
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Nahla Hwalla | + 1921 word(s) | 1921 | 2021-01-07 03:27:09 | | | |
| 2 | Dean Liu | -466 word(s) | 1455 | 2021-01-07 09:27:20 | | |
Video Upload Options
The pathophysiology behind obesity involves a myriad of genetic, epigenetic, physiological, and macroenvironmental factors that drive food intake and appetite and increase the obesity risk for susceptible individuals. Metabolically, food intake and appetite are regulated via intricate processes and feedback systems between the brain, gastrointestinal system, adipose and endocrine tissues that aim to maintain body weight and energy homeostasis but are also responsive to environmental cues that may trigger overconsumption of food beyond homeostatic needs.
1. Introduction
The obesity epidemic is one of the most predominant public health challenges of the century and its prevalence continues to rise globally with now more than 500 billion obese individuals around the world[1]. Being associated with over 60 comorbid conditions and at least 12 different types of cancers, obesity poses significant health and economic burdens on affected individuals as well as on the sustainability of public health systems in all parts of the world, but more profoundly in developing countries. The figures of obesity in the Middle East are staggering, particularly in the Gulf region, and the prevalence of female obesity surpasses 40% in some countries such as Egypt, Jordan and Saudi Arabia [2](Figure 1). Over the past decade, the prevalence of obesity in the Middle Eastern and North African (MENA) region has increased by more than 30% and has been accompanied by a concomitant surge in the prevalence of non-communicable diseases (NCDs). For example, the prevalence of type 2 diabetes, which is strongly related to that of obesity, was estimated to surpass 15% of the adult populations in certain countries of the MENA region such as Egypt (15.2%), Bahrain (16.3%), Qatar (15.5%), Saudi Arabia (18.3%), and the United Arab Emirates (15.4%)[3]. Moreover, according to the International Diabetes Federation (IDF), the MENA region had the highest world-age standardized prevalence of diabetes globally in 2019 at 12.9% (95% confidence interval 7.2–17.6%)[3]. The dramatic and exponential rises in prevalences of obesity, diabetes and other NCDs call for immediate interventions addressing these serious epidemics. Data on the prevalence of obesity in different countries of the MENA region and the world from World Health Organization (WHO) 2016 data records are summarized in Figure 1 and Figure 2.
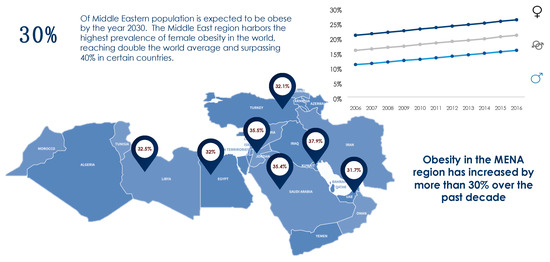
Figure 1. Prevalence of obesity in the MENA region based on WHO (2016) records[2].
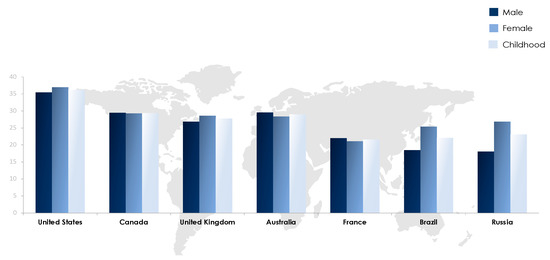
Figure 2. Prevalence of obesity in selected countries worldwide based on WHO (2016) records[2].
2. The Epigenetic Effects of Diet
A plethora of studies have related different nutrients, dietary patterns, and lifestyle choices to epigenetic changes that may have a pathogenic role in the development of diseases including obesity. The first 1000 days of life from conception represent a window of epigenetic plasticity whereby environmental cues such as maternal malnutrition or high fat diet could modulate fetal growth, organ maturation and susceptibility to disease[4]. There exist historical records linking poor maternal nutrition to the development of diseases and metabolic derangements such as diabetes, CVD and cognitive disorders in both the offspring and even the grand-offspring. The most prominently known evidence on the transgenerational heritability of epigenetic changes is the Dutch famine in 1944 whereby starvation in one generation led to poor health outcomes in subsequent generations. The DNA isolated from these individuals presented increased expression of insulin growth factor 2 (IGF2) and below-average methylation of its gene, among other epigenetic modifications, that persisted for six decades later[5]. These epigenetic changes were associated with a number of metabolic disorders including elevated total cholesterol, triglycerides, LDL-C, LDL-C to high-density lipoprotein cholesterol (HDL-C) ratio, and lower levels of HDL-C[6]. The fetal origins of adulthood disease have in fact been linked to both maternal and paternal diets and the linking mechanisms are suggested to include the methylation of gametes, mitochondrial dysfunction and oxidative stress in response to a restricted nutritional intake. Other nutritional factors known to have epigenetic effects are summarized in Table 1.
