59, 1039–1044.
- Ducci, F.; Goldman, D. The genetic basis of addictive disorders. Psychiatry Clin. N. Am. 2012, 35, 495–519.
- Cloninger, C.R.; Bohman, M.; Sigvardsson, S. Inheritance of alcohol abuse. Cross-fostering analysis of adopted men. Arch. Gen. Psychiatry 1981, 38, 861–868.
- Ho, M.K.; Goldman, D.; Heinz, A.; Kaprio, J.; Kreek, M.J.; Li, M.D.; Munafò, M.R.; Tyndale, R.F. Breaking barriers in the genomics and pharmacogenetics of drug addiction. Clin. Pharmacol. Ther. 2010, 88, 779–791.
- Jensen, K.P. A Review of Genome-Wide Association Studies of Stimulant and Opioid Use Disorders. Mol. Neuropsychiatry 2016, 2, 37–45.
- Goldberg, L.R.; Gould, T.J. Multigenerational and transgenerational effects of paternal exposure to drugs of abuse on behavioral and neural function. Eur. J. Neurosci. 2019, 50, 2453–2466.
- Bird, A. Perceptions of epigenetics. Nature 2007, 447, 396–398.
- Jaenisch, R.; Bird, A. Epigenetic regulation of gene expression: How the genome integrates intrinsic and environmental signals. Nat. Genet. 2003, 33, 245–254.
- Yohn, N.L.; Bartolomei, M.S.; Blendy, J.A. Multigenerational and transgenerational inheritance of drug exposure: The effects of alcohol, opiates, cocaine, marijuana, and nicotine. Prog. Biophys. Mol. Biol. 2015, 118, 21–33.
- Vassoler, F.M.; Toorie, A.M.; Byrnes, E.M. Multi-, Inter-, and Transgenerational Effects of Drugs of Abuse on Behavior. Curr. Top. Behav. Neurosci. 2019, 42, 247–258.
- Skinner, M.K. Environmental epigenetic transgenerational inheritance and somatic epigenetic mitotic stability. Epigenetics 2011, 6, 838–842.
- Hanson, M.A.; Skinner, M.K. Developmental origins of epigenetic transgenerational inheritance. Environ. Epigenet. 2016, 2.
- Conradt, E.; Flannery, T.; Aschner, J.L.; Annett, R.D.; Croen, L.A.; Duarte, C.S.; Friedman, A.M.; Guille, C.; Hedderson, M.M.; Hofheimer, J.A.; et al. Prenatal Opioid Exposure: Neurodevelopmental Consequences and Future Research Priorities. Pediatrics 2019, 144.
- Barker, D.J. Fetal origins of coronary heart disease. BMJ 1995, 311, 171–174.
- Skogen, J.C.; Overland, S. The fetal origins of adult disease: A narrative review of the epidemiological literature. JRSM Short Rep. 2012, 3, 59.
- Finnegan, L.P. Effects of maternal opiate abuse on the newborn. Fed. Proc. 1985, 44, 2314–2317.
- Towers, C.V.; Hyatt, B.W.; Visconti, K.C.; Chernicky, L.; Chattin, K.; Fortner, K.B. Neonatal Head Circumference in Newborns with Neonatal Abstinence Syndrome. Pediatrics 2019, 143.
- Attarian, S.; Tran, L.C.; Moore, A.; Stanton, G.; Meyer, E.; Moore, R.P. The neurodevelopmental impact of neonatal morphine administration. Brain Sci. 2014, 4, 321–334.
- Chiang, Y.C.; Hung, T.W.; Lee, C.W.; Yan, J.Y.; Ho, I.K. Enhancement of tolerance development to morphine in rats prenatally exposed to morphine, methadone, and buprenorphine. J. Biomed. Sci. 2010, 17, 46.
- Lacroix, I.; Berrebi, A.; Garipuy, D.; Schmitt, L.; Hammou, Y.; Chaumerliac, C.; Lapeyre-Mestre, M.; Montastruc, J.L.; Damase-Michel, C. Buprenorphine versus methadone in pregnant opioid-dependent women: A prospective multicenter study. Eur. J. Clin. Pharmacol. 2011, 67, 1053–1059.
