| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ana I. Flores | + 3606 word(s) | 3606 | 2021-01-04 09:25:49 | | | |
| 2 | Vicky Zhou | Meta information modification | 3606 | 2021-01-13 02:25:40 | | | | |
| 3 | Paz de la Torre | + 4 word(s) | 3610 | 2021-01-14 12:06:37 | | | | |
| 4 | Paz de la Torre | + 4 word(s) | 3610 | 2021-01-14 12:15:58 | | |
Video Upload Options
Perinatal tissues refer to tissues that are discarded at birth, such as the placenta, umbilical cord, cord blood, and amniotic fluid, and different stem and progenitor cell types can be isolated from these tissues. Regenerative medicine has found in the perinatal medical wastes one of the most promising sources of various cells and tissues for use in cell therapy and tissue engineering, both in experimental and clinical settings. The primary source of perinatal stem cells is cord blood. Cord blood has been a well-known source of hematopoietic stem/progenitor cells. Other perinatal tissues contain non-hematopoietic cells with potential therapeutic value. Indeed, in advanced perinatal cell therapy trials, mesenchymal stromal cells are the most commonly used.
1. Introduction
The overall interest in stem cells is due to their specific biological nature and their therapeutic potential, since they can divide and self-renew without limits to maintain their population and develop into many different cell types promoting the repair response of damaged tissues. The primary source of perinatal stem cells is cord blood. Cord blood has been a well-known source of hematopoietic stem/progenitor cells since 1974. Biobanked cord blood has been used to treat different hematological and immunological disorders for over 30 years. In addition to hematopioietic cells, non-hematopoietic cells with potential therapeutic value can be isolated from the perinatal tissues that are routinely discarded as medical waste. In fact, in advanced perinatal cell therapy trials, the most used are mesenchymal stem cells (MSC). The placenta is a complex and temporary organ that forms the interface between the fetus and the mother responsible for fetal development and which ceases to function at week 40 of pregnancy. The two main components of the placenta, the fetal and the maternal, must interact efficiently to achieve a healthy pregnancy. The main functions of the placenta are to ensure the supply of nutrients to the fetus, remove metabolic products and prevent immune rejection to the conceptus. The placenta also has major endocrine functions and acts as a selective barrier protecting the fetus from maternal and environmental stressors, such as maternal hormones, xenobiotics, pathogens, and parasites. Different stem and progenitor cell types can be isolated from the different parts of the placenta making it a particularly interesting tissue for regenerative medicine. The umbilical cord (UC) that attaches the embryo to the placenta, guaranteeing the continuous supply of nutrients and oxygen to the fetus, is also an important source of mesenchymal stem cells (MSC) forming the mucoid connective tissue surrounding the umbilical vessels named Wharton’s jelly. Amniotic fluid protects a developing fetus in the uterus and contains a heterogeneous cell population according to their morphology and growth, as well as in vitro biochemical characteristics and in vivo potential.
To get clinical benefit from the variety of stem cells in the perinatal environment, it is essential to study and define the most suitable cell to apply to a specific treatment, characterizing its safety and its ability to repair, replace or restore the biological function of the damaged tissue or organ. Many reviews collect knowledge about the characteristics of the different perinatal stem cells and there are also numerous clinical trials using perinatal-derived cells in a variety of diseases based on the benefits found in the use of these cells in preclinical models of human diseases [1]. The ClinicalTrials.gov (http://www.clinicaltrial.gov) and the EU Clinical Trials Register (http://www.clinicaltrialsregister.eu) are two of the twelve international trial registries where clinical trials of advanced cell therapies using perinatal cells can be found [2]. Although some of the completed trials have not yet published results, several others have demonstrated the safety and therapeutic benefits of perinatal stem cells.
2. Stem Cells in the Perinatal Tissues
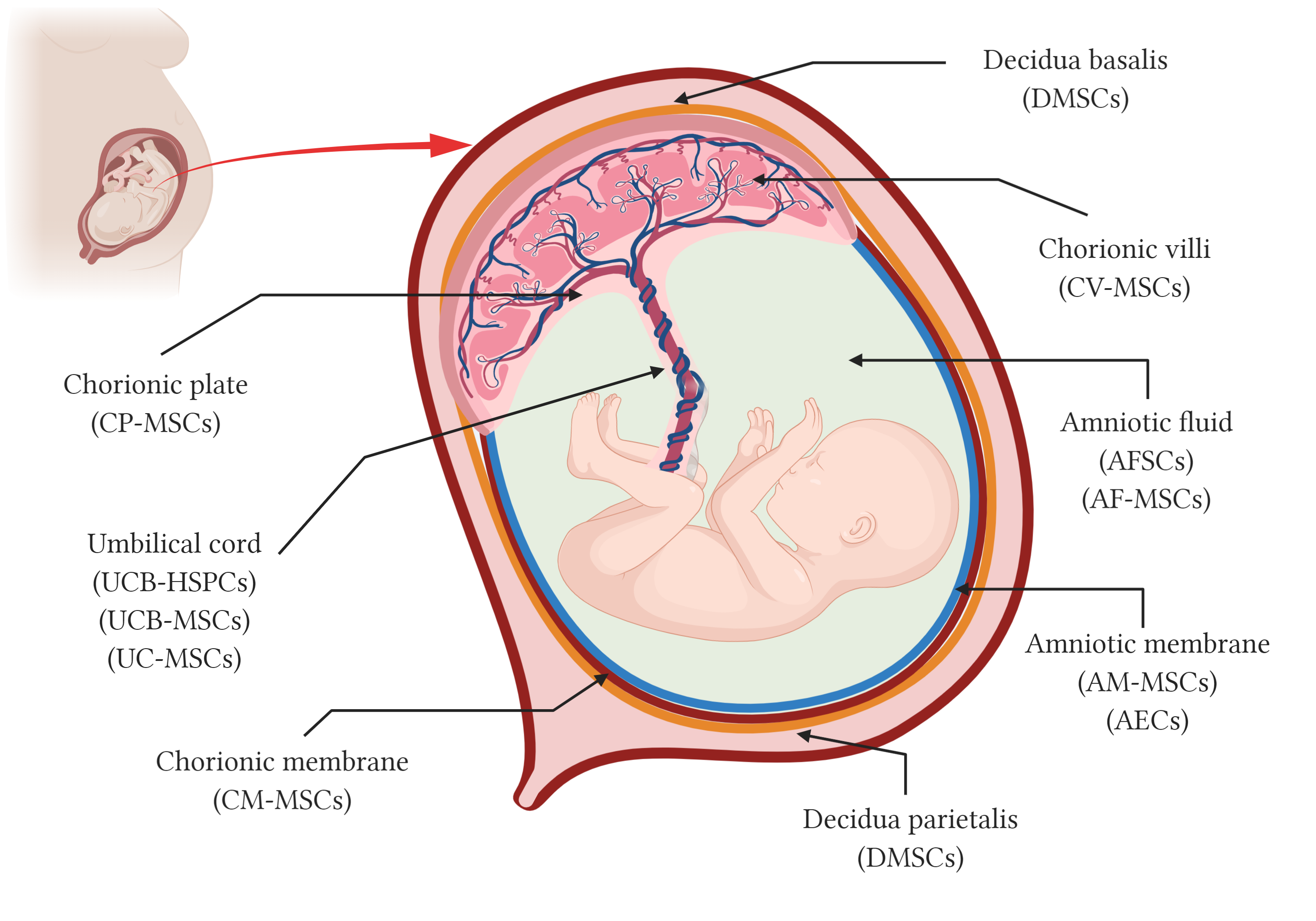
Although umbilical cord blood has been used in transplants for over 30 years, the use of the placenta and the fetal annexes as a source of stem cells started around 10–15 years ago. In addition to the hematopoietic stem cells from the cord blood (HSC), different types of stem cells as epithelial stem cells, trophoblasts and mesenchymal stromal cells (MSC) can be isolated from perinatal structures including the amniotic and the chorionic membranes, the chorionic villi, the chorionic plate and the decidua, in the placenta, the umbilical cord, and the amniotic fluid (Figure 1). The most well-known perinatal cell types are perhaps the hematopoietic stem cells (HSC) from umbilical cord blood and mesenchymal stromal cells (MSC) isolated from umbilical cord blood and tissue, also known as Wharton’s jelly. The amniotic membrane that covers the placenta and the umbilical cord has a mixture of MSC and epithelial stem cells. Other parts of the placenta such as chorion membrane and the decidua, and even the amniotic fluid, are all rich sources of stem and progenitor cells, collectively known as perinatal cells [3].
