| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Antonio ZUORRO | + 1754 word(s) | 1754 | 2021-01-08 07:22:02 | | | |
| 2 | Karina Chen | Meta information modification | 1754 | 2021-01-11 04:38:48 | | |
Video Upload Options
Microalgal biomass has gained a significant role in the development of dif-ferent high-end (nutraceuticals, colorants, food supplements, and pharmaceuticals) and low-end products (biodiesel, bioethanol, and biogas) due to its rapid growth and high carbon-fixing effi-ciency. Therefore, microalgae are considered a useful and sustainable resource to attain energy security while reducing our current reliance on fossil fuels. From the technologies available for obtaining biofuels using microalgae biomass, thermochemical processes (pyrolysis, Hydrothermal Liquefaction (HTL), gasification) have proven to be processed with higher viability, because they use all biomass. However, due to the complex structure of the biomass (lipids, carbohydrates, and proteins), the obtained biofuels from direct thermochemical conversion have large amounts of heteroatoms (oxygen, nitrogen, and sulfur). As a solution, catalyst-based processes have emerged as a sustainable solution for the increase in biocrude production.
1. Introduction
Biofuels are broadly classified by generations. First-generation (1st gen) biofuels are produced from food feedstock (corn, sugarcane, soybean, potato, beet, soybeans, coconut, sunflower, rapeseed, palm oil, switchgrass, Jatropha, Camelina, Cassava). Although 1st gen is considered a sustainable source of energy due to the reduction on greenhouse gas (GHG) emissions, specific details such as their competition with food supply, high requirement of government subsidies, large amounts of non-sustainable fertilizers, and environmental concerns due to the loss of biodiversity linked to the promotion of deforestation for large monoculture areas [1] hinder their true impact as a cleaner and more sustainable option over fossil fuels.
Second-generation (2nd gen) was conceived as a partial solution of several draw-backs of 1st gen biofuels. This generation relies on nonfood items such as cellulosic biomass, straw, manure, used cooking oil, and other non-conventional sources, which usually finish in landfills once their useful portion has been removed [2]. However, 2nd gen is still not industrially profitable due to biomass complexity and problems as-sociated with its production, storage, and transportation [3].
Third-generation (3rd gen) focuses on the upgrade of aquatic feedstock, such as microalgal and cyanobacterial biomass, into different fuels. Microalgae have been praised as a better solution for the energy problem due to specific qualities of algal production: (i) do not compete with human and animal food stock, (ii) harvesting can be done through the year, (iii) can employ saline and wastewater, (iv) have better growth rate than higher plants, (v) can convert up to 183 G tons of CO2 to produce 100 G tons of biomass in comparison to higher plants such as wood crops (165 G tons of CO2 to produce 100 G tons of biomass) [4], and (vi) the concentration of transforma-ble metabolites (lipids and carbohydrates) is stable in the biomass. First, the selected strain had to be cultured until it reaches the largest possible biomass concentration in the photobioreactor; once reached, the biomass is removed from the culture media (centrifugation, flocculation, filtration, and other techniques) and dried. Then, the dried biomass is ready to be used as feedstock for several biofuels (biodiesel, bioetha-nol, biogas, and so on). These different sections have been the main topic of research over the last 20 years, attracting the attention of different universities, research cen-ters, and energy companies worldwide such as Ecopetrol (Colombia), Exxon Mobile, Shell (US), Petrobras (Brazil), and Total (France).
2. How the Production of Algae-Based Biofuels Changed over Time
Several companies worldwide such as Solix biofuels, Corbion (previously known as Terravia or Solazyme), Cellana, Sapphire Energy, Seambiotic, Oil Fox, Synthetic genomics, Euglena, and others started the race for algae-based biofuels. However, after years of research, none of the companies proved the economic balance of algal-based biofuels [3]. The latter can be due to several problems identified through the last decade. First, the microalgal biodiversity is so vast that after ten years of research, we are still far from identifying the total diversity of algae and cyanobacteria [15]. Another problem related to the strains is the stability of their growth on industrial photobioreactors and the synthesis of the target metabolite [5][6].
