
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | María Vallet-Regí | + 3291 word(s) | 3291 | 2020-12-29 09:17:55 | | | |
| 2 | Catherine Yang | Meta information modification | 3291 | 2021-01-12 11:22:24 | | |
Video Upload Options
Mesoporous silica nanoparticles (MSNs) have been widely employed as drug carriers owing to their exquisite physico-chemical properties. Mesoporous material Solid and porous material, with natural or synthetic character, with an average pore size between microporous (less than 2 nm) and macroporous (more than 50 nm). The pore structure can be ordered or not and provides an extremely high surface area in a relatively small amount of material According to IUPAC notation, the mesoporous category is midway between the pore sizes that define microporous materials, up to 2 nm, and macroporous, pore diameters greater than 50 nm. They have a large number of applications in the fields of catalysis, molecular separation, drug release or chemical sensors among others, as a result of the network of porous cavities in their internal structure. - Examples: ordered mesoporous silica materials, carbon molecular sieves, porous organic / inorganic hybrid materials and porous metal oxides.
1. Introduction
Mesoporous silica materials have been widely studied since researchers from Waseda University [1] and the Mobil Oil Corporation [2] first reported them back in the early 90s. These bulk mesoporous materials offered (a) adjustable porous structures, (b) tunable and narrow pore size distributions (2–30 nm), (c) high pore volumes (ca. 1 cm3/g), (d) high specific surface areas (up to 1500 m2/g) and (e) high silanol density that allows further functionalization [3][4]. Given their excellent physico-chemical properties, mesoporous silica materials have been widely employed in many different fields, including catalysis [5][6], energy storage [7][8], or heavy metal adsorption [9][10], among others.
In addition to those applications, the field of drug delivery has greatly benefitted from the use of this type of materials since Prof. Vallet-Regí and coworkers first reported their suitability to host and release therapeutic payloads in 2001 [11]. Given their excellent properties and promising biomedical features, scientists focused on translating such properties to the nanoscale dimension, leading to mesoporous silica-based nanoparticles (MSNs) resembling the physico-chemical characteristics found in the bulk materials and paving the way to multiple biomedical applications. Examples of these applications include bone tissue regeneration [12][13], antibacterial treatment [14][15], controlled drug delivery [16][17], gene transfection [18][19] or as templates for carbon-based biomaterials [20][21], among others. Aside from the above-mentioned biomedical applications, MSNs have been extensively employed as drug delivery carriers for cancer treatment. In this sense, research has focused on two main aspects namely, achieving on-demand drug delivery and addressing the nanoparticles specifically to cancer cells.
Because MSNs present an open porous structure, therapeutics can be easily loaded within the silica matrix. However, for the same reason, it is very easy for them to diffuse out of the pores, leading to nonspecific drug distribution and side effects. A smart approximation to overcome such drawback is the use of stimuli-responsive gatekeepers, which are molecular structures able to block the pore entrances to avoid drug leakage and open them only upon application of a specific stimulus at the tumor. The origin of the stimuli can be external (using specific equipment) or internal (due to up-/downregulated values of biomarkers). Examples of external stimuli are light, ultrasounds, or magnetic fields. Examples of internal stimuli include acid pH, overexpressed enzymes, or upregulated redox species. The study of stimuli-responsive mesoporous silica nanomaterials is beyond the scope of this review and the reader is encouraged to check out this information elsewhere [22][23][24].
2. A Step Ahead: Subcellular Cancer Cell Targeting
2.1. Targeting the Cytoplasm: Endosomal Escape
Aside from accumulating in the tumor or being preferentially internalized by tumoral cells, nanoparticles should ideally be able to release their cargo properly within the cells. However, when nanoparticles are internalized through endocytosis they may end up sequestered within the acidic endosomes and lysosomes. This may have two main consequences: (1) the acidic environment might degrade the payload and (2) membrane-impermeable and/or poorly membrane-permeable therapeutics might not be able to exert the therapeutic action. There are two main approaches for inducing the disruption of the endo-lysosome, those internally triggered and those externally triggered.
2.2. Mitochondrial Targeting
One of the main drawbacks of current antitumoral drugs, such as doxorubicin or topotecan, is the development of drug resistance, which renders the drugs useless and leads to the failure of the treatments. Given that each chemotherapeutic exerts its action on a particular organelle, delivering the drugs near such a particular organelle may help to avoid drug resistance. In this sense, researchers have paid much attention to mitochondrial targeting, as mitochondria are involved in cell apoptosis, cell metabolism, and ROS generation [25][26].