Figure 1. Schematic representation of perinatal tissues and perinatal stem cells. Anatomy of the human term placenta and its fetal annexes representing the main regions from which different types of perinatal stem cells have been isolated. AMSCs, amniotic membrane mesenchymal stromal cells; AEC, amniotic membrane epithelial cells; CMSCs, chorionic membrane mesenchymal stromal cells; CP-MSCs, chorionic plate mesenchymal stem cells; CV-MSCs, chorionic villi mesenchymal stromal cells; AFC, amniotic fluid cells; AFSC, amniotic fluid stem cells; AF-MSC, amniotic fluid mesenchymal stromal cells; UCB-HSPC, umbilical cord blood hematopoietic stem/progenitor cells; UCB-MSC, umbilical cord blood mesenchymal stromal cells; UC-MSCs, umbilical cord mesenchymal stromal cells; and DMSC, decidua-derived mesenchymal stromal cells. Created with BioRender.com.
2.1. Amniotic Fluid
Amniotic fluid (AF) contains stem cells that can be isolated and used in the future for clinical therapeutic purposes. AF is harvested in the second trimester of pregnancy, between the fifteenth and nineteenth week of gestation, during routine amniocentesis for prenatal diagnosis testing. The remaining sample is used for cell stem cell isolation (Figure 1). AF contains a heterogeneous cell population according to their morphologies and growth, in vitro biochemical characteristics and in vivo potential. AF mainly includes three types of cells: epithelioid (E) type cells derived from fetal skin and urine, amniotic fluid (AF) type derived from the fetal membranes and trophoblast, and fibroblastic (F) type cells derived from fibrous connective tissues and dermal fibroblasts [4], which vary proportionately in line with gestational age [5]. Based on plastic adherence, two populations of amniotic fluid cells can be isolated: the amniotic fluid mesenchymal stem cells (AFMSC) and the amniotic fluid stromal cells (AFSC).
AF-MSC are isolated from the second and third trimester AF and present characteristics of MSC. The differentiation potential of AFMSCs includes the mesodermal lines of adipocytes and osteocytes, as well as neuronal cells [6][7][8].
AFSCs are a population of multipotent stem cells able to differentiate into mesoderm (bone, fat, cartilage, muscle, hematopoietic), endodermal (endothelial, hepatic) and ectodermal lineages (neuronal) [9][10][11][12][13]. AFSC have already demonstrated therapeutic potential for cardiovascular (ischemia-reperfusion injury, myocardial infarction), gastrointestinal (necrotizing enterocolitis), hematopoietic (congenital hematological diseases), musculo-skeletal (muscular dystrophy, regenerate bone in collagen alginate scaffolds), neurological (Krabbe globoid leukodystrophy, traumatic brain/ nerve injury, stroke, in utero treatment of spina bifida), respiratory (hyperoxia lung injury, lung hypoplasia) and urinary disorders (acute tubular necrosis, Alport syndrome) [14][15].
2.2. Amniotic Membrane
The amniotic membrane (AM) is the inner layer of the amniotic sac or extra-embryonic fetal membranes and is composed of three layers: an epithelial monolayer, an acellular basement layer, and a mesenchymal cell layer (Figure 1). AM is usually collected at term pregnancies after birth. AM includes two cell types, the amniotic membrane mesenchymal stromal cells (AMSC) and the amniotic epithelial cells (AEC) derived from the amniotic mesenchymal and the amniotic epithelial layers, respectively [16][17].
Besides differentiating into the characteristic mesodermal lineages (osteogenic, chondrogenic, adipogenic), AMSC have the ability to differentiate into other cell types, such as neural and glial cells, skeletal muscle cells, cardiomyocytes, pancreatic and hepatic cells [18]. AM-MSCs have been used to treat lung fibrosis [19] and musculoskeletal disorders [20].
AEC are multipotent cells with the capacity to differentiate toward cells of the three germ layers [21]. AEC efficiently differentiate in vitro into osteocytes, adipocytes, cardiomyocytes, and myocytes (mesodermal), pancreatic and hepatic cells (endodermal), neural, and astrocytic cells (ectodermal). The therapeutic effects of AEC have been studied in a broad variety of pathologies including ocular diseases [22], lung fibrosis [19], familial hypercholesterolaemia [23], cardiovascular pathologies [24], liver fibrosis [25], musculoskeletal disorders [26], and neurological diseases such as spinal cord injuries [27], Parkinson's disease [28], traumatic brain injury [29] and multiple sclerosis [30].
In addition, intact human AM is a biocompatible scaffold with adequate mechanical properties, low immunogenicity, and anti-inflammatory, anti-microbial, and anti-fibrotic properties [31]. It has been explored for a variety of clinical applications such as skin wounds [32], endometrial fibrosis [33], reconstruction of the oral cavity [34], and ocular diseases [35].
2.3. Chorionic Membrane
The chorionic membrane (CM) is the outer layer of the human extra-embryonic fetal membranes and connects the fetus to the maternal tissues (Figure 1). The CM is in close contact with the decidua and is separated from the amniotic membrane by a spongy layer of collagen fibers. The CM is composed of two layers: a mesenchymal layer and a trophoblastic layer from where MSCs from the chorionic membrane (CMSC) can be isolated [36][37]. CMSC have the multipotent capacity to differentiate into mesodermal (adipocytes, osteocytes), endodermal (pancreatic-like cells), and ectodermal (neuronal-like cells) lineage cells [38]. In recent years, CM has also been explored for use as a bioactive scaffold alone or together with AM in tissue engineering and regenerative medicine strategies for wound healing, burns, bone, and vascular diseases [39].