Table 1. Different strains studied for biodiesel production.
|
Strain |
Lipids (wt %) |
Carbohydrates (wt %) |
Proteins (wt %) |
Reference |
|
Arthrospira platensis |
30.23 |
31.89 |
16.81 |
[7] |
|
Auxenochlorella protothecoides |
42 |
26 |
30 |
[8] |
|
Botryococcus braunii |
45 |
10 |
44 |
[9] |
|
60 |
20 |
18 |
[10] |
|
|
Chlamydomonas reinhardtii |
22.11 |
52.2 |
23.69 |
[11] |
|
Ch. reinhardtii CC-400 |
28.5 |
n/a |
n/a |
[12] |
|
Ch. Reinhardtii CC-4349 |
64.25 |
n/a |
n/a |
[13] |
|
Chlorella sp G-9 |
36.5 |
n/a |
n/a |
[14] |
|
C. kessleri |
20 |
18.7 |
53.8 |
[15] |
|
C. pyrenoidosa |
19.8 |
14.8 |
57.3 |
[16] |
|
C. vulgaris UTEX 259 |
28 |
35 |
20 |
[17] |
|
C. vulgaris UTEX 1803 |
12 |
36 |
41 |
[18] |
|
C. vulgaris Mutant (UV715) |
41 |
n/a |
n/a |
[19] |
|
Chlorococcum oleofaciens |
20 |
42 |
35 |
[20] |
|
Dunaliella tertiolecta |
15 |
10 |
56 |
[21] |
|
Nannochloropsis gaditana |
17.6 |
n/a |
24.1 |
[22] |
|
Pseudokirchneriella Subcapitata |
40 |
20 |
30 |
[20] |
|
Phaeodactylum tricornutum |
55.7 |
9 |
22 |
[23] |
|
Scenedesmus almeriensis |
13.1 |
n/a |
30 |
[24] |
|
S. obliquus |
32.5 |
n/a |
n/a |
[25] |
|
24.9 |
n/a |
n/a |
[26] |
|
|
35 |
22 |
32 |
||
|
Tetraselmis suecica |
9.03 |
20 |
37.27 |
[29] |
|
25.07 |
17.52 |
42.05 |
[30] |
Limited studies reported that few species of microalgae and cyanobacteria possess an inherent capacity for lipid synthesis and storage (Table 1). Initially, the studies focused on applying industrially relevant strains such as Spirulina (Arthorspira) [7], Auxenochlorella [8], Botryococcus [9][10], Chlamydomonas [11][12][13], Chlorella [14][15][16][17][18][19], Dunaliella [24], Scenedesmus [28][29][30][31][32][33], and Tetraselmis [34][35]. Over time, other strain with a unique capacity for the synthesis of lipids and hydrocarbons such as Botryococcus braunii [9][10] were isolated and identified, and more recently, the scientific community has opted for the production of mutant strains with large lipid storage [12][13].
Microalgae can be produced under autotrophic, mixotrophic, or heterotrophic conditions. Different systems for the production of algae are available for their culture under the three conditions, as mentioned earlier [31]. Autotrophic systems are the most common, since the algae only require light as an energy source and dissolved CO2 as a source of carbon. Usually, algae growth under autotrophic systems can be produced in open or closed photobioreactors. Open ponds are the simplest of all systems for algal production, and it requires low energy inputs. It has easy maintenance; however, it is severely affected by seasonal variations and is prone to contamination by other microbes [32]. Mixotrophic and heterotrophic production of algae requires the addition of organic carbon sources (glucose, acetate, and others), which can lead to contamination by the presence of bacteria and fungi; therefore, these systems require closed photobioreactors (PBR). Closed PBR offers several advantages over open systems: (i) aseptic growth conditions, (ii) increased cell concentration due to better light distribution, (iii) improved pH control, and (iv) reduced water loss due to evaporation. However, their operation cost, maintenance, and energy inputs are considerably higher than in open ponds [32].
