1000/1000
Hot
Most Recent

Microfluidic lab-on-a-chip cell culture techniques have been gaining popularity by offering the possibility of reducing the amount of samples and reagents with greater control over the cellular microenvironment. Polydimethylsiloxane (PDMS) is the commonly used polymer for microfluidic cell culture devices because of the cheap and easy fabrication techniques, non-toxicity, biocompatibility, high gas permeability, and optical transparency.
Microfluidic technology, also known as lab-on-a-chip or micro total analysis system (μTAS), was applied in cell biology more than 20 years ago. Microfluidic techniques are powerful tools in cell culture because of its ability to create complex and controllable cellular microenvironment in microchannels [1]. This technology can provide a complex cell-based bioassay platform by integrating several steps such as fluid control, cell culture, cell capture, cell-cell, and cell-matrix interaction, cell lysis, cell signaling, and detection of biochemicals in a single device [2]. Successful cell culture in microfluidic devices depends on the characteristics of the substrate materials. A broad range of polymers, such as polycarbonate [3], polystyrene [4], polymethyl-methacrylate [5][6], cyclic olefin polymers [7][8], and polydimethylsiloxane (PDMS) [9][10][11][12][13] have been used for fabricating microfluidic cell culture devices. Among them, PDMS has been gaining popularity because of the relatively low-cost and easy fabrication procedures as well as good mechanical stability [14].
PDMS is a silicon-based synthetic polymer, consisting of the repeating unit of Si-O molecules with two organic methyl groups attached to silicon. PDMS possess distinctive properties, including low elasticity, low thermal conductivity, high electrical resistance, chemical inertness, non-toxicity, non-flammability, and porosity [15]. Some intrinsic properties, such as biocompatibility, optical transparency, and gas permeability can explain the acceptability of PDMS widely in microfluidic devices for bioassay and real-time imaging [13]. PDMS elastomer is transparent in the optical spectrum with wavelengths from 240 nm to 1100 nm [15]. The refraction index of PDMS is 1.4, making it compatible with various optical imaging methods [15]. Bright-field imaging technique can precisely track, and image small molecules or a single cell in the microfluidic device even at high frame rates [16]. On the other hand, the highly porous structure of PDMS allows for exchanging essential gasses (O2 and CO2) in a controlled manner for both short- and long-term cell cultures [13].
The main drawback of PDMS microfluidic devices in cell biology is the intrinsic high surface hydrophobicity. Due to its hydrophobic nature, the PDMS surface possesses poor wettability with the aqueous solvent [17]. However, most of the biological experiments performed in microchannels need an aqueous solution or a mixture of organic and aqueous solutions [15][18][19]. Cellular attachment is strongly influenced by the physicochemical properties of PDMS, while the attachment might vary depending on the cell types [20]. Moreover, hydrophobicity might lead to the absorption/adsorption of non-specific small molecules and biomolecules present in the cell media or secrete from the cells on the PDMS surface [21]. Cell signaling and behavior might be highly affected because of the depletion of biomolecules and secreted soluble factors [15]. To overcome this limitation, several surface modification methods are developed to increase the hydrophilicity by improving the wettability of the PDMS surface for facilitating cellular adhesion and proliferation in microfluidic devices.
Various methods have been developed and employed for the fabrication of PDMS microfluidic devices, such as soft lithography, inkjet printing [22], and direct writing [23]. Among these, soft lithography is a commonly used technique in PDMS chip fabrication for cell culture [13][15]. Soft lithography provides a simple, but robust fabrication of microchannel with various patterns and high optical transparency [24].