2.2.1. Triphenylphosphine Derivatives to Target Mitochondria
Because the mitochondrial membrane is negatively charged, the use of positively charged substances seems appealing for accumulating the cytotoxics within the mitochondrial matrix. In this sense, current approximations for improving mitochondrial internalization include linking a lipophilic cation (alkyltriphenylphosphonium and others) to a bioactive compound. Among them, Triphenylphosphine derivatives (TPP) are one of the most promising and widely used mitochondria-targeting ligands, especially in combination with MSNs [25][26].
Qu Q et al. reported TPP-functionalized MSNs (MSNP-PPh3) loaded with doxorubicin [27]. Co-localization of mitochondria and MSNs was demonstrated in HeLa tumoral cells by fluorescence analysis, showing the targeting efficacy. The authors also demonstrated the effective release of doxorubicin to mitochondria by MSNP-PPh3 and a successful escape from lysosomes. The DOX-loaded nanomaterial led to reduced cellular adenosine triphosphate (ATP) production and mitochondria membrane potential, indicating mitochondria dysfunction and resulting in a significant reduction in HeLa cell viability. Cai X et al. reported a new class of mitochondria-targeted three-dimensional (3D) dendritic MSN nanospheres for the delivery of high concentrations of the hydrophobic photosensitizer chlorin e6 (Ce6) in A549 lung cancer cells (Figure 1) [28]. Photodynamic therapy (PDT) in cancer presents some limitations, such as short lifetime or tumor hypoxia. To address the latter, the authors synthesized peroxidase-like nanozyme Pt NPs with ability to catalyze the conversion of intracellular H2O2 into oxygen in the tumoral cells. Then, MSNs were modified with the Pt NPs and further functionalized with TPP for mitochondrial targeting (Pt-DMSNs-TPP/Ce6). This nanocarrier induced an intracellular ROS burst in A549 cells due to the mitochondrial targeting capacity, resulting in cell apoptosis. Viability studies exhibited 80% of tumoral cell death induced by Pt-DMSN-TPP/Ce6 nanosystem upon light irradiation at 660 nm, eradicating the hypoxia problem. An alternative strategy was developed by Cheng R et al. [29] to clarify the mechanism of selenite (Na2SeO3)-mediated cancer cell death. For that purpose, the authors designed a mitochondria-targeted nanoprobe (Mito-N-D-MSN) able to monitor the changes in mitochondrial hydrogen selenide (H2Se) and superoxide anion (O2·-) changes simultaneously in HepG2 tumoral cells. This MSN-based nanodevice was loaded with two fluorescent nanoprobes and further modified with TPP. The in vitro results indicated that the mitochondrial H2Se content gradually increased, while the O2·- content remained unaffected in HepG2 cells under hypoxic environments, suggesting that the antitumoral mechanism of Na2SeO3 comprises non-oxidative stress in the real tumor microenvironment.
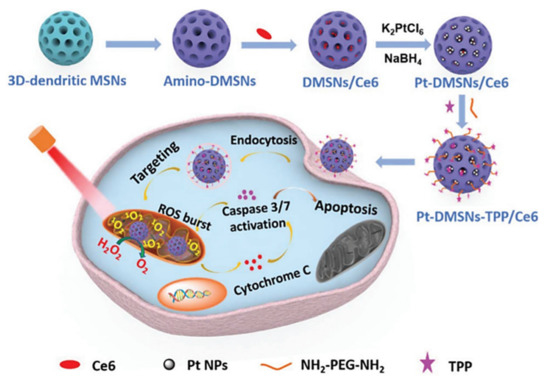
Figure 3. Schematic representation of mitochondria-targeted dendritic MSNs for photodynamic therapy. MSNs were functionalized with triphenylphosphonium (TPP) for mitochondrial targeting and loaded with the photosensitizer Ce6. Then, Pt nanoparticles showing peroxidase-like nanozyme activity were attached to the surface to catalyze the conversion of intracellular H2O2 into oxygen in the tumoral cells. Finally, Ce6 generated ROS upon light irradiation, leading to synergistic cell death. Reproduced from Ref. [28] with permission from The Royal Society of Chemistry.
Aside from endowing the MSNs with mitochondrial targeting, they can be modified to achieve sequential cell-to-organelle recognition. In this regard, Sun K et al. developed an iron oxide@SiO2 core-shell nanosystem functionalized with folate and TPP for membrane and subsequent mitochondrial targeting (Fe3O4@MSN-TPP/PEG-FA) for cancer therapy [30]. The nanocarrier was loaded with doxorubicin and the catalase inhibitor 3-amino-1,2,4-triazole (AT). Doxorubicin triggered nicotinamide adenine dinucleotide phosphate oxidases (NOXs) activation, which induced the formation of H2O2. Catalase inhibition led to the preservation of H2O2, forming hydroxyl radicals in the presence of the iron ions nearby mitochondria and exerting significant death on MGC-803 and MCF-7 cells.