2.4. Chorionic Plate
The chorionic plate is made up of the amniochorionic membrane and the fetal vessels (Figure 1). The stem cells are isolated from the closest region to the umbilical cord once the amniotic membrane is removed and the isolated cells have a mesenchymal type phenotype and known as chorionic plate MSC (CP-MSC) [40]. CP-MSC are able to differentiate into adipogenic, osteogenic, chondrogenic, and hepatogenic lineages. The therapeutic effect of CP-MSC has been studied in hepatic diseases [41][42], neurological disorders such as optic nerve injury [43], and ovarian dysfunction [44].
2.5. Chorionic Villi
Chorionic villi (CV) are finger-like projections that sprout from the chorion, and together with the maternal tissue of the basal plate form the placenta (Figure 1). CV is a source of cells with a typical morphology and phenotype of multipotent mesenchymal stromal cells (CV-MSC) [25]. CV-MSC differentiate toward adipocytes, osteocytes, chondrocytes, neurons, and hepatocyte lineage under appropriate induction conditions [45][46]. CV-MSC have been used to prevent endothelial dysfunction associated with diabetes and cardiovascular disease [47] in an in vitro model of breast cancer [48] and in cartilage tissue engineering [49].
2.6. Umbilical Cord
The umbilical cord (UC) attaches the embryo to the placenta guaranteeing the continuous supply of nutrients and oxygen to the fetus during pregnancy (Figure 1). UC is composed of two umbilical arteries, one umbilical vein and a mucoid connective tissue surrounding the umbilical vessels (i.e., Wharton’s jelly). UC is an important source of both hematopoietic stem/progenitor cells (HSPC) and mesenchymal stromal cells (MSC) [50][51][52].
HSPC are multipotent cells that have self‐renewal capacity and the ability to differentiate into all the different blood cell types (i.e., white blood cells, red blood cells, and platelets) that comprise the blood‐forming system during the hematopoiesis process. HSPC from UCB have been widely used in clinical settings for the treatment of severe hematological disorders, such as leukemia and Wiskott–Aldrich syndrome, and for regeneration of healthy blood cells after chemotherapy both in family-related and family-unrelated UC blood patients [53][54].
In addition, the HSPC, MSC-like cells can be collected from UCB although they are present at very low frequency [55][56][57][58]. In fact, MSC are only successfully isolated from approximately 40% of UCB units [51]. UC blood MSCs can be differentiated into osteocytes, chondrocytes, and adipocytes [57][58].
MSC are also isolated from Wharton´s Jelly (UC-MSC), the tissue surrounding the umbilical cord vessels by enzymatic digestion or explant methods. UC-MSC are multipotent cells that can be differentiated toward cell types from all germ layers such as adipocytes, osteoblasts, chondrocytes, skeletal myocytes, cardiomyocytes, neuronal cells, hepatocyte, insulin-producing cells, endothelial cells, and germ-like cells [59][60][61][62][63][64][65][66][67][68]. UC-MSC have been extensively used in the treatment of numerous pathologies such as autoimmune diseases, immunologic post-transplant complications, lung injury, cardiovascular diseases, liver pathologies, musculoskeletal disorders, diabetes mellitus, and neurodegenerative disorders (reviewed in [69]).
2.7. Decidua
The decidua is the maternal component of placental tissues and is divided into three regions: the decidua basalis that originates at the site of embryo implantation, the decidua capsularis that encloses the embryo, and the decidua parietalis that covers the rest of the uterus and fuses with the decidua capsularis by the fourth month of pregnancy (Figure 1). Both decidua basalis and decidua parietalis are a source of MSCs known as decidua-derived mesenchymal stromal cells (DMSC) [70][71]. DMSC are multipotent cells that can be differentiated in vitro toward multiple cell types from all germ layers such as adipocytes, osteoblasts, chondrocytes, skeletal and cardiac myocytes, neuronal cells, hepatocytes and pulmonary cells [70][72][73]. DMSC have been used to treat breast cancer affecting their growth and development [74], in multiple sclerosis modulating the clinical course decreased inflammatory infiltration of the central nervous system [75], in diabetes protecting endothelial cells from the toxic effects of high glucose [76], and in preeclampsia reducing inflammation, tissue damage and blood pressure [77].
3. Immunological Properties of Perinatal Stem Cells
A clear advantage of the use of perinatal derived cells in regenerative medicine lies in their low immunogenicity. It is known that the placenta plays an important role during pregnancy by modulating the maternal immune system and offering immunological protection to the fetus, and perinatal stem cells are in a state of immune tolerance. Perinatal stem cells do not express HLA class II antigens (HLA-DR) or the co-stimulatory molecules CD40, CD80, and CD86 that are required for T cell activation [78][79]. However, HLA-DR expression increases after in vitro stimulation with IFN-ɣ or when cultured without serum. In addition, perinatal stem cells express HLA-G, a non-classical MHC class I molecule, which is known to inhibit natural killer (NK) cells and CD8+T CD4+T cell proliferation [80]. HLA-G expression is also induced by IFN-g on perinatal stem cells [81], although the precise role of IFN-g on its immunomodulatory functions is still unclear.
Besides affecting the innate immune response, perinatal stem cells also affect the adaptive immune system and show potent immunosuppressive properties. Perinatal stem cells suppress the in vitro proliferation differentiation, and the function of immune cells such as T cells, dendritic cells (DC), and NK cells. This ability to suppress immune cells was observed in a cell–cell contact, in a trans-well system and using conditioned media suggesting that the immunomodulatory activity of perinatal stem cells is provided by a paracrine mechanism [75][82]. Several molecules are involved in the paracrine effect of perinatal stem cells which include the secretion of prostaglandin E2 indoleamine 2, 3-dioxygenase, NO, transforming growth factor-1, hepatocyte growth factor, and leukemia inhibitory factor, insulin like growth factor, and interleukin IL-10 [83][78]. The low immunogenicity and immunomodulatory properties of perinatal stem cells encourages their use in allogeneic clinical applications and in inflammatory and autoimmune diseases.
4. Nanotechnology for Perinatal Stem Cells
Nanotechnology used to treat diseases and prevent health issues is called Nanomedicine [84]. In the continuous search for a successful regenerative medicine, nanomedicine linked to stem cell-based strategies appears as a useful tool capable of improving the replacement of injured or damaged tissues. In the field of stem cells and regenerative medicine, nanotechnology based approaches have been developed to control the differentiation process, to label and track transplanted cells, to improve the stem cell regenerative process and to facilitate drug delivery [85][86]. Several scaffolds based on hydrogels, nanofibers, nanotubes and nanoparticles (NPs) have been used to control stem cell proliferation and differentiation.