Soft lithography involves a group of patterning methods, such as imprinting, casting, and embossing with the elastomeric master mould or stamp [25]. PDMS exhibits a relatively low glass transition temperature and liquid at room temperature that makes it suitable to fabricate a replica from the master mould [13][17]. The two major steps in soft lithography are photolithography and replica moulding. Photolithography is used to generate the master mould. A photosensitive emulsion called a photoresist is deposited on a silicon wafer and exposed to UV light through a photomask. To dissolve the unexposed regions, a developing reagent is used, and then finally releases the bas-relief structure of the master mould for PDMS fabrication [17]. A silicon master mould can be used several times for replica moulding. Replica moulding can be performed at ambient temperature. In general, liquid PDMS prepolymer is mixed with a curing agent at a ratio of 10:1 (base: curing agent). This ratio provides the optimum mechanical properties and biocompatibility for cell culture [15][26]. Mixing of PDMS prepolymer with the curing agent activates the polymer chains and transforms the liquid materials into the solid elastomer. The time of PDMS curing normally depends on the temperature. PDMS can be cured within an hour at 75 °C while it can take 24 h at room temperature. After curing, the PDMS device is peeled off from the master mould and small inlet and outlet holes are punched. At the final stage, the PDMS device is generally sealed to itself or another flat surface both reversibly, or irreversibly [27]. After bonding, the device is cured for 10 min at 75 °C and becomes ready to use. Figure 1 shows the step-by-step fabrication procedure of a PDMS device by replica moulding.
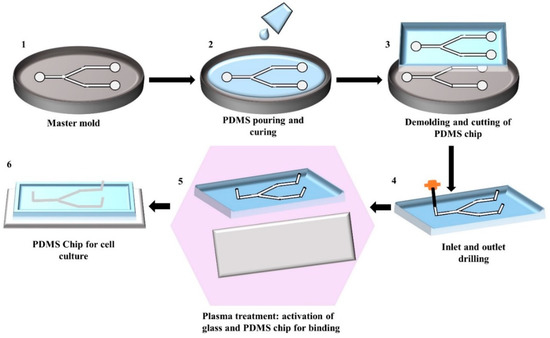
Figure 1. Illustration of the step-by-step fabrication process of a PDMS chip by replica moulding: (1) Generating silicon master mould using photolithography; (2) Pouring of the mixture of PDMS prepolymer and curing agent into the master mould and allowing it to solidify; (3) Peeling of the solidified PDMS from the master mould and cutting it into an appropriate shape; (4) Punching the inlet and outlet holes; (5) Activating the PDMS and glass surface by plasma treatment for facilitating the bonding; (6) Binding and curing of PDMS chip bonded on glass ready to use.
However, the common problem associated with the soft lithography technique is the deformation of patterns during demoulding [24]. This mould based technique requires an expensive photolithography technique to design the master mould that increases the production cost [28]. However, this technique does not require any clean-room environment during chip fabrication, the photolithographic master mould preparation needs to be done inside the clean-room environment [29]. Moreover, trained personnel and a well-equipped lab are required to perform this multi-step fabrication procedure.
The hydrophobicity of PDMS is associated with the organic methyl groups present in the chemical structure of PDMS. Hydrophobicity of PDMS leads to poor wettability and limits the cell adhesion on the PDMS surface. Wettability is defined as the ability of the liquid to maintain contact with a solid surface and quantified by measuring the water contact angle (WCA). A surface with a WCA smaller than 90 °C is referred to as a hydrophilic surface, while WCA greater than this corresponds to a hydrophobic surface [30]. The WCA of PDMS is approximately 108 °C ± 7 °C [31], which makes the cell adhesion difficult on the PDMS surface. Surface modification treatment is required to increase the hydrophilicity of the PDMS surface for optimal ECs adhesion. The most commonly used PDMS surface modification method is plasma treatment because the process is relatively simple and short [32][33][34]. Oxygen, nitrogen, argon, hydrogen bromide, and chlorine gasses are mainly used in plasma treatment [18]. Among all, oxygen plasma treatment shows the most rapid increase of the hydrophilicity of the PDMS surface by removing hydrocarbon groups and introducing polar silanol (SiOH) groups via oxidization [18][35]. On the other hand, PDMS coating with extracellular matrix (ECM) proteins such as collagen, gelatin, and/or fibronectin alter the surface roughness and provide a natural moiety for cell anchoring and survival [36]. Besides, chemical treatment of PDMS surface has been introduced because of ECM protein degradation, as well as instability under shear stress [37]. Chemically modified PDMS surface provides a strong and stable covalent linkage to cell adhesion moieties. Furthermore, the combination of different modification techniques shows better wettability and cell adhesions. This section discusses different surface modification techniques for ECs adhesion, and provides a summary of the recent studies (Table 1), with major pros and cons of different treatments.