A similar approximation was reported by Wang L et al., who developed TPP-functionalized MSNs coated with a folic-acid targeted, pH-controlled lipid bilayer [31]. The particles were loaded with 2,2’-azobis[2-(2-imidazolin-2-yl) propane] dihydrochloride (AIPH) and the liposomes were loaded with docetaxel, forming the final core-shell system by self-assembly throughout the liposomes hydration (AIPH/MSN-TPP@Lipo/DTX-FA). AIPH/MSN-TPP@Lipo/DTX-FA nanosystem successfully internalized in breast tumoral cells through FA receptor-mediated endocytosis. The liposomes were destabilized by the acid pH of the lysosomes and docetaxel was released. Then, the AIPH/MSN-TPP nanoparticles escaped the lysosomes and targeted mitochondria, releasing AIPH and inducing temperature-activated alkyl radicals burst (oxidative damage), showing synergistic effect both in vitro and in vivo. The selective recognition of tumoral cells can also be accomplished using hyaluronic acid to target overexpressed CD44 receptors. In this sense, Naz S et al. reported enzyme-responsive MSNs bearing TPP on the surface and further functionalized with hyaluronic acid (HA) as membrane targeting and pore capping agent [32]. HA triggered the internalization in CD44 receptor-positive cells and predominantly accumulated in mitochondria thanks to TPP. Then, HAase, which is overexpressed in cancer cells, induced the degradation of HA and triggered DOX release in MGC-803 tumoral cells, leading to significant cell death.
2.2.2. Other molecular Agents to Target Mitochondria
Even though most of the systems are based on using TPP for targeting the mitochondria, there are some other approximations. For instance, Ahn J et al. proposed the use of guanidinium derivatives (GA) as mitochondrial targeting agents (Figure 2) [33]. They designed GA-targeted, DOX-loaded Fe3O4@SiO2 nanoparticles (DOX/GA-Fe3O4@MSNs) The nanosystem showed competent mitochondria-targeting capability in a co-localization, microscopic, and fluorometric analysis. DOX/GA-Fe3O4@MSNs induced a significant decrease in HeLa tumoral cell viability while the GA-Fe3O4@MSNs did not induce any change. Remarkably, GA induced higher mitochondrial targeting than that observed for a control group of such nanoparticles functionalized with TPP, highlighting the high affinity of GA. Another approach was reported by Liu J et al., who developed mitochondria-targeted, silica-coated gold nanorods (AuNR@MSN) by functionalizing the surface with the mitochondria-targeting peptide RLA ([RLARLAR]2) [34]. The hybrid was loaded with indocyanine green for dual photodynamic and photothermal therapy and the pores were capped with β-cyclodextrin and further functionalized with RLA. In addition, a charge-reversible polymer was hosted to provide stealth property. In this context, the weak acidity in the tumor tissue could produce the dissociation of the charge-reversible polymer, exposing the RLA peptide for cell internalization and subsequent mitochondria accumulation in MCF-7 tumor cells. After 808 nm NIR light irradiation, AuNR@MSN-ICG-RLA/CS (DMA)-PEG induced tumor cell apoptosis in vitro because of ROS generation and local hyperthermia via mitochondria. These results were confirmed in vivo where this nanodevice showed antitumoral effect with minimal side effect, after specific accumulation at tumor location, improving combination therapy of PDT and PTT.
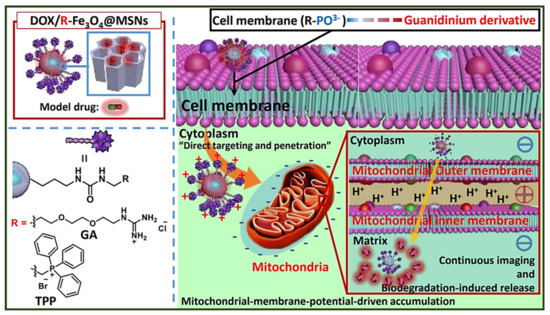
Figure 2. Schematic representation of MSNs functionalized with guanidinium derivatives for mitochondrial targeting. The GA-functionalized MSNs accumulated faster into the mitochondria, compared with the TPP derivatives. Reproduced from Ref. [33] with permission from The Royal Society of Chemistry.
2.2.3. Compounds Acting on Mitochondria
Hu J et al. [35] proposed a new approach for enhanced tumor chemotherapy based on ROS-triggered self-accelerating drug release MSNs (T/D@RSMSNs). The particles were loaded with DOX and α-tocopheryl succinate (α-TOS) and gated by PEGylated β-cyclodextrins through a ROS-cleavable thioketal linker. α-TOS is a vitamin E analog that acts as a ROS mediator after interacting with the mitochondrial respiratory complex II. T/D@RSMSNs released doxorubicin and α-TOS and induced an increase in intracellular ROS concentration in human breast cancer (MCF-7) cells, triggering the cleavage of the thioketal linker TK linkage for further doxorubicin release, both in tumoral MCF-7 cells in vitro studies and in BALB/c nude mice tumor xenograft models, decreasing tumor weight and volume, compared with others similar nanosystems based on ROS-responsive strategies.
