
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yaya Zhang | + 1861 word(s) | 1861 | 2021-01-06 09:30:51 | | | |
| 2 | Dean Liu | -567 word(s) | 1294 | 2021-01-07 02:13:42 | | |
Video Upload Options
Terahertz (THz) spectroscopy and imaging technology have seen significant developments in the fields of biology, medical diagnosis, food safety, and nondestructive testing. Label-free diagnosis of malignant tumours has been obtained and also achieved significant development in THz biomedical imaging.
1. Introduction
Terahertz (THz) occupies a frequency range from 0.1 to 10 THz in the electromagnetic spectrum between millimeter and infrared waves, and the corresponding wavelength range is between 30 µm and 3 mm. Due to their properties, THz waves have far-reaching research value and broad application prospects in the field of biomedical imaging[1][2]. Researchers have conducted a number of studies on THz imaging of in vivo biological tissues, including brain tumours, teeth, bone density testing, liver cancer, and burnt skin tissues[3]. The rapid development of THz imaging technology in biomedical applications is mainly due to four excellent properties. Firstly, THz waves have stronger penetrating ability than visible and infrared radiation, so that they can achieve perspective imaging for many opaque non-polar substances[4], such as ceramics, paper, wood, plastics, and non-polar liquids. THz imaging and detection technology is mostly used for the epidermis of tissues and some organs. It can also penetrate the epidermis of animals to the depth of hundreds of microns to image superficial tissues in vivo. THz imaging can serve as a novel technique that complements conventional medical imaging modalities, such as magnetic resonance imaging and X-ray imaging. Secondly, THz radiation has nondestructive properties[5] because their energy is one millionth of the X-ray wave energy, making them suitable for the live detection of human and other organisms without ionising their component elements and breaking their structure. Thirdly, the vibrational and rotational energy levels of most biological macromolecules are located at the bandgap of THz, and every molecule has its own unique signature and fingerprint spectrum[6], allowing researchers to identify molecular structures and analyse substance components. Lastly, THz radiation have a strong absorption effect with water[7], which can be used to distinguish cancer tissues from normal tissues based on the difference of their water content.
According to different detection and signal processing methods, THz detection technology can be divided into spectral detection technology and imaging technology. The principle of THz spectrum detection technology is that the spectrum of most low-frequency biomolecule motion (such as hydrogen bonds, molecular vibration and rotation, and van der Waals force) and the biomolecules have their own unique THz fingerprint spectrum characteristics so that researchers can identify biomolecular samples by analysing the difference of the absorption coefficient or reflection coefficient[6]. The THz Time Domain Spectroscopy (THz-TDS) system can distinguish diseased and normal tissues by analysing the peaks, troughs, absorption spectra, and specific principal component information of the time domain signal. THz-TDS has been widely studied with macromolecules, cells, cancer tissues and other biological structures [8][9][10][11]. However, the THz-TDS detection area is associated with the size of the radiation spot, and the tissues are not completely uniform, which often affects the accuracy of the detection outcomes in clinical medicine. In contrast, CW THz imaging technology can be more accurate and visualised to identify the difference between lesions and normal tissues. Therefore, THz imaging for biological tissues samples detection has more advantages.
Increased attention has been paid by researchers to THz biomedical imaging in recent years. The key application of THz imaging technology in the field of biomedicine is to extract information such as the absorption or refractive index distribution and morphological characteristics of biological samples from the THz intensity and phase images. In 1995, Hu and Nuss demonstrated the first THz imaging system and successfully imaged a chip and leaf by transmission single-point scanning imaging [12]. According to the difference of the working mode of the THz sources, the THz imaging technology is divided into terahertz pulsed imaging (TPI) and continuous-wave (CW THz) THz imaging. The photoconductive method and the optical rectification method are commonly used to generate THz pulses in the THz-TDS systems. The photoconductive method uses high-speed photoconductor materials as the transient current source to generate THz radiation by a self-mode-locked Ti sapphire laser. The optical rectification method utilises the reverse process of the electro-optic effect of ZeTe or LiTaO3 to generate THz pulses, and the power of the radiation is related to the incident radiation power and energy conversion efficiency. The measurement of the TPI is a coherent measurement method, which can obtain both the amplitude and phase information. To detect THz waveforms with the time-domain pulse THz systems, both the electro-optic sampling method and the photoconductive antenna technology are commonly adopted. The electro-optical sampling method has a wide detected bandwidth, so it is suitable for full-field imaging[13][14]. The photoconductive receiver, by using the coherent delay scanning detection method, can obtain the real-time power of the THz waveform. This method can improve the signal-to-noise ratio (SNR) of the system, but the structures of the TPI systems are complex.