Table 1. Summary of the extensively used PDMS surface modification treatment for improving cell adhesion.
|
Method |
Hydrophilicity of PDMS |
Type of Cell Used |
Adhesion of Cells |
Flow Conditions |
Pros |
Cons |
References |
|
Plasma treatment |
Increases as WCA decreases by approximately 30° |
Human primary pulmonary arterial endothelial cells |
100% confluency was achieved after 3 days on plasma-treated PDMS surface |
Confluency was equivalent in both static and flow condition |
Relatively inexpensive Easy to perform. Time-efficient. |
The hydrophilicity of the oxygen plasma-treated PDMS surface is temporary and gradual hydrophobic recovery is shown over time. It is not suitable for long term cell adhesion. |
|
|
Coating with ECM protein-Collagen |
Type I Collagen increases the hydrophilicity to the greatest extent among extracellular matrix (ECM)proteins |
Human umbilical vein endothelial cells (HUVECs) |
Both cell lines were able to attach and proliferate after initial seeding |
Stable under static conditions for a few days |
Good adsorption of collagen onto PDMS among ECM proteins Good modulation of ECs morphology Increases the hydrophilicity of PDMS to one of the greatest extents amongst reagents Exhibits good adhesion of ECs |
Cell detachment occurs after a few days due to the formation of cell clusters Type IV Collagen is a poor reagent for seeding EC Might not be stable under high flow rates as ECs begin to detach at flow rates above 10 μL/min |
|
|
Endothelial cells derived from Human induced pluripotent stem cells (iPSC-ECs) |
More cell activity than HUVEC under flow conditions of 10 μL/min |
||||||
|
Human dermal microvascular endothelial cells |
Confluent layer formed |
Not specified |
|||||
|
HUVECs |
Good adhesion as confluency achieved after an hour |
Cells were stable at flow rates of 5–10 μL/min |
|||||
|
Coating with ECM protein-Gelatin |
Increases the hydrophilicity by increasing the surface roughness |
Sheep Carotid Arterial endothelial cells |
Poor adhesion of endothelial cells (ECs) as compared to other ECM proteins |
Cells were adherent when exposed to the shear stress of 1 dyne/cm2 |
Able to maintain the activity of cells for the longest duration |
Cell aggregation A high tendency for cells to dissociate from PDMS |
|
|
HUVECs, |
Good adhesion |
||||||
|
Coating with ECM protein-Fibronectin |
Hydrophilicity increases significantly |
Sheep Carotid Arterial ECs |
Good adhesion |
Adhesion lasts for a few days without exposure to flow. |
Second among the ECM proteins in seeding ECs The highest rate of reagent adsorption onto PDMS |
Fibronectin is an ECM protein that can lead to cell dissociation |
|
|
HeLa ECs |
Better than gelatin in terms of adhesion |
||||||
|
Human aortic ECs |
Unable to reach confluency |
||||||
|
HUVECs |
The same extent of adhesion as oxygen-fibronectin |
Stable to flow rates at 7.5 mL/min |
|||||
|
Bovine Aortic ECs |
The same extent of adhesion as oxygen-fibronectin |
95% detachment after 2 weeks under static flow |
|||||
|
Coating with biopolymer-Laminin |
Increases but not as much as ECM protein. |
HUVECs |
Poor adhesion of ECs as compared to ECM protein. |
Stable underflow at 5 dyne/cm2 |
Good adhesion |
Spreading of cells over the laminin-modified surface is slow. Might change the cell morphology. |
[61] |
|
Chemical treatment-APTES ((3-aminopropyl) triethoxysilane) |
Increases as WCA decreases by approximately 70° |
HUVECs |
Cells proliferated with the increase in incubation time |
Good stability and adhesion under shear stress (0.5 mm/s) |
Chemical treatment is not prone to degradation Forms amine groups, which is suitable for HUVECs adhesion |
Weaker increase in hydrophilicity as compared to ECM proteins |
|
|
Vascular ECs |
Cell adhesion observed |
||||||
|
Chemical Treatment-PDA (Polydopamine) |
Increases as WCA decreases by 50% |
Vascular ECs Human cerebral microvascular ECs |
Improved adhesion and proliferation for both cell lines |
Poorer response when exposed to flow compared to fibronectin |
Significant increase in hydrophilicity Non-toxic to cells Long term stability for cell culture |
Effect of PDA on cells is poorly understood Seldom used in ECs seeding |