Aside from conventional chemotherapeutics, researchers have paid attention to the encapsulation of less typical drugs with antitumoral activity. In this regard, Choi et al. developed PEGylated polyaminoacid-capped MSNs (CMSN-PEG) for celastrol delivery to cancer cells [36]. Celastrol is extracted from the traditional Chinese medicinal plant Tripterygium wilfordii and several studies have shown antitumoral effects in vitro and in vivo by targeting mitochondria. The celastrol-loaded nanosystem showed controlled drug release and induced apoptosis of SCC-7, SH-SY5Y, and BT-474 tumoral cell lines in vitro, related to decreased HIF-1α protein levels and other proteins implicated in mitochondrial apoptosis pathway. In vivo, this novel nanosystem significantly decreased solid tumor presence in SCC7 tumor-bearing xenograft model, inducing a rise in apoptotic markers and stimulating the reduction of CD31 and Ki67 proliferation markers. An alternative tumor tissue-specific drug is Umbelliferone, a natural coumarin derivative. Kundu M et al. [37] designed umbelliferone-loaded MSNs capped with pH-sensitive poly acrylic acid (PAA) and further functionalized with folic acid (Umbe@MSN-PAA-FA). Umbe@MSN-PAA-FA nanosystem induced a decrease in cell viability due to both oxidative stress and mitochondrial damage in MCF-7 cells, compared with free Umbelliferone. This nanohybrid displayed discretely drug loading capability, enhanced tumoral cell targeting, and effective pH-controlled Umbelliferone release properties. Thanks to the optimal drug bioavailability in the tumoral mass, Umbe@MSN-PAA-FA nanocarrier showed a significant decrease in tumor size on an in vivo solid tumor model in Swiss albino mice, compared with free umbelliferone, without negative effects in other vital organs.
Luo GF et al. [38] attached a TPP-modified antibiotic peptide (KLAKLAK)2 to the surface of topotecan-loaded MSNs via disulfide bonds. Then, a charge reversal polyanion poly(ethylene glycol)-blocked-2,3-dimethylmaleic anhydride-modified poly(L-lysine) (PEG-PLL(DMA)) was added to the surface via electrostatic interactions. In this manner, the DMA block degraded in the acidic tumor microenvironment, removing the outer shielding layer and triggering the uptake in increasing ubiquitous KERATIN- forming tumor cell line HeLa. Finally, the TPP-modified peptide was released from nanodevice by the overexpressed glutathione, targeting the mitochondria and showing synergistic specific cancer cell death.
An alternative new antibacterial peptide delivery strategy was designed by Cao J et al. as a cancer treatment approach [39]. Given that many antibacterial peptides lead to in hemolysis in vivo, Cao J et al. synthesized a novel antibacterial peptide RGD-hylin a1 with reduced hemolysis and loaded it within the pores of the MSNs (RGD-hylin a1-MSN). This nanocarrier released RGD-hylin a1 in a pH-controlled manner at acid pH, inducing the reduction of mitochondrial membrane potential and leading to cancer cell death, and non-effect in pH = 7. In vivo, this nanocarrier reduced (50–60%) solid tumor volume and weight after intravenous administration in tumor-bearing mice at low dosage via intravenous injection compared with control non-treated group, without inducing toxicity.
As shown in the review, MSNs have been widely engineered for targeting the mitochondria with extraordinary efficacy, increasing cancer cell death and decreasing tumor growth and size in vitro and in vivo. Nonetheless, it should be mentioned that these nanosystems also find application in other diseases, such as Alzheimer’s disease (AD). In this sense, several AD therapies have focused on amyloid-β targeted treatments, but recent studies have demonstrated that tau pathway is connected with clinical expansion of AD signs, indicating that can be a possible therapeutic target. In this regard, Chen Q et al. loaded the MSNs with the tau aggregation inhibitor methylene blue (MB) and further functionalized them with a hyperphosphorylated tau binding agent (Amino-T807) [40] as well as ultrasmall ceria nanocrystals (CeNCs) and iron oxide nanocrystals (IONCs). CeNCs exhibited high ROS activity and antioxidant properties in mitochondrial oxidative-stress-induced damage therapy in Alzheimer’s disease, acting as tau hyperphosphorylation inhibitors. Both in vitro and in vivo results showed that this complex nanocarrier decreased Alzheimer’s disease consequences, diminishing mitochondrial oxidative stress and inhibiting tau hyperphosphorylation and neuronal apoptosis, yielding a synergistically improved therapeutic alternative.