Table 1. Key dietary factors with associated epigenetic metabolic effects.
| Nutritional Factors | Potential Epigenetic Mechanisms | Associated Metabolic Outcomes |
|---|---|---|
| Caloric restriction/undernutrition | DNA methylation Histone modifications miRNA |
Increased risk of obesity and metabolic disorders (including insulin resistance and diabetes) in adulthood |
| Fatty Acids | ||
| Unsaturated FA (MUFA, n-3 PUFA) |
DNA methylation Histone modifications miRNA |
Prevention of metabolic derangements (lipid metabolism disturbances, inflammation, and insulin resistance) or chronic diseases (obesity, diabetes, non-alcoholic fatty liver disease, CVD risk and some types of cancer) (Becerra, 2019) |
| n-6 PUFA, Saturated and trans FA | DNA methylation Histone modifications miRNA |
Associated with the presence or development of obesity, diabetes, inflammatory profile, atherosclerosis, hyperglycemia, insulin resistance, lipid alterations, lipotoxicity, dysregulation of lipid metabolism, and abnormal lipid accumulation (Becerra, 2019) |
| SCFA | DNA methylation Histone modifications Interaction with Gut Microbiome |
Mixed effects |
| Methyl donors (e.g., folate, methionine, choline) * | DNA methylation | Increased risk of metabolic disorders including insulin resistance, diabetes and obesity |
| Probiotics/Fibers | Production of SCFA (same as SCFA) | Same as SCFA |
| Vitamins and minerals (e.g., retinol, ascorbate, tocopherol) | DNA methylation Histone modifications |
Antioxidant effects |
| Polyphenols and other plant compounds | Inhibition of DNA hypermethylation Histone modification miRNA |
May protect against carcinogenesis and obesity on adulthood |
| Dietary Patterns | ||
| High fat diet/Western diet | DNA methylation of obesity related genes leading to changes in genes expression and appetite regulation | - Imbalance in fatty acids causing oxidative stress and inflammatory state - Increased risk of obesity and metabolic disorders (including insulin resistance and diabetes) in adulthood |
| Mediterranean diet | DNA methylation | Protective against CVD risk and cancer |
| Intermittent Fasting | Improve the expression of anti-oxidant and anti-inflammatory genes | May be protective against oxidative stress |
Even though perinatal period contributes most significantly to phenotypic plasticity, evidence suggests that dietary factors after birth and even during adulthood may also lead to epigenetic changes. For instance, in a study by Yuan et al, lactation in rodents has been shown to cause epigenetic changes that are linked to adult obesity. Milk lipids activate the nuclear receptor PPARα (peroxisome proliferator-activated receptor) which is known to regulate transcription at the level of the liver. PPARα has been shown to induce PPARα dependent demethylation of Fgf21 (fibroblast growth factor 21) which is a liver hormone involved in a multitude of metabolic roles including body weight and energy homeostasis. The study correlates the demethylation of Fgf21 with a decreased risk of diet-induced obesity in older rodents thus highlighting a link between breastfeeding and suppression of obesity. Additionally, in a study by Madkour et al. intermittent fasting during Ramadan has been shown to improve the expression of anti-oxidant and anti-inflammatory genes (TFAM, SOD2, and Nrf2) and thus may have a protective role against oxidative stress which is a primary etiological factor in multiple metabolic disorders including obesity[11].