- Odegaard, K.E.; Schaal, V.L.; Clark, A.R.; Koul, S.; Gowen, A.; Sankarasubramani, J.; Xiao, P.; Guda, C.; Lisco, S.J.; Yelamanchili, S.V.; et al. Characterization of the intergenerational impact of in utero and postnatal oxycodone exposure. Transl. Psychiatry 2020, 10, 329.
- Oei, J.L. Adult consequences of prenatal drug exposure. Intern. Med. J. 2018, 48, 25–31.
- Nygaard, E.; Slinning, K.; Moe, V.; Walhovd, K.B. Cognitive function of youths born to mothers with opioid and poly-substance abuse problems during pregnancy. Child. Neuropsychol. 2017, 23, 159–187.
- Vidal, S.I.; Vandeleur, C.; Rothen, S.; Gholam-Rezaee, M.; Castelao, E.; Halfon, O.; Aubry, J.M.; Ferrero, F.; Preisig, M. Risk of mental disorders in children of parents with alcohol or heroin dependence: A controlled high-risk study. Eur. Addict. Res. 2012, 18, 253–264.
- Rey, R.; Wallace, L.E.; Cadden, J.A.; Cadden, S.W.; Brieger, G.H. The History of Pain; Harvard University Press: Cambridge, MA, USA, 1995.
- Ksir, C.J.; Carl, D.; Hart, L. Drugs, Society, and Human Behavior; McGraw-Hill Education: New York, NY, USA, 2017.
- Cooper, T.E.; Chen, J.; Wiffen, P.J.; Derry, S.; Carr, D.B.; Aldington, D.; Cole, P.; Moore, R.A. Morphine for chronic neuropathic pain in adults. Cochrane Database Syst. Rev. 2017, 5.
- Beltrán-Campos, V.; Silva-Vera, M.; García-Campos, M.L.; Díaz-Cintra, S. Effects of morphine on brain plasticity. Neurologia 2015, 30, 176–180.
- Manchikanti, L.; Helm, S.; Fellows, B.; Janata, J.W.; Pampati, V.; Grider, J.S.; Boswell, M.V. Opioid epidemic in the United States. Pain Physician 2012, 15, ES9–ES38.
- Dasgupta, N.; Beletsky, L.; Ciccarone, D. Opioid Crisis: No Easy Fix to Its Social and Economic Determinants. Am. J. Public Health 2018, 108, 182–186.
- Lee, M.R.; Jayant, R.D. Penetration of the blood-brain barrier by peripheral neuropeptides: New approaches to enhancing transport and endogenous expression. Cell Tissue Res. 2019, 375, 287–293.
- Scholl, L.; Seth, P.; Kariisa, M.; Wilson, N.; Baldwin, G. Drug and Opioid-Involved Overdose Deaths-United States, 2013-2017. MMWR Morb. Mortal Wkly. Rep. 2018, 67, 1419–1427.
- Nelson, L.S.; Juurlink, D.N.; Perrone, J. Addressing the Opioid Epidemic. JAMA 2015, 314, 1453–1454.
- Odegaard, K.E.; Chand, S.; Wheeler, S.; Tiwari, S.; Flores, A.; Hernandez, J.; Savine, M.; Gowen, A.; Pendyala, G.; Yelamanchili, S.V. Role of Extracellular Vesicles in Substance Abuse and HIV-Related Neurological Pathologies. Int. J. Mol. Sci. 2020, 21, 6765.
- Pacifici, G.M. Metabolism and pharmacokinetics of morphine in neonates: A review. Clinics (Sao Paulo) 2016, 71, 474–480.
- Chen, M.; Zhao, Y.; Yang, H.; Luan, W.; Song, J.; Cui, D.; Dong, Y.; Lai, B.; Ma, L.; Zheng, P. Morphine disinhibits glutamatergic input to VTA dopamine neurons and promotes dopamine neuron excitation. Elife 2015, 4.
- NIDA. Heroin DrugFacts; National Institute on Drug Abuse Website. Available online: https://www.drugabuse.gov/publications/drugfacts/heroin (accessed on 21 November 2019).
- Chahl, L.A. Opioids-mechanisms of action. Exp. Clin. Pharmacol. 1996, 19.
- Freye, E. Opioids in Medicine: A Comprehensive Review on the Mode of Action and the Use of Analgesics in Different Clinical Pain States; Springer Science & Business Media: Dordrecht, The Netherlands, 2008.