Chorion-derived MSCs grown and differentiated over gold-coated collagen nanofibers (GCNFs) showed a significant increase in proliferation and a more advanced differentiated state for neuronal and cardiac differentiation, compared to control without substrate. The differentiation could be further accelerated by electrical stimulation due to the characteristics of these electrically conductive GCNFs [87]. Hydrogel matrix prepared by polymerizing carbon nanotubes into collagen type I supported the differentiation of human decidua parietalis stem cells into neural cells serving as a tool for future applications to obtain mature neurons at the site of injury [88]. AFC cultured in unmodified hydrogel-based scaffolds showed high levels of osteogenic differentiation, whereas AFC cultured over hydrogels coated with extracellular matrix (ECM)-derived oligopeptides maintained the pluripotency suggesting that interactions with ECM are important to support or inhibit the differentiation ability of ASC [89]. The treatment of bone loss and nonunion fractures is still a great challenge to achieve. UC-MSC showed increased osteogenesis and angiogenesis capacity in vivo when seeded in a recently developed nanocomposite scaffold made of a bioactive glass/gelatin mixture to treat critical size calvarial defects [90]. The combination of nanotechnology and perinatal-derived MSC could be a relevant and highly promising research field and provide significant contributions in the area of the musculoskeletal disorders.
The field of nanomedicine is now focusing on developing nanocarriers for targeted drug delivery combined with on-site drug release. Achieving targeted delivery of medication will improve efficacy and reduce side effects on non-target tissues. NPs have been widely investigated as carriers for targeted drug delivery to treat cancer. However, nanotechnology has not yet achieved a long tern stability of the nanoparticles, nor a specific localization in tumor tissues. Human MSC from the decidua of the human placenta (DMSC) have been observed migrating toward tumors in a preclinical model of breast cancer [74]. This characteristic makes them excellent cellular vehicles for drug-loaded nanoparticles that could also be modified to have a controlled release of the payload to avoid side effects and the premature death of the carrier cells [91][92]. Different strategies can also be used to load the nanoparticles with both, genes and drugs to improve therapeutic strategy and these nanoparticles and perinatal MSC can be used as transporters to release the therapeutic molecule on-demand [93][94].
For the use of perinatal cells in cell therapy applications there is a lack of reliable methods to monitor their biodistribution and pharmacokinetics once transplanted. There are only a few studies in which these parameters are investigated in perinatal cells, such as the use of fluorescent nanodiamonds to trace and quantify the biodistribution of MSC derived from the choriodecidual membrane of human placenta in miniature pigs [95]; or the use of polyethylene glycol-coated superparamagnetic iron oxide nanoparticles to label the placenta-MSC and to track their migration and distribution pattern into a clinically relevant glioblastoma mouse model [96].
In the long term, additional studies are necessary to provide insights into how research findings related to nanotechnology-based therapies can be applied to the use of perinatal derived stem cells. It is expected that new and exciting nanotechnology-perinatal stem cell platforms will arise.
5. Future Directions and New Prospects
The rapid advances in basic stem cell research using perinatal-derived cells and several reports of effective cell-based interventions in animal models of human disease have created high expectations for their use in regenerative medicine and cell therapies. There are many questions to be resolved to help the understanding of the role of perinatal stem cells in the human body and different therapeutic strategies have been used to treat diseased, injured, or aged tissues. However, there are many obstacles when translating in vitro science to the in vivo preclinical environment in order to take full advantage of their effect later in clinical settings [97].
Although some advances in the understanding of MSC biology have been made, several questions regarding the use of some of the cells derived from perinatal tissues have to be resolved before their clinical use. The most frequently perinatal-derived cells used in humans are UC-MSC, and MSC have different functional properties depending on how they are isolated, expanded, and administered [98]. A deeper understanding of the characteristics, properties, and function of the different stem cells derived from the perinatal tissues would help to decide which to use to treat each particular disease.
It is also important to reach a better understanding of their paracrine mechanisms of action and their immunomodulatory properties. In addition, it is essential to determine the best culture conditions for their isolation and expansion under good manufacturing practices (GMP), the cell dose to use, and the regimen treatment for clinical approaches all requiring authorization from the European Medicines Agency (EMA) in Europe and the FDA in the United States. It is now well known that placental-derived stem cells exert their effects mostly due to paracrine mediators which act on endogenous cells to induce tissue repair and/or regeneration. Therefore, it is essential to identify which molecules are responsible for their therapeutic effects, as a cell-free treatment would be possible and would have several advantages such as safety concerns of cell transplantation and would also promote the differentiation of quiescent resident stem cells into the injured tissue. The therapeutic mechanisms of the different types of stem cells isolated from the placenta are poorly understood.
Umbilical cord blood has been used and biobanked to treat hematological disorders for over 30 years. Recently, other perinatal stem cells have been used in patients in several indications including acute myocardial infarction, stroke, cancer, rheumatoid arthritis, and most recently to treat COVID-19 pneumonia with 26 registered clinical trials. Given that most clinical trials testing advanced perinatal cell therapies in humans are at an early stage, it is easy to think that this field is set to grow rapidly in the years to come. It is to be expected that UCB biobanks will also be important in the growing field of advanced perinatal cell therapies and will help to ensure their effectiveness and safety by having perinatal cells reach the clinic maintaining their properties and biological function.
The combination of nanotechnology and perinatal stem cell research is a new and highly promising field that could have a significant impact on human healthcare. However, the use of perinatal-derived MSCs in combination with nanoengineered devices and structures for cell therapy and tissue regeneration is still in its infancy, and more intensive in vivo and in vitro research is still required to be applied in humans.
References
- De la Torre, P.; Pérez-Lorenzo, M.J.; Flores, A.I. Human Placenta-Derived Mesenchymal Stromal Cells: A Review from Basic Research to Clinical Application. In Stromal Cells—Structure, Function, and Therapeutic Implications; Valarmathi, M.T., Ed.; IntechOpen: Rijeka, Italy, 2018.
- Couto, P.S.; Bersenev, A.; Verter, F. The first decade of advanced cell therapy clinical trials using perinatal cells (2005–2015). Regen. Med. 2017, 12, 953–968.
- Silini, A.R.; Masserdotti, A.; Papait, A.; Parolini, O. Shaping the Future of Perinatal Cells: Lessons From the Past and Interpretations of the Present. Front. Bioeng. Biotechnol. 2019, 7, 75.
- Silini, A.R.; Di Pietro, P.; Lang, I.; Alviano, F.; Banerjee, A.; Basile, M.; Borutinskaitė, V.V.; Eissner, G.; Gellhaus, A.; Giebel, B.; et al. Perinatal derivatives: Where do we stand? A roadmap of the human placenta and consensus for tissue and cell nomenclature. Front. Bioeng. Biotechnol. 2020. Provisionally accepted.
- Hoehn, H.; Bryant, E.M.; Fantel, A.G.; Martin, G.M. Cultivated cells from diagnostic amniocentesis in second trimester pregnancies. III. The fetal urine as a potential source of clonable cells. Humangenetik 1975, 29, 285–290.
- Moraghebi, R.; Kirkeby, A.; Chaves, P.; Ronn, R.E.; Sitnicka, E.; Parmar, M.; Larsson, M.; Herbst, A.; Woods, N.B. Term amniotic fluid: An unexploited reserve of mesenchymal stromal cells for reprogramming and potential cell therapy applications. Stem Cell Res. Ther. 2017, 8, 190.
- Tsai, M.S.; Lee, J.L.; Chang, Y.J.; Hwang, S.M. Isolation of human multipotent mesenchymal stem cells from second-trimester amniotic fluid using a novel two-stage culture protocol. Hum. Reprod. 2004, 19, 1450–1456.