2.3. Nuclear Targeting
As previously mentioned, the problems that chemotherapeutics have to face are drug resistance and subsequent cell exocytosis. In this sense, drug-loaded nanocarriers can also be functionalized for targeting the nucleus, delivering their cargo nearby their target [26][41]. It is well-known that there are numerous pore complexes of 20-70 nm in diameter on the nuclear membrane, which are the specific passages through which substances of up to 40-60 kDa diffuse from and to the cytoplasm. Given that the passive diffusion through the nuclear envelope is restricted by the size of those complexes, nanoparticles need a nuclear localization signal or sequence (NLS), which are peptides for recognizing specific transport receptors to initiate the trans-nuclear membrane penetration process [42]. The internalization into the nucleus is mediated by importins. First, NLS peptides bind importin α and, then, importin β binds them to form a complex that finally transports the target compound into the nucleus [43].
Wu M et al. reported the synthesis of PEI-coated, TAT-targeted MSNs for nuclear transfection of a model plasmid that encoded green fluorescent protein [44]. They demonstrated that the combination of PEI + TAT provided the highest plasmid loading capacity, probably due to the highly positive surface, and protected the nucleic acids from degradation. Finally, they demonstrated the nuclear targeting and efficiently encoded the green protein without affecting the viability of the cells (Figure 3).
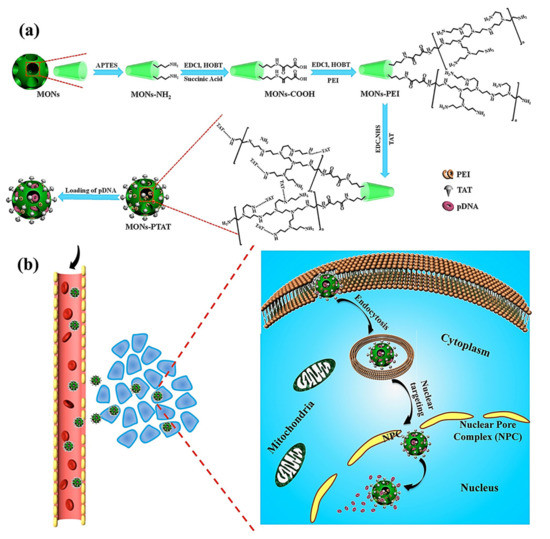
Figure 3. (a) Schematic representation of TAT-targeted MSNs for nucleic acid transfection. The plasmid was loaded into the PEI-TAT mesh, leading to enhanced nuclear accumulation and encoding green florescence protein through a model plasmid. (b) Schematic representation of the mode of action of the nanocarrier. Reproduced from Ref. [44] with permission from Wiley.
The most extended strategy involves the design of dual-targeted nanoparticles bearing a membrane and a nuclear targeting for sequential cell-to-organelle recognition. In this regard, Zhao J et al. reported MSNs whose surface was functionalized with a fluorescent derivative of the HIV-1 transactivator (NLS) peptide (TAT-FITC), for nuclear targeting, and the peptide YSA-BHQ1 for binding the EphA2 membrane receptor [43]. In addition, citraconic anhydride was used to invert the charge of the TAT peptide in neutral or weak alkaline conditions so that the positively charged YSA peptide could combine with TAT through electrostatic attraction, avoiding nonspecific TAT interactions with normal cells. The nanocarrier was successfully internalized by MCF-7 tumoral cells via YSA recognition. Then, the acid pH of the lysosomes cleaved the citraconic anhydride and triggered the lysosomal escape and subsequent nuclear translocation, where DOX was rapidly released, inducing an apoptotic effect in the cells. Wu Z et al. synthesized dual-targeted, enzyme-responsive MSNs for anticancer therapy and imaging [45]. They doped the MSNs during the synthesis with Eu3+ and Gd3+ for carrying out bioimaging. Then, TAT was attached to the surface and the particles were further coated with HA to mask the peptide as well as to provide membrane targeting and gatekeeping. The in vitro evaluation showed that HA degraded upon a lysosome HA decomposition by HAase, inducing the exposition of nuclear target TAT, releasing camptothecin into the nucleus and stimulating tumoral cell death.
Murugan C et al. developed topotecan-loaded MSNs functionalized with the TAT peptide, to which RGD-functionalized poly(acrylic acid) and citraconic anhydride-metformin were added [46]. The latter components acted as gatekeepers and as masking agents of the positively charged TAT. The nanosystem was effectively internalized by MDA-MB-231 tumoral cells via integrin targeting. The lysosomal pH triggered metformin drug release and exposed again the TAT peptide, achieving endosomal escape and nuclear translocation. Then, topotecan was successfully released into the nucleus, leading to synergistic antitumoral effect and reducing tumor growth without significant toxicity associated with mice.