The CW THz imaging systems work in a narrow frequency band and utilise the scattering effect of the THz wave on the edge of the internal defect of the samples by detecting the distribution of the intensity of the scattering effect. The average output power of CW THz is higher than that of the THz pulse sources, although the peak output of the pulse sources can be higher than 14 mW[8]. Furthermore, the geometry of the CW THz imaging systems is compact and real time without additional pump optical detection elements. Due to the above advantages, the CW THz imaging technology is more suitable for biomedical detection. The CW THz sources include optically pumped THz gas lasers [15], backward-wave oscillators[16], quantum cascade lasers (QCL)[17], and Gunn diodes. The detector can be divided into point scanning and area array detection according to the working principle. The former includes the Schottky diode[18] and Golay cell[19], and the latter includes microbolometer arrays and pyroelectric cameras[20][21]. Correspondingly, the CW THz imaging technology mainly includes CW THz single-point scanning imaging, full-field imaging, and CW THz three-dimensional imaging. Each has its different imaging system and advantages that are suitable for specific application conditions. The following sections will introduce the characteristics of these methods and their developments in the biomedical field.
2. CW THz Tomography Imaging
The idea of CW THz tomography imaging comes from the X-ray computed tomography (CT). CW THz computed tomography (THz-CT) is another interesting approach for three-dimensional (3D) THz imaging. The penetrability in THz radiation is poorer than X-ray, which provides a better contrast for biological soft tissue imaging. The internal structure of samples can be reflected more accurately, giving comprehensive three-dimensional information of biological tissues. THz-CT is a non-destructive detection method[22]. It detects the one-dimensional Fourier transform of the incident beam or scattering field at different projection angles and then calculates the one-dimensional Fourier transform of the projection of each angle. Finally, these data are combined to construct the two-dimensional Fourier transform of the cross-sectional image of the object. The commonly used data processing method is the filter back projection (FBP) algorithm.
In 2014, Kashiwagi et al. described a THz-CT imaging system based on an IJJ-THz emitter at 440 GHz and a bolometer detector as shown in Figure 12 [23]. The emitted beam was converted to the parallel beam by the first off-axis parabolic mirror, PM1; then, it is focused on the object plane by the second off-axis parabolic mirror, PM2. The sample was a dried pea containing three seeds. The object was placed on the turntable and rotated 360° with a rotating speed of 10 mm/s. The scanning time was 10 min. The typical heart of a pea was seen to be made up of three ventricles, each of them with a thin-wall compose of triangular endothelium, and all of them pressed together. Three round seeds in the different ventricles were also visible at the bottom of the pea.
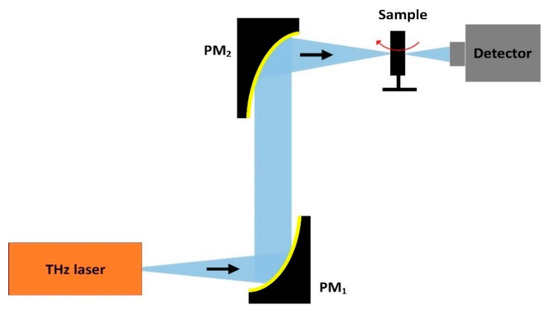
Figure 12. Schematic of the THz-computed tomography (CT) imaging system.
References
- Erik, B.; Heinz-Wilhelm, H.; Maurice, F. Terahertz Techniques, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–28.
- Lee, Y. Principles of Terahertz Science and Technology; Springer: New York, NY, USA, 2009; pp. 1–41.
- Peralta, X.G.; Lipscomb, D.; Wilmink, G.J.; Echchgadda, I. Terahertz spectroscopy of human skin tissue models with different melanin content. Biomed. Opt. Express 2019, 10, 2942–2955.
- Pawar, A.Y.; Sonawane, D.D.; Erande, K.B.; Derle, D.V. Terahertz technology and its applications. Drug Invent. Today 2013, 5, 157–163.
- Fan, S.; He, Y.; Ung, B.; Pickwell-MacPherson, E. The growth of biomedical terahertz research. Appl. Phys. 2014, 47, 374009.
- Ho, L.; Pepper, M.; Taday, P.F. Terahertz spectroscopy: Signatures and fingerprints. Nat. Photonics 2008, 2, 541–543.