|
|
Chemical Treatment-PEG (Poly (ethylene glycol)) |
Increases as WCA decreases by approximately 57° |
HUVECs |
Adhesion was similar to non-modified PDMS. |
Poor cell adhesion underflow |
Stable for long term culture when used to encapsulate cells |
Poor adhesion when used as a coating reagent |
|
|
(iPSC-ECs) |
When encapsulated with PEG, cells were stable for at least 2 weeks |
||||||
|
Chemical treatment-Silica-Titanium |
Increases but less than ECM proteins |
HUVECs |
Good adhesion of cells |
Not specified |
Does not degrade easily as ECM proteins |
Certain combinations of silica-titanium could present a hostile environment for cells |
|
|
Combination treatment-Oxygen Plasma + Fibronectin |
Increases as WCA decreases by approximately 80° |
HUAECs |
The same extent of adhesion as fibronectin Confluency reached |
Stable adhesion at a physiological flow rate (0.5 mm/s) |
Increases the hydrophilicity of PDMS to a huge extent |
Cell dissociation in long term cell culture |
|
|
Combination treatment-PEG + RGDS (Arg–Gly–Asp–Ser) peptides |
Increases |
HUVECs |
87% of cells coverage observed |
Stable at low flow rates of 0.3 µL/min |
Good adhesion of cells Cells increase with increasing RGDS density |
The combination is not commonly used as ECM proteins |
|
|
Combination Treatment-TEOS (tetraethylorthosilane) + Fibronectin |
Increases |
Primary Pulmonary Artery ECs |
Adhesion of cells was achieved |
Stable under low flow rates of 0.1 mL/h |
Good adhesion of cells |
The detachment of cells might occur at high flow rates |
[70] |
PDMS is the most widely used polymer for the fabrication of microfluidic cell culture devices. The material catches the interest of biomedical researchers because of its chemical inertness and biocompatibility. Easy and low-cost fabrication methods also enhance the use of PDMS in lab-on-a-chip technology. The successful operation of PDMS microfluidic lab-on-a-chip mostly depends on the cell growth and proliferation in the chip. However, the intrinsic hydrophobicity of PDMS can disrupt the optimal cellular adhesion inside the device. Cell adhesion and proliferation might depend on the ratio of the PDMS prepolymer and curing agents and cell types.
Different types of surface modification treatments are performed to increase the hydrophilicity by improving the wettability of the PDMS surface for successful endothelial cells (ECs) attachment. Plasma treatment is the most commonly used modification method for PDMS, but the rapid hydrophobic recovery of the surface limits long-term cell attachment. Coating with different extracellular matrix (ECM) proteins deliberates an easy modification platform, while the increase of wettability varies among different proteins. Moreover, easy dissociation of coating protein underflow is commonly observed. The chemical treatment gives a strong binding affinity to cells with PDMS surface. However, using a chemical could be harsh to cells and cytotoxicity must be checked carefully before use. Surface treated with charged molecules can bind with ECs by electrostatic interaction and can improve the adhesion propensity to some extent. Physical modification of PDMS surface, such as altering surface roughness can improve the cellular adhesion, but this method is only suitable for short-term cell culture.
As one treatment method has some advantages and disadvantages over other methods, it is important to combine different methods together to maximise cell adhesion. However, one set of modifications is not effective for all types of cell lines. Therefore, careful selection of methods and reagents are important for durable and cytocompatible PDMS modification for longer cell culture in dynamic conditions. On the other hand, PDMS elastomer with different topographies modification could directly use in chip fabrication. This might help to omit the surface treatment complexity in a micrometer scale and ease cell seeding inside the chips. Although a PDMS chip for cell culture is still a new area, continuous research for material and method selection, as well as designing new materials for achieving required PDMS properties is indispensable.