- Bossolasco, P.; Montemurro, T.; Cova, L.; Zangrossi, S.; Calzarossa, C.; Buiatiotis, S.; Soligo, D.; Bosari, S.; Silani, V.; Deliliers, G.L.; et al. Molecular and phenotypic characterization of human amniotic fluid cells and their differentiation potential. Cell Res. 2006, 16, 329–336.
- Roubelakis, M.G.; Pappa, K.I.; Bitsika, V.; Zagoura, D.; Vlahou, A.; Papadaki, H.A.; Antsaklis, A.; Anagnou, N.P. Molecular and proteomic characterization of human mesenchymal stem cells derived from amniotic fluid: Comparison to bone marrow mesenchymal stem cells. Stem Cells Dev. 2007, 16, 931–952.
- Tsai, M.S.; Hwang, S.M.; Tsai, Y.L.; Cheng, F.C.; Lee, J.L.; Chang, Y.J. Clonal amniotic fluid-derived stem cells express characteristics of both mesenchymal and neural stem cells. Biol. Reprod. 2006, 74, 545–551.
- Zagoura, D.S.; Trohatou, O.; Bitsika, V.; Makridakis, M.; Pappa, K.I.; Vlahou, A.; Roubelakis, M.G.; Anagnou, N.P. AF-MSCs fate can be regulated by culture conditions. Cell Death Dis. 2013, 4, e571.
- Savickiene, J.; Treigyte, G.; Baronaite, S.; Valiuliene, G.; Kaupinis, A.; Valius, M.; Arlauskiene, A.; Navakauskiene, R. Human Amniotic Fluid Mesenchymal Stem Cells from Second- and Third-Trimester Amniocentesis: Differentiation Potential, Molecular Signature, and Proteome Analysis. Stem Cells Int. 2015, 2015, 319238.
- De Coppi, P.; Bartsch, G., Jr.; Siddiqui, M.M.; Xu, T.; Santos, C.C.; Perin, L.; Mostoslavsky, G.; Serre, A.C.; Snyder, E.Y.; Yoo, J.J.; et al. Isolation of amniotic stem cell lines with potential for therapy. Nat. Biotechnol. 2007, 25, 100–106.
- Loukogeorgakis, S.P.; De Coppi, P. Concise Review: Amniotic Fluid Stem Cells: The Known, the Unknown, and Potential Regenerative Medicine Applications. Stem Cells 2017, 35, 1663–1673.
- Srivastava, M.; Ahlawat, N.; Srivastava, A. Amniotic Fluid Stem Cells: A New Era in Regenerative Medicine. J. Obstet. Gynecol. India 2018, 68, 15–19.
- Moore, R.; Silver, R.; Moore, J. Physiological apoptotic agents have different effects upon human amnion epithelial and mesenchymal cells. Placenta 2003, 24, 173–180.
- Casey, M.L.; Macdonald, P.C. Interstitial collagen synthesis and processing in human amnion: A property of the mesenchymal cells. Biol. Reprod. 1996, 55, 1253–1260.
- Manuelpillai, U.; Moodley, Y.; Borlongan, C.V.; Parolini, O. Amniotic membrane and amniotic cells: Potential therapeutic tools to combat tissue inflammation and fibrosis? Placenta 2011, 32 (Suppl. 4), S320–S325.
- Cargnoni, A.; Gibelli, L.; Tosini, A.; Signoroni, P.B.; Nassuato, C.; Arienti, D.; Lombardi, G.; Albertini, A.; Wengler, G.S.; Parolini, O. Transplantation of allogeneic and xenogeneic placenta-derived cells reduces bleomycin-induced lung fibrosis. Cell Transplant. 2009, 18, 405–422.
- Liu, D.; Hui, H.; Chai, X.; Wang, B.; Qiu, J. Construction of tissue-engineered cartilage using human placenta-derived stem cells. Sci. China Life Sci. 2010, 53, 207–214.
- Ilancheran, S.; Michalska, A.; Peh, G.; Wallace, E.M.; Pera, M.; Manuelpillai, U. Stem cells derived from human fetal membranes display multilineage differentiation potential. Biol. Reprod. 2007, 77, 577–588.
- Nakamura, T.; Inatomi, T.; Sotozono, C.; Ang, L.P.; Koizumi, N.; Yokoi, N.; Kinoshita, S. Transplantation of autologous serum-derived cultivated corneal epithelial equivalents for the treatment of severe ocular surface disease. Ophthalmology 2006, 113, 1765–1772.
- Takahashi, S.; Ohsugi, K.; Yamamoto, T.; Shiomi, M.; Sakuragawa, N. A novel approach to ex vivo gene therapy for familial hypercholesterolemia using human amniotic epithelial cells as a transgene carrier. Tohoku J. Exp. Med. 2001, 193, 279–292.
- Fujimoto, K.L.; Miki, T.; Liu, L.J.; Hashizume, R.; Strom, S.C.; Wagner, W.R.; Keller, B.B.; Tobita, K. Naive rat amnion-derived cell transplantation improved left ventricular function and reduced myocardial scar of postinfarcted heart. Cell Transpl. 2009, 18, 477–486.
- Manuelpillai, U.; Tchongue, J.; Lourensz, D.; Vaghjiani, V.; Samuel, C.S.; Liu, A.; Williams, E.D.; Sievert, W. Transplantation of human amnion epithelial cells reduces hepatic fibrosis in immunocompetent CCl(4)-treated mice. Cell Transpl. 2010, 19, 1157–1168.
- Kawamichi, Y.; Cui, C.H.; Toyoda, M.; Makino, H.; Horie, A.; Takahashi, Y.; Matsumoto, K.; Saito, H.; Ohta, H.; Saito, K.; et al. Cells of extraembryonic mesodermal origin confer human dystrophin in the mdx model of Duchenne muscular dystrophy. J. Cell Physiol. 2010, 223, 695–702.
- Sankar, V.; Muthusamy, R. Role of human amniotic epithelial cell transplantation in spinal cord injury repair research. Neuroscience 2003, 118, 11–17.
- Park, S.; Kim, E.; Koh, S.E.; Maeng, S.; Lee, W.D.; Lim, J.; Shim, I.; Lee, Y.J. Dopaminergic differentiation of neural progenitors derived from placental mesenchymal stem cells in the brains of Parkinson’s disease model rats and alleviation of asymmetric rotational behavior. Brain Res. 2012, 1466, 158–166.
- Chen, Z.; Tortella, F.C.; Dave, J.R.; Marshall, V.S.; Clarke, D.L.; Sing, G.; Du, F.; Lu, X.C. Human amnion-derived multipotent progenitor cell treatment alleviates traumatic brain injury-induced axonal degeneration. J. Neurotrauma 2009, 26, 1987–1997.
- Fisher-Shoval, Y.; Barhum, Y.; Sadan, O.; Yust-Katz, S.; Ben-Zur, T.; Lev, N.; Benkler, C.; Hod, M.; Melamed, E.; Offen, D. Transplantation of placenta-derived mesenchymal stem cells in the EAE mouse model of MS. J. Mol. Neurosci. 2012, 48, 176–184.