After biomass production, the cells are harvested from the media. Due to their nature, microalgal cells have a small size and low specific gravity; therefore, their concentration and harvesting are energy and time-intensive [33]. Several techniques are available at industrial scale such as centrifugation, filtration, flocculation, flotation, electroflotation, and so on [36]. However, the method’s selection and application lie on the technical and economic analysis since some of them can be extremely expensive and energy-intensive for the production of algal-based biofuels [34]. Once the biomass is removed from the media, most of the cell water content must be removed via spray drying, drum drying, freeze-drying, or solar drying to avoid any interference with the extraction [31]. Following drying comes the extraction of lipids and carbohydrates, which is considered as the crucial step that inhibits the industrial-scale production of algae-based biofuels [34]. The microalgal cell wall is made of polysaccharides and cellulose synthesized from silicic acid [35], and it must be broken in order to release both lipids and carbohydrates; as a consequence, only a fraction of the biomass is used in biofuel process production. Therefore, biodiesel and bioethanol production are still not economically feasible due to the high cost and energy inputs in almost all stages [37]. Other biofuels such as biogas and biohydrogen have gained attention as sustainable alternatives for energy production using microalgal biomass.
3. Biochemical Conversion for Third-Generation Biofuel
The biochemical conversion of algal biomass into third-generation biofuels are divided into biodiesel, bioethanol, biogas, and biohydrogen. Biodiesel from algae requires the extraction and conversion of lipidic fraction into low atomic weight compounds, biodegradable fatty acid methyl esters (FAME), for hands ready usage in engines through transesterification [38]. In the transesterification reaction in the presence of a chemical (acid, alkali) or biological (lipase) catalyst [39], methanol or ethanol is used to increase the reaction rate and maintain a balance change toward the production of fatty acid esters with glycerol as a by-product [40]. The biodiesel derived from algal biomass has a petrodiesel-like calorific value (39–41 MJ/kg) [41]; it also has a higher percentage of unsaturated fatty acid compared to saturated fatty acid, which is a prerequisite for fuel engineering [42]. A higher degree of unsaturation leads to better cold flow; however, insoluble particle production is simultaneously increased [43].
Microalgae are an alternative resource for bioethanol production as they showed higher productivity than certain feedstocks for bioethanol production, such as sugarcane and corn [53]. Several strains accumulate carbohydrates in excess (mainly as insoluble starch and cellulose, with the absence of lignin) of up to 50% of their dry weight (DW) [44]. These carbohydrates are not readily fermentable to bioethanol [45]; thus, pretreatment processes, including chemical (acid and alkaline) or enzymatic hydrolysis, are crucial [46][47][48].
There are many pretreatment methods (acid, basic, and enzymatic hydrolysis); however, their cost can significantly contribute up to 30% of the total cost of bioethanol production [49]. Acid hydrolysis is quicker and cheaper under high temperatures and pressures but can decompose sugar into inhibitors [50][51][52]. Conversely, under mild temperatures and pressure, enzymatic hydrolysis can be achieved, but it is slower, more costly, and still involves physical or chemical pretreatment [53].
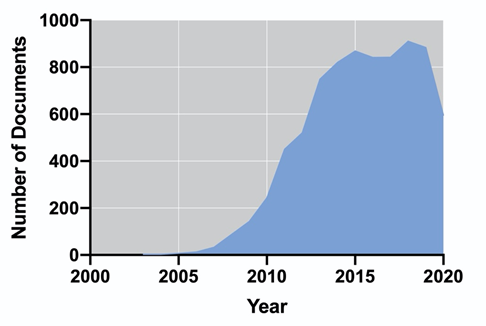
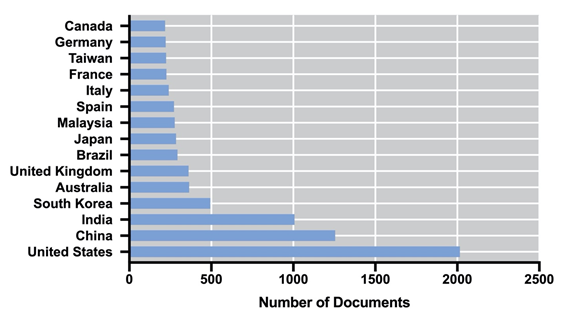
Biogas is produced via a sequence of biochemical processes converting the organic material: hydrolysis, fermentation, acetogenesis, and methanogenesis, also known as anaerobic digestion (AD) [54]. In this process, the whole biomass is used for the production of methane (55–75%) and carbon dioxide (25–45%) [55]; therefore, the energy performance is higher in comparison to biodiesel and bioethanol [56]. Additionally, nutrients such as organic nitrogen or phosphorus may be mineralized and subsequently recycled for algae cultivation [57]. Unlike biogas, biohydrogen is produced via their metabolic pathways along with the cell growth; therefore, it does not require further processing of the biomass (i.e., harvesting, dewatering, drying, and extraction), and it is considered clean and renewable, with higher energy production (142 MJ/Kg) [58]. Biohydrogen can be obtained by photofermentation, dark fermentation, direct and indirect biophotolysis [59]; however, hydrogen production cannot be achieved amidst effective photosynthesis, as oxygen inactivates hydrogenase [60]. The Research and Development on algal-based biofuels is a field that, in recent years, has been maintained with a considerable number of publications. Figure 1 shows the number of publications per year in the last 18 years, according to the Scopus database (Elsevier). It is possible to observe an exponential increase in the number of publications between 2006 and 2015. Since 2016, the number of documents has remained almost constant up to a final number of 8022 (including accepted manuscripts for 2021). The United States, China, India, South Korea, and the United Kingdom dominate the scientific publication on algal-based biofuels.