Aside from using NLS peptides, the nucleus can be targeted by taking advantage of the properties of gold nanoclusters, which show unique nuclei staining properties. In this sense, Croissant et al. [47] reported the synthesis of gemcitabine/doxorubicin-loaded MSNs functionalized with BSA-coated gold nanoclusters (MSN-AuNC@BSA) acting as gatekeepers via electrostatic interactions. Drug release took place at acid pH owing to the disruption of such interactions, triggering HeLa tumoral cell death. In addition, the BSA-coated nanoclusters were released at acid pH as well, being able to stain the nuclei for cancer cell imaging. The authors showed a robust specific fluorescence of the nanocarrier in the tumors induced in mice, indicating the high value of this type nanotheranostic platform based on MSNs and gold-protein clusters in cancer therapy and diagnosis.
References
- Yanagisawa, T.; Shimizu, T.; Kuroda, K.; Kato, C. The preparation of alkyltrimethylammonium-kanemite complexes and their conversion to microporous materials. Bull. Chem. Soc. Jpn. 1990, 63, 988–992.
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712.
- Hoffmann, F.; Cornelius, M.; Morell, J.; Fröba, M. Silica-based mesoporous organic-inorganic hybrid materials. Angew. Chem. Int. Ed. 2006, 45, 3216–3251.
- Vallet-Regí, M.; Balas, F.; Arcos, D. Mesoporous materials for drug delivery. Angew. Chem. Int. Ed. 2007, 46, 7548–7558.
- Yan, Z.; Meng, H.; Shi, L.; Li, Z.; Kang, P. Synthesis of mesoporous hollow carbon hemispheres as highly efficient Pd electrocatalyst support for ethanol oxidation. Electrochem. Commun. 2010, 12, 689–692.
- Serrano, E.; Linares, N.; García-Martínez, J.; Berenguer, J.R. Sol–Gel Coordination Chemistry: Building Catalysts from the Bottom-Up. ChemCatChem 2013, 5, 844–860.
- Zhang, Y.; Zheng, S.; Zhu, S.; Ma, J.; Sun, Z.; Farid, M. Evaluation of paraffin infiltrated in various porous silica matrices as shape-stabilized phase change materials for thermal energy storage. Energy Convers. Manag. 2018, 171, 361–370.
- Mitran, R.A.; Berger, D.; Munteanu, C.; Matei, C. Evaluation of Different Mesoporous Silica Supports for Energy Storage in Shape-Stabilized Phase Change Materials with Dual Thermal Responses. J. Org. Chem. C 2015, 119, 15177–15184.
- Walcarius, A.; Mercier, L. Mesoporous organosilica adsorbents: Nanoengineered materials for removal of organic and inorganic pollutants. J. Mater. Chem. 2010, 20, 4478–4511.
- Sangvanich, T.; Morry, J.; Fox, C.; Ngamcherdtrakul, W.; Goodyear, S.; Castro, D.; Fryxell, G.E.; Addleman, R.S.; Summers, A.O.; Yantasee, W. Novel Oral Detoxification of Mercury, Cadmium, And Lead with Thiol-Modified Nanoporous Silica. ACS Appl. Mater. Interfaces 2014, 6, 5483–5493.
- Vallet-Regí, M.; Rámila, A.; Del Real, R.P.; Pérez-Pariente, J. A new property of MCM-41: Drug delivery system. Chem. Mater. 2001, 13, 308–311.
- Jia, Y.; Zhang, P.; Sun, Y.; Kang, Q.; Xu, J.; Zhang, C.; Chai, Y. Regeneration of large bone defects using mesoporous silica coated magnetic nanoparticles during distraction osteogenesis. Nanomed. Nanotechnol. Biol. Med. 2019, 21, 102040.
- Gisbert-Garzarán, M.; Lozano, D.; Vallet-Regí, M.; Manzano, M. Self-Immolative Polymers as novel pH-responsive gate keepers for drug delivery. RSC Adv. 2017, 7, 132–136.
- Colilla, M.; Izquierdo-Barba, I.; Vallet-Regí, M. The Role of Zwitterionic Materials in the Fight against Proteins and Bacteria. Medicines 2018, 5, 125.
- Vallet-Regí, M.; Lozano, D.; González, B.; Izquierdo-Barba, I. Biomaterials against Bone Infection. Adv. Healthc. Mater. 2020, 9, 2000310.