- Niknejad, H.; Peirovi, H.; Jorjani, M.; Ahmadiani, A.; Ghanavi, J.; Seifalian, A.M. Properties of the amniotic membrane for potential use in tissue engineering. Eur. Cell Mater. 2008, 15, 88–99.
- Song, M.; Wang, W.; Ye, Q.; Bu, S.; Shen, Z.; Zhu, Y. The repairing of full-thickness skin deficiency and its biological mechanism using decellularized human amniotic membrane as the wound dressing. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 77, 739–747.
- Chen, X.; Zhou, Y. Preventive effects of transplantation of oral mucosal epithelial cells seeded on a decellularized amniotic membrane in a model of intrauterine adhesion. Int. J. Clin. Exp. Pathol. 2018, 11, 1510–1519.
- Fenelon, M.; Catros, S.; Fricain, J.C. What is the benefit of using amniotic membrane in oral surgery? A comprehensive review of clinical studies. Clin. Oral Investig. 2018, 22, 1881–1891.
- Jirsova, K.; Jones, G.L.A. Amniotic membrane in ophthalmology: Properties, preparation, storage and indications for grafting-a review. Cell Tissue Bank. 2017, 18, 193–204.
- Portmann-Lanz, C.B.; Schoeberlein, A.; Huber, A.; Sager, R.; Malek, A.; Holzgreve, W.; Surbek, D.V. Placental mesenchymal stem cells as potential autologous graft for pre- and perinatal neuroregeneration. Am. J. Obstet. Gynecol. 2006, 194, 664–673.
- Soncini, M.; Vertua, E.; Gibelli, L.; Zorzi, F.; Denegri, M.; Albertini, A.; Wengler, G.S.; Parolini, O. Isolation and characterization of mesenchymal cells from human fetal membranes. J. Tissue Eng. Regen. Med. 2007, 1, 296–305.
- Battula, V.L.; Bareiss, P.M.; Treml, S.; Conrad, S.; Albert, I.; Hojak, S.; Abele, H.; Schewe, B.; Just, L.; Skutella, T.; et al. Human placenta and bone marrow derived MSC cultured in serum-free, b-FGF-containing medium express cell surface frizzled-9 and SSEA-4 and give rise to multilineage differentiation. Differentiation 2007, 75, 279–291.
- Deus, I.A.; Mano, J.F.; Custódio, C.A. Perinatal tissues and cells in tissue engineering and regenerative medicine. Acta Biomater. 2020, 110, 1–14.
- Huang, Q.; Yang, Y.; Luo, C.; Wen, Y.; Liu, R.; Li, S.; Chen, T.; Sun, H.; Tang, L. An efficient protocol to generate placental chorionic plate-derived mesenchymal stem cells with superior proliferative and immunomodulatory properties. Stem Cell Res. Ther. 2019, 10, 301.
- Lee, M.J.; Jung, J.; Na, K.H.; Moon, J.S.; Lee, H.J.; Kim, J.H.; Kim, G.I.; Kwon, S.W.; Hwang, S.G.; Kim, G.J. Anti-fibrotic effect of chorionic plate-derived mesenchymal stem cells isolated from human placenta in a rat model of CCl(4)-injured liver: Potential application to the treatment of hepatic diseases. J. Cell Biochem. 2010, 111, 1453–1463.
- Lee, Y.B.; Choi, J.H.; Kim, E.N.; Seok, J.; Lee, H.J.; Yoon, J.H.; Kim, G.J. Human Chorionic Plate-Derived Mesenchymal Stem Cells Restore Hepatic Lipid Metabolism in a Rat Model of Bile Duct Ligation. Stem Cells Int. 2017, 2017, 5180579.
- Chung, S.; Rho, S.; Kim, G.; Kim, S.R.; Baek, K.H.; Kang, M.; Lew, H. Human umbilical cord blood mononuclear cells and chorionic plate-derived mesenchymal stem cells promote axon survival in a rat model of optic nerve crush injury. Int. J. Mol. Med. 2016, 37, 1170–1180.
- Li, J.; Yu, Q.; Huang, H.; Deng, W.; Cao, X.; Adu-Frimpong, M.; Yu, J.; Xu, X. Human chorionic plate-derived mesenchymal stem cells transplantation restores ovarian function in a chemotherapy-induced mouse model of premature ovarian failure. Stem Cell Res. Ther. 2018, 9, 81.
- Ventura Ferreira, M.S.; Bienert, M.; Muller, K.; Rath, B.; Goecke, T.; Oplander, C.; Braunschweig, T.; Mela, P.; Brummendorf, T.H.; Beier, F.; et al. Comprehensive characterization of chorionic villi-derived mesenchymal stromal cells from human placenta. Stem Cell Res. Ther. 2018, 9, 28.
- Chien, C.C.; Yen, B.L.; Lee, F.K.; Lai, T.H.; Chen, Y.C.; Chan, S.H.; Huang, H.I. In vitro differentiation of human placenta-derived multipotent cells into hepatocyte-like cells. Stem Cells 2006, 24, 1759–1768.
- Basmaeil, Y.S.; Al Subayyil, A.M.; Khatlani, T.; Bahattab, E.; Al-Alwan, M.; Abomaray, F.M.; Kalionis, B.; Alshabibi, M.A.; AlAskar, A.S.; Abumaree, M.H. Human chorionic villous mesenchymal stem/stromal cells protect endothelial cells from injury induced by high level of glucose. Stem Cell Res. Ther. 2018, 9, 238.
- Alshareeda, A.T.; Rakha, E.; Alghwainem, A.; Alrfaei, B.; Alsowayan, B.; Albugami, A.; Alsubayyil, A.M.; Abomraee, M.; Mohd Zin, N.K. The effect of human placental chorionic villi derived mesenchymal stem cell on triple-negative breast cancer hallmarks. PLoS ONE 2018, 13, e0207593.
- Zhang, X.; Mitsuru, A.; Igura, K.; Takahashi, K.; Ichinose, S.; Yamaguchi, S.; Takahashi, T.A. Mesenchymal progenitor cells derived from chorionic villi of human placenta for cartilage tissue engineering. Biochem. Biophys. Res. Commun. 2006, 340, 944–952.
- Pipes, B.; Tsang, T.; Peng, S.-X.; Fiederlein, R.; Graham, M.; Harris, D. Telomere length changes after umbilical cord blood transplant. Transfusion 2006, 46, 1038–1043.
- Yoshioka, S.; Miura, Y.; Iwasa, M.; Fujishiro, A.; Yao, H.; Miura, M.; Fukuoka, M.; Nakagawa, Y.; Yokota, A.; Hirai, H.; et al. Isolation of mesenchymal stromal/stem cells from small-volume umbilical cord blood units that do not qualify for the banking system. Int. J. Hematol. 2015, 102, 218–229.
- Chen, G.; Yue, A.; Ruan, Z.; Yin, Y.; Wang, R.; Ren, Y.; Zhu, L. Human umbilical cord-derived mesenchymal stem cells do not undergo malignant transformation during long-term culturing in serum-free medium. PLoS ONE 2014, 9, e98565.