3. The Way Forward
Despite the great body of research, the evidence comparing different dietary approaches in the management of obesity is challenged by a number of factors namely limited follow up data on long term effectiveness and adherence to dietary interventions which constitutes a huge gap in knowledge on the effectiveness of any intervention. The frameworks behind regulation of body weight and food intake are far from being completely understood and recent research has identified a high degree of inter-individual variability in the vulnerability to the obesogenic environment and the metabolic response to food and weight loss interventions, asserting that when it comes to obesity treatment, one size does not fit all[12]. Current efforts are focused on gathering all relevant genetic, epigenetic, phenotypic, medical and nutritional information in the aim of integrating them into multi-dimensional individualized recommendations that take into account individual preferences as well as metabolic profiles to maximize adherence. Excessive adiposity results from a pre-programmed genetic, epigenetic, metabolic and even gut microbial makeup, and influenced by environmental factors, which complicates all attempts for prevention and treatment of obesity and its associated comorbidities. Nevertheless, lifestyle modification and proper nutrition remain a crucial part of the solution to obesity and an effective approach that can greatly influence much of the modifiable risk factors. At the population level, whole system approaches (WSA) that recognize the complexity and multifaceted nature of obesity are being increasingly used to address this epidemic at the national, regional and international levels. The main features of WSA involve acknowledging the multifactorial drivers of obesity, coordinating actions between multiple stakeholders including non-healthcare related players, operating at all levels of governance and targeting all age groups[13]. A systematic review by Bagnall et al. (2019) reported a range of positive health outcomes resulting from the adoption of WSA in the context of obesity and related public health issues in multiple countries; these include improvements in BMI, awareness and health behaviors among others[13]. Such findings highlight the importance of addressing the food and built environments as essential drivers of obesity and NCDs and adopting a holistic approach that targets food systems from production to consumption in curbing down obesity and its associated morbidities. Future research should continue to explore the options to optimize dietary approaches in order to deliver more integrative, personalized and country-specific nutritional guidance.
References
- Null, N. Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet 2016, 387, 1377–1396.
- Global Health Observatory. Prevalence of Obesity among Adults, BMI ≥ 30, Age-Standardized—Estimates by WHO Region [Online]. 2016. Available online: https://apps.who.int/gho/data/view.main.REGION2480A?lang=en (accessed on 19 October 2020).
- International Diabetes Federation. IDF Diabetes Atlas Ninth Edition 2019; International Diabetes Federation: Brussels, Belgium, 2019.
- Gabbianelli, R.; Bordoni, L.; Morano, S.; Agius, J.C.; Lalor, J. Nutri-Epigenetics and Gut Microbiota: How Birth Care, Bonding and Breastfeeding Can Influence and Be Influenced? Int. J. Mol. Sci. 2020, 21, 5032.
- Heijmans, B.T.; Tobi, E.W.; Stein, A.D.; Putter, H.; Blauw, G.J.; Susser, E.S.; Slagboom, P.E.; Lumey, L.H. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc. Natl. Acad. Sci. USA 2008, 105, 17046–17049.
- Shen, L.; Li, C.; Wang, Z.; Zhang, R.; Shen, Y.; Miles, T.; Wei, J.; Zou, Z. Early-life exposure to severe famine is associated with higher methylation level in the IGF2 gene and higher total cholesterol in late adulthood: The Genomic Research of the Chinese Famine (GRECF) study. Clin. Epigenetics 2019, 11, 88.
- Li, Y. Epigenetic Mechanisms Link Maternal Diets and Gut Microbiome to Obesity in the Offspring. Front. Genet. 2018, 9, 342.
- Milagro, F.; Mansego, M.; De Miguel, C.; Martinez, J.A. Dietary factors, epigenetic modifications and obesity outcomes: Progresses and perspectives. Mol. Asp. Med. 2013, 34, 782–812.
- Keleher, M.R.; Zaidi, R.; Hicks, L.; Shah, S.; Xing, X.; Li, D.; Wang, T.; Cheverud, J. A high-fat diet alters genome-wide DNA methylation and gene expression in SM/J mice. BMC Genom. 2018, 19, 888.
- Yuan, X.; Tsujimoto, K.; Hashimoto, K.; Kawahori, K.; Hanzawa, N.; Hamaguchi, M.; Seki, T.; Nawa, M.; Ehara, T.; Kitamura, Y. Epigenetic modulation of Fgf21 in the perinatal mouse liver ameliorates diet-induced obesity in adulthood. Nat. Commun. 2018, 9, 636.
- Madkour, M.I.; El-Serafi, A.T.; Jahrami, H.A.; Sherif, N.M.; Hassan, R.E.; Awadallah, S.; Faris, M.A.-I.E. Ramadan diurnal intermittent fasting modulates SOD2, TFAM, Nrf2, and sirtuins (SIRT1, SIRT3) gene expressions in subjects with overweight and obesity. Diabetes Res. Clin. Pr. 2019, 155, 107801.
- Lowe, M.R.; Benson, L.; Singh, S. Individual differences in within-subject weight variability: There’s a signal in the noise. Physiol. Behav. 2020, 226, 113112.
- Bagnall, A.-M.; Radley, D.; Jones, R.; Gately, P.; Nobles, J.; Van Dijk, M.; Blackshaw, J.; Montel, S.; Sahota, P. Whole systems approaches to obesity and other complex public health challenges: A systematic review. BMC Public Health 2019, 19, 8.