- Wang, Y.; Zhao, Q.; Han, N.; Bai, L.; Li, J.; Liu, J.; Che, E.; Hu, L.; Zhang, Q.; Jiang, T.; et al. Mesoporous silica nanoparticles in drug delivery and biomedical applications. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 313–327.
- Giret, S.; Wong Chi Man, M.; Carcel, C. Mesoporous-Silica-Functionalized Nanoparticles for Drug Delivery. Chem. A Eur. J. 2015, 21, 13850–13865.
- Slowing, I.I.; Vivero-Escoto, J.L.; Wu, C.-W.; Lin, V.S. Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Adv. Drug Deliv. Rev. 2008, 60, 1278–1288.
- Paris, J.L.; Vallet-Regí, M. Mesoporous Silica Nanoparticles for Co-Delivery of Drugs and Nucleic Acids in Oncology: A Review. Pharmaceutics 2020, 12, 526.
- Gisbert-Garzarán, M.; Berkmann, J.C.; Giasafaki, D.; Lozano, D.; Spyrou, K.; Manzano, M.; Steriotis, T.; Duda, G.N.; Schmidt-Bleek, K.; Charalambopoulou, G.; et al. Engineered pH-Responsive Mesoporous Carbon Nanoparticles for Drug Delivery. ACS Appl. Mater. Interfaces 2020.
- Huang, X.; Wu, S.; Du, X. Gated mesoporous carbon nanoparticles as drug delivery system for stimuli-responsive controlled release. Carbon N. Y. 2016, 101, 135–142.
- Moreira, A.F.; Dias, D.R.; Correia, I.J. Stimuli-responsive mesoporous silica nanoparticles for cancer therapy: A review. Microporous. Mesoporous. Mater. 2016, 236, 141–157.
- Gisbert-Garzarán, M.; Vallet-Regí, M. Influence of the Surface Functionalization on the Fate and Performance of Mesoporous Silica Nanoparticles. Nanomaterials 2020, 10, 916.
- Argyo, C.; Weiss, V.; Bräuchle, C.; Bein, T. Multifunctional Mesoporous Silica Nanoparticles as a Universal Platform for Drug Delivery Multifunctional Mesoporous Silica Nanoparticles as a Universal Platform for Drug Delivery. Chem. Mater. 2014, 26, 435–451.
- Smith, S.A.; Selby, L.I.; Johnston, A.P.R.; Such, G.K. The Endosomal Escape of Nanoparticles: Toward More Efficient Cellular Delivery. Bioconjug. Chem. 2018, 30, 263–272.
- Catalán, M.; Olmedo, I.; Faúndez, J.; Jara, J.A. Medicinal chemistry targeting mitochondria: From new vehicles and pharmacophore groups to old drugs with mitochondrial activity. Int. J. Mol. Sci. 2020, 21, 8684.
- Qu, Q.; Ma, X.; Zhao, Y. Targeted delivery of doxorubicin to mitochondria using mesoporous silica nanoparticle nanocarriers. Nanoscale 2015, 7, 16677–16686.
- Cai, X.; Luo, Y.; Song, Y.; Liu, D.; Yan, H.; Li, H.; Du, D.; Zhu, C.; Lin, Y. Integrating in situ formation of nanozymes with three-dimensional dendritic mesoporous silica nanospheres for hypoxia-overcoming photodynamic therapy. Nanoscale 2018, 10, 22937–22945.
- Cheng, R.; Kong, F.; Tong, L.; Liu, X.; Xu, K.; Tang, B. Simultaneous Detection of Mitochondrial Hydrogen Selenide and Superoxide Anion in HepG2 Cells under Hypoxic Conditions. Anal. Chem. 2018, 90, 8116–8122.
- Sun, K.; Gao, Z.; Zhang, Y.; Wu, H.; You, C.; Wang, S.; An, P.; Sun, C.; Sun, B. Enhanced highly toxic reactive oxygen species levels from iron oxide core–shell mesoporous silica nanocarrier-mediated Fenton reactions for cancer therapy. J. Mater. Chem. B 2018, 6, 5876–5887.
- Wang, L.; Niu, X.; Song, Q.; Jia, J.; Hao, Y.; Zheng, C.; Ding, K.; Xiao, H.; Liu, X.; Zhang, Z.; et al. A two-step precise targeting nanoplatform for tumor therapy via the alkyl radicals activated by the microenvironment of organelles. J. Control. Release 2020, 318, 197–209.
- Naz, S.; Wang, M.; Han, Y.; Hu, B.; Teng, L.; Zhou, J.; Zhang, H.; Chen, J. Enzyme-responsive mesoporous silica nanoparticles for tumor cells and mitochondria multistage-targeted drug delivery. Int. J. Nanomedicine 2019, 14, 2533–2542.