(a)
(b)
Figure 1. Evolution of the number of publications from 2003 to 2020 on algal biofuels (a) and their country of origin (b).
References
- Kargbo, H.; Harris, J.S.; Phan, A.N. “Drop-in” Fuel Production from Biomass: Critical Review on Techno-Economic Feasibility and Sustainability. Renew. Sustain. Energy Rev. 2021, 135, 110168. [Google Scholar] [CrossRef]
- Raheem, A.; Wan Azlina, W.A.K.G.; Taufiq Yap, Y.H.; Danquah, M.K.; Harun, R. Thermochemical Conversion of Microalgal Biomass for Biofuel Production. Renew. Sustain. Energy Rev. 2015, 49, 990–999. [Google Scholar] [CrossRef]
- Chowdhury, H.; Loganathan, B. Third-Generation Biofuels from Microalgae: A Review. Curr. Opin. Green Sustain. Chem. 2019, 20, 39–44. [Google Scholar] [CrossRef]
- Huang, C.-H.; Tan, C.-S. A Review: CO2 Utilization. Aerosol Air Qual. Res. 2014, 14, 480–499. [Google Scholar] [CrossRef]
- Costa, J.A.V.; de Freitas, B.C.B.; Lisboa, C.R.; Santos, T.D.; de Fraga Brusch, L.R.; de Morais, M.G. Microalgal Biorefinery from CO2 and the Effects under the Blue Economy. Renew. Sustain. Energy Rev. 2019, 99, 58–65. [Google Scholar] [CrossRef]
- Quintero-Dallos, V.; García-Martínez, J.B.; Contreras-Ropero, J.E.; Barajas-Solano, A.F.; Barajas-Ferrerira, C.; Lavecchia, R.; Zuorro, A. Vinasse as a Sustainable Medium for the Production of Chlorella vulgaris UTEX 1803. Water 2019, 11, 1526. [Google Scholar] [CrossRef]
- Hena, S.; Znad, H.; Heong, K.T.; Judd, S. Dairy Farm Wastewater Treatment and Lipid Accumulation by Arthrospira platensis. Water Res. 2018, 128, 267–277. [Google Scholar] [CrossRef]
- Polat, E.; Yüksel, E.; Altınbaş, M. Mutual Effect of Sodium and Magnesium on the Cultivation of Microalgae Auxenochlorella protothecoides. Biomass Bioenergy 2020, 132, 105441. [Google Scholar] [CrossRef]
- Gouveia, J.D.; Ruiz, J.; van den Broek, L.A.M.; Hesselink, T.; Peters, S.; Kleinegris, D.M.M.; Smith, A.G.; van der Veen, D.; Barbosa, M.J.; Wijffels, R.H. Botryococcus braunii Strains Compared for Biomass Productivity, Hydrocarbon and Carbohydrate Content. J. Biotechnol. 2017, 248, 77–86. [Google Scholar] [CrossRef]
- Barajas-Solano, A.F.; Guzmán-Monsalve, A.; Kafarov, V. Effect of Carbon-Nitrogen Ratio for the Biomass Production, Hydrocarbons and Lipids on Botryoccus braunii UIS 003. Chem. Eng. Trans. 2016, 49, 247–252. [Google Scholar] [CrossRef]
- Banerjee, S.; Ray, A.; Das, D. Optimization of Chlamydomonas reinhardtii Cultivation with Simultaneous CO2 Sequestration and Biofuels Production in a Biorefinery Framework. Sci. Total Environ. 2020, 143080. [Google Scholar] [CrossRef] [PubMed]
- Kao, P.-H.; Ng, I.-S. CRISPRi Mediated Phosphoenolpyruvate Carboxylase Regulation to Enhance the Production of Lipid in Chlamydomonas reinhardtii. Bioresour. Technol. 2017, 245, 1527–1537. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.S.; Jeong, J.; Nguyen, T.H.T.; Kim, J.Y.H.; Jin, E.; Sim, S.J. Targeted Knockout of Phospholipase A2 to Increase Lipid Productivity in Chlamydomonas reinhardtii for Biodiesel Production. Bioresour. Technol. 2019, 271, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Yang, H.-L.; Li, C.; Peng, Y.-Y.; Lu, M.-M.; Jin, W.-H.; Bao, J.-J.; Guo, Y.-M. Effect of Organic Carbon to Nitrogen Ratio in Wastewater on Growth, Nutrient Uptake and Lipid Accumulation of a Mixotrophic Microalgae Chlorella sp. Bioresour. Technol. 2019, 282, 118–124. [Google Scholar] [CrossRef]