- Bornstein, R.; Flores, A.I.; Montalban, M.A.; del Rey, M.J.; de la Serna, J.; Gilsanz, F. A modified cord blood collection method achieves sufficient cell levels for transplantation in most adult patients. Stem Cells 2005, 23, 324–334.
- Ballen, K.K.; Gluckman, E.; Broxmeyer, H.E. Umbilical cord blood transplantation: The first 25 years and beyond. Blood 2013, 122, 491–498.
- Mareschi, K.; Biasin, E.; Piacibello, W.; Aglietta, M.; Madon, E.; Fagioli, F. Isolation of human mesenchymal stem cells: Bone marrow versus umbilical cord blood. Haematologica 2001, 86, 1099–1100.
- Wexler, S.A.; Donaldson, C.; Denning-Kendall, P.; Rice, C.; Bradley, B.; Hows, J.M. Adult bone marrow is a rich source of human mesenchymal ‘stem’ cells but umbilical cord and mobilized adult blood are not. Br. J. Haematol. 2003, 121, 368–374.
- Yang, S.E.; Ha, C.W.; Jung, M.; Jin, H.J.; Lee, M.; Song, H.; Choi, S.; Oh, W.; Yang, Y.S. Mesenchymal stem/progenitor cells developed in cultures from UC blood. Cytotherapy 2004, 6, 476–486.
- Amati, E.; Sella, S.; Perbellini, O.; Alghisi, A.; Bernardi, M.; Chieregato, K.; Lievore, C.; Peserico, D.; Rigno, M.; Zilio, A.; et al. Generation of mesenchymal stromal cells from cord blood: Evaluation of in vitro quality parameters prior to clinical use. Stem Cell Res. Ther. 2017, 8, 14.
- Baksh, D.; Yao, R.; Tuan, R.S. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells 2007, 25, 1384–1392.
- Karahuseyinoglu, S.; Cinar, O.; Kilic, E.; Kara, F.; Akay, G.G.; Demiralp, D.O.; Tukun, A.; Uckan, D.; Can, A. Biology of stem cells in human umbilical cord stroma: In situ and in vitro surveys. Stem Cells 2007, 25, 319–331.
- Conconi, M.T.; Burra, P.; Di Liddo, R.; Calore, C.; Turetta, M.; Bellini, S.; Bo, P.; Nussdorfer, G.G.; Parnigotto, P.P. CD105(+) cells from Wharton’s jelly show in vitro and in vivo myogenic differentiative potential. Int. J. Mol. Med. 2006, 18, 1089–1096.
- Kadivar, M.; Khatami, S.; Mortazavi, Y.; Shokrgozar, M.A.; Taghikhani, M.; Soleimani, M. In vitro cardiomyogenic potential of human umbilical vein-derived mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2006, 340, 639–647.
- Fu, Y.S.; Cheng, Y.C.; Lin, M.Y.; Cheng, H.; Chu, P.M.; Chou, S.C.; Shih, Y.H.; Ko, M.H.; Sung, M.S. Conversion of human umbilical cord mesenchymal stem cells in Wharton’s jelly to dopaminergic neurons in vitro: Potential therapeutic application for Parkinsonism. Stem Cells 2006, 24, 115–124.
- Fu, Y.S.; Shih, Y.T.; Cheng, Y.C.; Min, M.Y. Transformation of human umbilical mesenchymal cells into neurons in vitro. J. Biomed. Sci. 2004, 11, 652–660.
- Campard, D.; Lysy, P.A.; Najimi, M.; Sokal, E.M. Native umbilical cord matrix stem cells express hepatic markers and differentiate into hepatocyte-like cells. Gastroenterology 2008, 134, 833–848.
- Wu, L.F.; Wang, N.N.; Liu, Y.S.; Wei, X. Differentiation of Wharton’s jelly primitive stromal cells into insulin-producing cells in comparison with bone marrow mesenchymal stem cells. Tissue Eng. Part A 2009, 15, 2865–2873.
- Wu, K.H.; Zhou, B.; Lu, S.H.; Feng, B.; Yang, S.G.; Du, W.T.; Gu, D.S.; Han, Z.C.; Liu, Y.L. In vitro and in vivo differentiation of human umbilical cord derived stem cells into endothelial cells. J. Cell Biochem. 2007, 100, 608–616.
- Latifpour, M.; Shakiba, Y.; Amidi, F.; Mazaheri, Z.; Sobhani, A. Differentiation of human umbilical cord matrix-derived mesenchymal stem cells into germ-like cells. Avicenna J. Med. Biotechnol. 2014, 6, 218–227.
- Nasadyuk, C.M. Umbilical cord stem cells: Biological characteristics, approaches to banking and clinical application. Cell Organ Transplantol. 2016, 4, 230–235.
- Macias, M.I.; Grande, J.; Moreno, A.; Dominguez, I.; Bornstein, R.; Flores, A.I. Isolation and characterization of true mesenchymal stem cells derived from human term decidua capable of multilineage differentiation into all 3 embryonic layers. Am. J. Obstet. Gynecol. 2010, 203, 495.e9–495.e23.
- Abomaray, F.M.; Al Jumah, M.A.; Alsaad, K.O.; Jawdat, D.; Al Khaldi, A.; AlAskar, A.S.; Al Harthy, S.; Al Subayyil, A.M.; Khatlani, T.; Alawad, A.O.; et al. Phenotypic and Functional Characterization of Mesenchymal Stem/Multipotent Stromal Cells from Decidua Basalis of Human Term Placenta. Stem Cells Int. 2016, 2016, 5184601.
- Cerrada, A.; de la Torre, P.; Grande, J.; Haller, T.; Flores, A.I.; Perez-Gil, J. Human decidua-derived mesenchymal stem cells differentiate into functional alveolar type II-like cells that synthesize and secrete pulmonary surfactant complexes. PLoS ONE 2014, 9, e110195.
- Bornstein, R.; Macias, M.I.; de la Torre, P.; Grande, J.; Flores, A.I. Human decidua-derived mesenchymal stromal cells differentiate into hepatic-like cells and form functional three-dimensional structures. Cytotherapy 2012, 14, 1182–1192.
- Vegh, I.; Grau, M.; Gracia, M.; Grande, J.; de la Torre, P.; Flores, A.I. Decidua mesenchymal stem cells migrated toward mammary tumors in vitro and in vivo affecting tumor growth and tumor development. Cancer Gene Ther. 2013, 20, 8–16.
- Bravo, B.; Gallego, M.I.; Flores, A.I.; Bornstein, R.; Puente-Bedia, A.; Hernandez, J.; de la Torre, P.; Garcia-Zaragoza, E.; Perez-Tavarez, R.; Grande, J.; et al. Restrained Th17 response and myeloid cell infiltration into the central nervous system by human decidua-derived mesenchymal stem cells during experimental autoimmune encephalomyelitis. Stem Cell Res. Ther. 2016, 7, 43.
- Basmaeil, Y.S.; Bahattab, E.; Alshabibi, M.A.; Abomaray, F.M.; Abumaree, M.; Khatlani, T. Human Decidua Basalis mesenchymal stem/stromal cells reverse the damaging effects of high level of glucose on endothelial cells in vitro. J. Cell Mol. Med. 2020.