- Wang, Y.; Minamide, H.; Tang, M.; Notake, T.; Ito, H. Study of water concentration measurement in thin tissues with terahertz-wave parametric source. Opt. Express 2010, 18, 15504–15512.
- Yardimci, T.; Cakmakyapan, S.; Hemmati, S.; Jarrahi, M. A High-Power Broadband Terahertz Source Enabled by Three Dimensional Light Confinement in a Plasmonic Nanocavity. Sci. Rep. 2017, 7, 4166.
- Cheon, H.; Yang, H.J.; Lee, S.H.; Kim, Y.; Son, J.H. Terahertz molecular resonance of cancer DNA. Sci. Rep. 2016, 6, 37103.
- Reid, C.B.; Reese, G.; Gibson, A.; Wallace, V.P. Terahertz Time-Domain Spectroscopy of Human Blood. IEEE Trans. Terahertz Sci. Technol. 2013, 3, 363–367.
- Alibadi, A.; Macgrogan, G.; Grzyb, J.; Guillet, J.P.; Mavarani, L.; Mounaix, P.; Hillger, P.; Cassar, Q.; Zimmer, T.; Pfeiffer, U.R. Pilot study of freshly excised breast tissue response in the 300–600 GHz range. Biomed. Opt. Express 2018, 9, 2930–2942.
- Hu, B.B.; Nuss, M.C. Imaging with terahertz waves. Opt. Lett. 1995, 16, 1716–1718.
- Schall, M.; Helm, H.; Keiding, S.R. Far Infrared Properties of Electro-Optic Crystals Measured by THz Time-Domain Spectroscopy. Int. J. Infrared Millim. Waves 1999, 20, 595–604.
- Good, J.T.; Holland, D.B.; Finneran, I.A.; Carroll, P.B.; Kelley, M.J.; Blake, G.A. A decade-spanninghigh-resolution asynchronous optical sampling terahertz time-domain and frequency comb spectrometer. Rev. Sci. Instrum. 2015, 86, 103–107.
- Pavlov, S.G.; HuBers, H.W.; Riemann, H.; Zhukavin, R.K.; Orlova, E.E.; Shastin, V.N. Terahertz optically pumped Si:Sb laser. J. Appl. Phys. 2002, 92, 5632–5634.
- He, W.; Zhang, L.; Bowes, D.; Yin, H.; Ronald, K.; Phelps, A.D.R.; Cross, A.W. Generation of broadband terahertz radiation using a backward wave oscillator and pseudospark-sourced electron beam. Appl. Phys. Lett. 2015, 133501.
- Wang, X.; Shen, C.; Jiang, T.; Zhan, Z.; Deng, Q.; Li, W.; Wu, W.; Yang, N.; Chu, W.; Duan, S. High-power terahertz quantum cascade lasers with 0.23 W in continuous wave mode. Aip. Adv. 2016, 6, 075210.
- Maestrini, A.; Thomas, B.; Wang, H.; Jung, C.; Treuttel, J.; Jin, Y.; Chattopadhyay, G.; Mehdi, I.; Beaudin, G. Schottky diode-based terahertz frequency multipliers and mixers. C. R. Phys. 2010, 11, 480–495.
- Golay Detectors. Available online: http://www.tydexoptics.com/pdf/Golay_Detectors.pdf (accessed on 25 October 2020).
- Behnken, B.N.; Karunasiri, G.; Chamberlin, D.R.; Robrish, P.R.; Faist, J. Real-time imaging using a 2.8 THz quantum cascade laser and uncooled infrared micrometer camera. Opt. Lett. 2008, 33, 440–442.
- Ophir THz Laser Measurement Products. Available online: https://www.ophiropt.com/laser-measurement/sites/default/files/Pyrocam_1.pdf (accessed on 25 October 2020).
- Stübling, E.; Rehn, A.; Siebrecht, T.; Bauckhage, Y.; Öhrström, L.; Eppenberger, P.; Balzer, J.; Ruhli, F.; Koch, M. Application of a robotic THz imaging system for sub-surface analysis of ancient human remains. Sci. Rep. 2019, 9, 3390.
- Kashiwagi, T.; Nakade, K.; Saiwai, Y.; Minami, H.; Kitamura, T.; Watanabe, C.; Ishida, K.; Sekimoto, S.; Asanuma, K.; Yasui, T.; et al. Computed tomography image using sub-terahertz waves generated from a high-Tc superconducting intrinsic Josephson junction oscillator. Appl. Phy. Lett. 2014, 104, 82603.