- Bauer, L.M.; Costa, J.A.V.; da Rosa, A.P.C.; Santos, L.O. Growth Stimulation and Synthesis of Lipids, Pigments and Antioxidants with Magnetic Fields in Chlorella kessleri Cultivations. Bioresour. Technol. 2017, 244, 1425–1432. [Google Scholar] [CrossRef]
- Cheng, P.; Chu, R.; Zhang, X.; Song, L.; Chen, D.; Zhou, C.; Yan, X.; Cheng, J.J.; Ruan, R. Screening of the Dominant Chlorella pyrenoidosa for Biofilm Attached Culture and Feed Production While Treating Swine Wastewater. Bioresour. Technol. 2020, 318, 124054. [Google Scholar] [CrossRef]
- Alavijeh, R.S.; Karimi, K.; Wijffels, R.H.; van den Berg, C.; Eppink, M. Combined Bead Milling and Enzymatic Hydrolysis for Efficient Fractionation of Lipids, Proteins, and Carbohydrates of Chlorella vulgaris Microalgae. Bioresour. Technol. 2020, 309, 123321. [Google Scholar] [CrossRef]
- Estévez-Landazábal, L.L.; Barajas-Solano, A.F.; Barajas-Ferreira, C.; Kafarov, V. Improvement of lipid productivity on Chlorella vulgaris using waste glycerol and sodium acetate. CTF Cienc. Tecnol. Futuro 2013, 5, 113–126. Available online: http://www.scielo.org.co/scielo.php?script=sci_arttext&pid=S0122-53832013000100009 (accessed on 29 November 2020).
- Sarayloo, E.; Simsek, S.; Unlu, Y.S.; Cevahir, G.; Erkey, C.; Kavakli, I.H. Enhancement of the Lipid Productivity and Fatty Acid Methyl Ester Profile of Chlorella vulgaris by Two Rounds of Mutagenesis. Bioresour. Technol. 2018, 250, 764–769. [Google Scholar] [CrossRef]
- Del Río, E.; García-Gómez, E.; Moreno, J.; Guerrero, M.G.; García-González, M. Microalgae for Oil. Assessment of Fatty Acid Productivity in Continuous Culture by Two High-Yield Strains, Chlorococcum oleofaciens and Pseudokirchneriella subcapitata. Algal Res. 2017, 23, 37–42. [Google Scholar] [CrossRef]
- Chinnasamy, S.; Bhatnagar, A.; Hunt, R.W.; Das, K.C. Microalgae Cultivation in a Wastewater Dominated by Carpet Mill Effluents for Biofuel Applications. Bioresour. Technol. 2010, 101, 3097–3105. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Silva, L.; López-González, D.; Garcia-Minguillan, A.M.; Valverde, J.L. Pyrolysis, Combustion and Gasification Characteristics of Nannochloropsis gaditana Microalgae. Bioresour. Technol. 2013, 130, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Balamurugan, S.; Li, D.-W.; Liu, Y.-H.; Zeng, H.; Wang, L.; Yang, W.-D.; Liu, J.-S.; Li, H.-Y. Glucose-6-Phosphate Dehydrogenase as a Target for Highly Efficient Fatty Acid Biosynthesis in Microalgae by Enhancing NADPH Supply. Metab. Eng. 2017, 41, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Priharto, N.; Ronsse, F.; Prins, W.; Carleer, R.; Heeres, H.J. Experimental Studies on a Two-Step Fast Pyrolysis-Catalytic Hydrotreatment Process for Hydrocarbons from Microalgae (Nannochloropsis gaditana and Scenedesmus almeriensis). Fuel Process. Technol. 2020, 206, 106466. [Google Scholar] [CrossRef]