- Chatterjee, P.; Chiasson, V.L.; Pinzur, L.; Raveh, S.; Abraham, E.; Jones, K.A.; Bounds, K.R.; Ofir, R.; Flaishon, L.; Chajut, A.; et al. Human placenta-derived stromal cells decrease inflammation, placental injury and blood pressure in hypertensive pregnant mice. Clin. Sci. (Lond.) 2016, 130, 513–523.
- Abumaree, M.H.; Abomaray, F.M.; Alshabibi, M.A.; AlAskar, A.S.; Kalionis, B. Immunomodulatory properties of human placental mesenchymal stem/stromal cells. Placenta 2017, 59, 87–95.
- Wassmer, C.H.; Berishvili, E. Immunomodulatory Properties of Amniotic Membrane Derivatives and Their Potential in Regenerative Medicine. Curr. Diabetes Rep. 2020, 20, 31.
- Kim, M.J.; Shin, K.S.; Jeon, J.H.; Lee, D.R.; Shim, S.H.; Kim, J.K.; Cha, D.H.; Yoon, T.K.; Kim, G.J. Human chorionic-plate-derived mesenchymal stem cells and Wharton’s jelly-derived mesenchymal stem cells: A comparative analysis of their potential as placenta-derived stem cells. Cell Tissue Res. 2011, 346, 53–64.
- Liu, K.J.; Wang, C.J.; Chang, C.J.; Hu, H.I.; Hsu, P.J.; Wu, Y.C.; Bai, C.H.; Sytwu, H.K.; Yen, B.L. Surface expression of HLA-G is involved in mediating immunomodulatory effects of placenta-derived multipotent cells (PDMCs) towards natural killer lymphocytes. Cell Transplant. 2011, 20, 1721–1730.
- Magatti, M.; Caruso, M.; De Munari, S.; Vertua, E.; De, D.; Manuelpillai, U.; Parolini, O. Human Amniotic Membrane-Derived Mesenchymal and Epithelial Cells Exert Different Effects on Monocyte-Derived Dendritic Cell Differentiation and Function. Cell Transplant. 2015, 24, 1733–1752.
- Arutyunyan, I.; Elchaninov, A.; Makarov, A.; Fatkhudinov, T. Umbilical Cord as Prospective Source for Mesenchymal Stem Cell-Based Therapy. Stem Cells Int. 2016, 2016, 6901286.
- De la Torre, P.; Flores, A.I. Nanotechnology and Mesenchymal Stem Cells for Regenerative Medicine. Glob. J. Nanomed. 2017, 1, 555559.
- Yi, D.K.; Nanda, S.S.; Kim, K.; Tamil Selvan, S. Recent progress in nanotechnology for stem cell differentiation, labeling, tracking and therapy. J. Mater. Chem. B 2017, 5, 9429–9451.
- De la Torre, P.; Perez-Lorenzo, M.J.; Alcazar-Garrido, A.; Flores, A.I. Cell-Based Nanoparticles Delivery Systems for Targeted Cancer Therapy: Lessons from Anti-Angiogenesis Treatments. Molecules 2020, 25, 715.
- Orza, A.; Soritau, O.; Olenic, L.; Diudea, M.; Florea, A.; Rus Ciuca, D.; Mihu, C.; Casciano, D.; Biris, A.S. Electrically conductive gold-coated collagen nanofibers for placental-derived mesenchymal stem cells enhanced differentiation and proliferation. ACS Nano 2011, 5, 4490–4503.
- Kim, T.; Sridharan, I.; Zhu, B.; Orgel, J.; Wang, R. Effect of CNT on collagen fiber structure, stiffness assembly kinetics and stem cell differentiation. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 49, 281–289.
- Muduli, S.; Lee, H.H.; Yang, J.S.; Chen, T.Y.; Higuchi, A.; Kumar, S.S.; Alarfaj, A.A.; Munusamy, M.A.; Benelli, G.; Murugan, K.; et al. Proliferation and osteogenic differentiation of amniotic fluid-derived stem cells. J. Mater. Chem. B 2017, 5, 5345–5354.
- Kargozar, S.; Mozafari, M.; Hashemian, S.J.; Brouki Milan, P.; Hamzehlou, S.; Soleimani, M.; Joghataei, M.T.; Gholipourmalekabadi, M.; Korourian, A.; Mousavizadeh, K.; et al. Osteogenic potential of stem cells-seeded bioactive nanocomposite scaffolds: A comparative study between human mesenchymal stem cells derived from bone, umbilical cord Wharton’s jelly, and adipose tissue. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 61–72.
- Paris, J.L.; de la Torre, P.; Manzano, M.; Cabanas, M.V.; Flores, A.I.; Vallet-Regi, M. Decidua-derived mesenchymal stem cells as carriers of mesoporous silica nanoparticles. In vitro and in vivo evaluation on mammary tumors. Acta Biomater. 2016, 33, 275–282.
- Paris, J.L.; de la Torre, P.; Victoria Cabanas, M.; Manzano, M.; Grau, M.; Flores, A.I.; Vallet-Regi, M. Vectorization of ultrasound-responsive nanoparticles in placental mesenchymal stem cells for cancer therapy. Nanoscale 2017, 9, 5528–5537.
- Paris, J.L.; de la Torre, P.; Cabanas, M.V.; Manzano, M.; Flores, A.I.; Vallet-Regi, M. Suicide-gene transfection of tumor-tropic placental stem cells employing ultrasound-responsive nanoparticles. Acta Biomater. 2019, 83, 372–378.
- Bonomi, A.; Silini, A.; Vertua, E.; Signoroni, P.B.; Cocce, V.; Cavicchini, L.; Sisto, F.; Alessandri, G.; Pessina, A.; Parolini, O. Human amniotic mesenchymal stromal cells (hAMSCs) as potential vehicles for drug delivery in cancer therapy: An in vitro study. Stem Cell Res. Ther. 2015, 6, 155.
- Su, L.J.; Wu, M.S.; Hui, Y.Y.; Chang, B.M.; Pan, L.; Hsu, P.C.; Chen, Y.T.; Ho, H.N.; Huang, Y.H.; Ling, T.Y.; et al. Fluorescent nanodiamonds enable quantitative tracking of human mesenchymal stem cells in miniature pigs. Sci. Rep. 2017, 7, 45607.
- Hsu, F.T.; Wei, Z.H.; Hsuan, Y.C.; Lin, W.; Su, Y.C.; Liao, C.H.; Hsieh, C.L. MRI tracking of polyethylene glycol-coated superparamagnetic iron oxide-labelled placenta-derived mesenchymal stem cells toward glioblastoma stem-like cells in a mouse model. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. 3), S448–S459.
- Dimmeler, S.; Ding, S.; Rando, T.A.; Trounson, A. Translational strategies and challenges in regenerative medicine. Nat. Med. 2014, 20, 814–821.
- Pittenger, M.F.; Discher, D.E.; Peault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal stem cell perspective: Cell biology to clinical progress. NPJ Regen. Med. 2019, 4, 22.