- Ahn, J.; Lee, B.; Choi, Y.; Jin, H.; Lim, N.Y.; Park, J.; Kim, J.H.; Bae, J.; Jung, J.H. Non-peptidic guanidinium-functionalized silica nanoparticles as selective mitochondria-targeting drug nanocarriers. J. Mater. Chem. B 2018, 6, 5698–5707.
- Liu, J.; Liang, H.; Li, M.; Luo, Z.; Zhang, J.; Guo, X.; Cai, K. Tumor acidity activating multifunctional nanoplatform for NIR-mediated multiple enhanced photodynamic and photothermal tumor therapy. Biomaterials 2018, 157, 107–124.
- Hu, J.-J.; Lei, Q.; Peng, M.-Y.; Zheng, D.-W.; Chen, Y.-X.; Zhang, X.-Z. A positive feedback strategy for enhanced chemotherapy based on ROS-triggered self-accelerating drug release nanosystem. Biomaterials 2017, 128, 136–146.
- Choi, J.Y.; Gupta, B.; Ramasamy, T.; Jeong, J.-H.; Jin, S.G.; Choi, H.-G.; Yong, C.S.; Kim, J.O. PEGylated polyaminoacid-capped mesoporous silica nanoparticles for mitochondria-targeted delivery of celastrol in solid tumors. Colloids Surfaces B Biointerfaces 2018, 165, 56–66.
- Kundu, M.; Chatterjee, S.; Ghosh, N.; Manna, P.; Das, J.; Sil, P.C. Tumor targeted delivery of umbelliferone via a smart mesoporous silica nanoparticles controlled-release drug delivery system for increased anticancer efficiency. Mater. Sci. Eng. C 2020, 116, 111239.
- Luo, G.-F.; Chen, W.-H.; Liu, Y.; Lei, Q.; Zhuo, R.-X.; Zhang, X.-Z. Multifunctional Enveloped Mesoporous Silica Nanoparticles for Subcellular Co-delivery of Drug and Therapeutic Peptide. Sci. Rep. 2014, 4, 6064.
- Cao, J.; Zhang, Y.; Shan, Y.; Wang, J.; Liu, F.; Liu, H.; Xing, G.; Lei, J.; Zhou, J. A pH-dependent Antibacterial Peptide Release Nano-system Blocks Tumor Growth in vivo without Toxicity. Sci. Rep. 2017, 7, 11242.
- Chen, Q.; Du, Y.; Zhang, K.; Liang, Z.; Li, J.; Yu, H.; Ren, R.; Feng, J.; Jin, Z.; Li, F.; et al. Tau-Targeted Multifunctional Nanocomposite for Combinational Therapy of Alzheimer’s Disease. ACS Nano 2018, 12, 1321–1338.
- Pustylnikov, S.; Costabile, F.; Beghi, S.; Facciabene, A. Targeting mitochondria in cancer: Current concepts and immunotherapy approaches. Transl. Res. 2018, 202, 35–51.
- Pan, L.; Shi, J. Chemical Design of Nuclear-Targeting Mesoporous Silica Nanoparticles for Intra-nuclear Drug Delivery. Chin. J. Chem. 2018, 36, 481–486.
- Zhao, J.; Zhao, F.; Wang, X.; Fan, X.; Wu, G. Secondary nuclear targeting of mesoporous silica nano-particles for cancer-specific drug delivery based on charge inversion. Oncotarget 2016, 7, 70100–70112.
- Wu, M.; Meng, Q.; Chen, Y.; Du, Y.; Zhang, L.; Li, Y.; Zhang, L.; Shi, J. Large-Pore Ultrasmall Mesoporous Organosilica Nanoparticles: Micelle/Precursor Co-templating Assembly and Nuclear-Targeted Gene Delivery. Adv. Mater. 2015, 27, 215–222.
- Wu, Z.-Y.; Lee, C.-C.; Lin, H.-M. Hyaluronidase-Responsive Mesoporous Silica Nanoparticles with Dual-Imaging and Dual-Target Function. Cancers 2019, 11, 697.
- Murugan, C.; Venkatesan, S.; Kannan, S. Cancer Therapeutic Proficiency of Dual-Targeted Mesoporous Silica Nanocomposite Endorses Combination Drug Delivery. ACS Omega 2017, 2, 7959–7975.
- Croissant, J.G.; Zhang, D.; Alsaiari, S.; Lu, J.; Deng, L.; Tamanoi, F.; AlMalik, A.M.; Zink, J.I.; Khashab, N.M. Protein-gold clusters-capped mesoporous silica nanoparticles for high drug loading, autonomous gemcitabine/doxorubicin co-delivery, and in-vivo tumor imaging. J. Control. Release 2016, 229, 183–191.