- Gupta, S.; Pawar, S.B. An Integrated Approach for Microalgae Cultivation Using Raw and Anaerobic Digested Wastewaters from Food Processing Industry. Bioresour. Technol. 2018, 269, 571–576. [Google Scholar] [CrossRef]
- Girard, J.-M.; Roy, M.-L.; Hafsa, M.B.; Gagnon, J.; Faucheux, N.; Heitz, M.; Tremblay, R.; Deschênes, J.-S. Mixotrophic Cultivation of Green Microalgae Scenedesmus Obliquus on Cheese Whey Permeate for Biodiesel Production. Algal Res. 2014, 5, 241–248. [Google Scholar] [CrossRef]
- Cuéllar-García, D.J.; Rangel-Basto, Y.A.; Urbina-Suarez, N.A.; Barajas-Solano, A.F.; Muñoz-Peñaloza, Y.A. Lipids production from Scenedesmus obliquus through carbon/nitrogen ratio optimization. J. Phys. Conf. Ser. 2019, 1388, 012043. [Google Scholar] [CrossRef]
- Cuéllar-García, D.J.; Rangel-Basto, Y.A.; Barajas-Solano, A.F.; Muñoz-Peñaloza, Y.A.; Urbina-Suarez, N.A. Towards the production of microalgae biofuels: The effect of the culture medium on lipid deposition. BioTechnologia 2019, 100, 273–278. [Google Scholar] [CrossRef]
- Andreotti, V.; Solimeno, A.; Rossi, S.; Ficara, E.; Marazzi, F.; Mezzanotte, V.; García, J. Bioremediation of Aquaculture Wastewater with the Microalgae Tetraselmis suecica: Semi-Continuous Experiments, Simulation and Photo-Respirometric Tests. Sci. Total Environ. 2020, 738, 139859. [Google Scholar] [CrossRef]
- Srivatsa, S.C.; Li, F.; Bhattacharya, S. Optimization of Reaction Parameters for Bio-Oil Production by Catalytic Pyrolysis of Microalga Tetraselmis suecica: Influence of Ni-Loading on the Bio-Oil Composition. Renew. Energy 2019, 142, 426–436. [Google Scholar] [CrossRef]
- Guiza-Franco, L.; Orozco-Rojas, L.G.; Sanchez-Galvis, M.; Garcia-Martinez, J.B.; Barajas-Ferreira, C.; Zuorro, A.; Barajas-Solano, A.F. Production of Chlorella vulgaris Biomass on UV-Treated Wastewater as an Alternative for Environmental Sustainability on High-Mountain Fisheries. Chem. Eng. Trans. 2018, 64, 517–522. [Google Scholar] [CrossRef]
- Merchuk, J.C. Chapter 5—Photobioreactor Design; Jacob-Lopes, E., Maroneze, M.M., Queiroz, M.I., Zepka, L.Q., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 101–126. [Google Scholar] [CrossRef]
- Sanchez-Galvis, E.M.; Cardenas-Gutierrez, I.Y.; Contreras-Ropero, J.E.; García-Martínez, J.B.; Barajas-Solano, A.F.; Zuorro, A. An Innovative Low-Cost Equipment for Electro-Concentration of Microalgal Biomass. Appl. Sci. 2020, 10, 4841. [Google Scholar] [CrossRef]
- Garcia-Martinez, B.; Ayala-Torres, E.; Reyes-Gomez, O.; Zuorro, A.; Barajas-Solano, A.; Barajas-Ferreira, C. Evaluation of a Two-Phase Extraction System of Carbohydrates and Proteins from Chlorella vulgaris UTEX 1803. Chem. Eng. Trans. 2016, 49, 355–360. [Google Scholar] [CrossRef]
- Kumar, R.; Ghosh, A.K.; Pal, P. Synergy of Biofuel Production with Waste Remediation along with Value-Added Co-Products Recovery through Microalgae Cultivation: A Review of Membrane-Integrated Green Approach. Sci. Total Environ. 2020, 698, 134169. [Google Scholar] [CrossRef] [PubMed]
- Castellanos-Estupiñan, M.; Sanchez-Galvis, M.; Garcia-Martinez, J.B.; Barajas-Ferreira, C.; Zuorro, A.; Barajas-Solano, A.F. Design of an Electroflotation System for the Concentration and Harvesting of Freshwater Microalgae. Chem. Eng. Trans. 2018, 64, 1–6. [Google Scholar] [CrossRef]
- Zuorro, A.; Malavasi, V.; Cao, G.; Lavecchia, R. Use of cell wall degrading enzymes to improve the recovery of lipids from Chlorella sorokiniana. Chem. Eng. J. 2019, 377, 120325. [Google Scholar] [CrossRef]
- Arun, J.; Gopinath, K.P.; SundarRajan, P.; Felix, V.; JoselynMonica, M.; Malolan, R. A Conceptual Review on Microalgae Biorefinery through Thermochemical and Biological Pathways: Bio-Circular Approach on Carbon Capture and Wastewater Treatment. Bioresour. Technol. Rep. 2020, 11, 100477. [Google Scholar] [CrossRef]
- Rangel-Basto, Y.A.; García-Ochoa, I.E.; Suarez-Gelvez, J.H.; Zuorro, A.; Barajas-Solano, A.F.; Urbina-Suarez, N.A. The Effect of Temperature and Enzyme Concentration in the Transesterification Process of Synthetic Microalgae Oil. Chem. Eng. Trans. 2018, 64, 331–336. [Google Scholar] [CrossRef]
- Kumar, M.; Sun, Y.; Rathour, R.; Pandey, A.; Thakur, I.S.; Tsang, D.C.W. Algae as Potential Feedstock for the Production of Biofuels and Value-Added Products: Opportunities and Challenges. Sci. Total Environ. 2020, 716, 137116. [Google Scholar] [CrossRef]
- Demirbas, A. Use of Algae as Biofuel Sources. Energy Convers. Manag. 2010, 51, 2738–2749. [Google Scholar] [CrossRef]
- Tripathi, R.; Singh, J.; Thakur, I.S. Characterization of Microalga Scenedesmus sp. ISTGA1 for Potential CO2 Sequestration and Biodiesel Production. Renew. Energy 2015, 74, 774–781. [Google Scholar] [CrossRef]
- Kumar, M.; Thakur, I.S. Municipal Secondary Sludge as Carbon Source for Production and Characterization of Biodiesel from Oleaginous Bacteria. Bioresour. Technol. Rep. 2018, 4, 106–113. [Google Scholar] [CrossRef]
- Dragone, G.; Fernandes, B.D.; Abreu, A.P.; Vicente, A.A.; Teixeira, J.A. Nutrient Limitation as a Strategy for Increasing Starch Accumulation in Microalgae. Appl. Energy 2011, 88, 3331–3335. [Google Scholar] [CrossRef]
- Ho, S.-H.; Huang, S.-W.; Chen, C.-Y.; Hasunuma, T.; Kondo, A.; Chang, J.-S. Bioethanol Production Using Carbohydrate-Rich Microalgae Biomass as Feedstock. Bioresour. Technol. 2013, 135, 191–198. [Google Scholar] [CrossRef] [PubMed]
- El-Dalatony, M.M.; Kurade, M.B.; Abou-Shanab, R.A.I.; Kim, H.; Salama, E.-S.; Jeon, B.-H. Long-Term Production of Bioethanol in Repeated-Batch Fermentation of Microalgal Biomass Using Immobilized Saccharomyces cerevisiae. Bioresour. Technol. 2016, 219, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Zhao, X.-Q.; Yen, H.-W.; Ho, S.-H.; Cheng, C.-L.; Lee, D.-J.; Bai, F.-W.; Chang, J.-S. Microalgae-Based Carbohydrates for Biofuel Production. Biochem. Eng. J. 2013, 78, 1–10. [Google Scholar] [CrossRef]
- Wang, H.; Ji, C.; Bi, S.; Zhou, P.; Chen, L.; Liu, T. Joint Production of Biodiesel and Bioethanol from Filamentous Oleaginous Microalgae Tribonema sp. Bioresour. Technol. 2014, 172, 169–173. [Google Scholar] [CrossRef]
- Talebnia, F.; Karakashev, D.; Angelidaki, I. Production of Bioethanol from Wheat Straw: An Overview on Pretreatment, Hydrolysis and Fermentation. Bioresour. Technol. 2010, 101, 4744–4753. [Google Scholar] [CrossRef]
- Baeyens, J.; Kang, Q.; Appels, L.; Dewil, R.; Lv, Y.; Tan, T. Challenges and Opportunities in Improving the Production of Bio-Ethanol. Prog. Energy Combust. Sci. 2015, 47, 60–88. [Google Scholar] [CrossRef]
- Hernández, D.; Riaño, B.; Coca, M.; García-González, M.C. Saccharification of Carbohydrates in Microalgal Biomass by Physical, Chemical and Enzymatic Pre-Treatments as a Previous Step for Bioethanol Production. Chem. Eng. J. 2015, 262, 939–945. [Google Scholar] [CrossRef]
- Barajas-Solano, A.F.; Gonzalez-Delgado, A.D.; Kafarov, V. Effect of Thermal Pre-Treatment on Fermentable Sugar Production of Chlorella vulgaris. Chem. Eng. Trans. 2014, 37, 655–660. [Google Scholar] [CrossRef]
- Rizza, L.S.; Smachetti, M.E.S.; Do Nascimento, M.; Salerno, G.L.; Curatti, L. Bioprospecting for Native Microalgae as an Alternative Source of Sugars for the Production of Bioethanol. Algal Res. 2017, 22, 140–147. [Google Scholar] [CrossRef]
- Córdova, O.; Santis, J.; Ruiz-Fillipi, G.; Zuñiga, M.E.; Fermoso, F.G.; Chamy, R. Microalgae Digestive Pretreatment for Increasing Biogas Production. Renew. Sustain. Energy Rev. 2018, 82, 2806–2813. [Google Scholar] [CrossRef]
- Jankowska, E.; Sahu, A.K.; Oleskowicz-Popiel, P. Biogas from Microalgae: Review on Microalgae’s Cultivation, Harvesting and Pretreatment for Anaerobic Digestion. Renew. Sustain. Energy Rev. 2017, 75, 692–709. [Google Scholar] [CrossRef]
- González-Fernández, C.; Sialve, B.; Bernet, N.; Steyer, J.P. Thermal Pretreatment to Improve Methane Production of Scenedesmus Biomass. Biomass Bioenergy 2012, 40, 105–111. [Google Scholar] [CrossRef]
- Sialve, B.; Bernet, N.; Bernard, O. Anaerobic Digestion of Microalgae as a Necessary Step to Make Microalgal Biodiesel Sustainable. Biotechnol. Adv. 2009, 27, 409–416. [Google Scholar] [CrossRef]
- Anwar, M.; Lou, S.; Chen, L.; Li, H.; Hu, Z. Recent Advancement and Strategy on Bio-Hydrogen Production from Photosynthetic Microalgae. Bioresour. Technol. 2019, 292, 121972. [Google Scholar] [CrossRef]
- Jiménez-Llanos, J.; Ramírez-Carmona, M.; Rendón-Castrillón, L.; Ocampo-López, C. Sustainable Biohydrogen Production by Chlorella sp. Microalgae: A Review. Int. J. Hydrogen Energy 2020, 45, 8310–8328. [Google Scholar] [CrossRef]
- Gimpel, J.A.; Specht, E.A.; Georgianna, D.R.; Mayfield, S.P. Advances in Microalgae Engineering and Synthetic Biology Applications for Biofuel Production. Curr. Opin. Chem. Biol. 2013, 17, 489–495. [Google Scholar] [CrossRef]
- Gimpel, J.A.; Specht, E.A.; Georgianna, D.R.; Mayfield, S.P. Advances in Microalgae Engineering and Synthetic Biology Applications for Biofuel Production. Curr. Opin. Chem. Biol. 2013, 17, 489–495. [Google Scholar] [CrossRef]