
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sandra Schlögl | + 3097 word(s) | 3097 | 2021-01-04 08:49:22 | | | |
| 2 | Camila Xu | Meta information modification | 3097 | 2021-01-06 09:30:35 | | |
Video Upload Options
Coumarin (2H-1-benzopyran-2-one) is an oxygen containing heterocycle and belongs to the subcategory of lactones.
1. Introduction
Coumarin (2H-1-benzopyran-2-one) is named after the French word for tonka bean (Dipteryx odorata) Coumarou as Vogel first extracted coumarin from tonka beans in 1820 [1]. Later, coumarin was also isolated from sweet clover, bison grass, and woodruff [2]. There exist six different basic natural types of coumarin: (a) simple coumarin derivatives, (b) dihydrofurano coumarin derivatives, (c) furano coumarin derivatives, (d) pyrano coumarin derivatives (linear and angular), (e) phenyl coumarin derivatives, and (f) bicoumarin derivatives (Figure 1) [3].
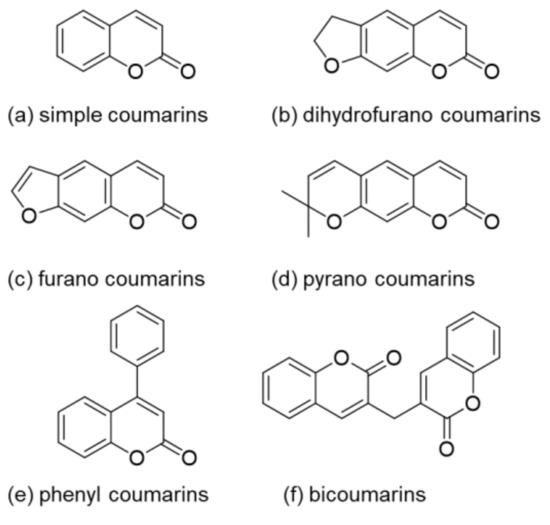
Figure 1. Structures of the different coumarin classes.
As they are secondary metabolites of bacteria, plants, and fungi [3][4], coumarins appear in many different natural sources such as essential oils, fruits, green tea, and other foods [3][5]. Although these natural compounds occur in various parts of different plants, their highest concentration can be found in fruits, roots, stems, and leaves. However, their concentration distribution is influenced by environmental and seasonal changes [6]. Apart from plants, microorganisms are also a natural source of coumarins. For instance, novobiocin and coumermycin have been extracted from Streptomyces and aflatoxins from Aspergillus species [7][8]. While aflatoxins are very toxic fungal metabolites [6], novobiocin and coumermycin are members of an antibiotics group as they are able to inhibit DNA gyrase. All members of this antibiotics group feature a 3-amino-4-hydroxy-coumarin moiety and a substituted deoxysugar [9].
The isolated coumarins are mostly biologically active and show antimicrobial, antibacterial, antifungal [3][10][11][12][13][14][15][16][17][18][19], and antiviral activity [17][18]. Others reveal antioxidant [17][20][21][22], anti-inflammatory [3][22], and/or anticorrosive [23] activity. There are also reports about the usage of coumarin derivatives for medical application. For example, some types of coumarin are used for Alzheimer disease treatment due to their ability to inhibit acetylcholinesterase (AchE) [17][18][24]. Moreover, authors reported anti-HIV [25][26], anticancer [5][17][18], and anticoagulant [3][4] activity.
Coumarins are not only interesting because of their bioactivity but also due to their photoreactivity. In 1902, Ciamician and Silber investigated the photodimerization of coumarin under UV light exposure (>300 nm), in ethanol, or in aqueous solutions (Figure 2) [27].
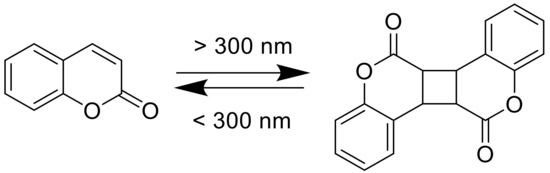
Figure 2. Photodimerization of coumarin.
During photoirradiation, four different types of dimers are being formed: anti head-to-head, anti head-to-tail, syn head-to-head, and syn head-to-tail. Krauch et al. investigated the photocleavage of anti-head-to-head dimers in dioxane with wavelength <310 nm, in 1966 (Figure 2) [28]. Additionally, coumarin and its derivatives are highly fluorescent in the visible light range. Researchers started their studies on photophysics of coumarin moieties in the 1940s. In the late 1950s, Wheelock and his coworkers showed a shift of the fluorescence band by substitutions on the coumarin structure [29][30]. These properties enable the use of coumarin molecules in numerous different application fields such as organic light emitting diodes (OLEDs) [31][32][33][34], optical data storages [35][36], laser dyes [37][38], or drug delivery systems [39][40][41][42][43][44].
Researchers from different areas, such as medicine, polymer science, or biology, are working on coumarins due to their versatile properties [29]. However, the isolation from natural resources is time consuming and not profitable. Therefore, the research regarding the synthesis of coumarin and its derivatives has gained increased attention. Lončarić et al. published a review of numerous synthesis strategies in 2020 involving Perkin reaction, Knoevenagel condensation, Pechmann condensation, Wittig reaction, Baylis-Hillman reaction, Claisen rearrangement, and Vilsmeier–Haack or Suzuki cross-coupling reaction, to name only a few examples [18].
Trenor et al. reviewed the use of coumarin moieties in the design of functional polymers in 2004. They focused on coumarin-containing polymers in electro-optical studies, the development of photoreversible systems, coumarin in biopolymers, polymerizations, chiral stationary phases for HPLC, and fluorescent tags and fluoroprobes [29]. Since 2004, there has been a steadily growing interest in using the versatility of coumarin chromophores in the design of functional polymers. This current contribution serves as an update to the previous review from Trenor et al. and will focus on the latest developments in coumarin functional polymers.
2. Photoreaction Mechanisms of Coumarin and Its Derivatives
Due to the excellent electronic, photophysical, and photochemical properties of coumarin and its derivatives, these chromophores can undergo various photoreactions and play a key role in different fields of applications. Prominent examples are photoactive surfaces relying on photofuses, controlled release of biochemical substances based on caged compounds, introduction of (reversibly) cross-linkable moieties in polymers, and generation of reactive chemical species by exploiting coumarin compounds as photoinitiators. Based on the relevant applications, this chapter has been divided into three parts in accordance with its reactivity and mechanisms involved.
2.1. Photocleavage of Coumarin-Caged Compounds and Photolabile Surfaces Bearing Functional Coumarin Groups (Photofuses)
The irreversible response of (coumarin-4-yl)methyl derivatives upon light exposure has been particularly exploited as a tool for temporally and spatially controlled probes of cell-based processes [45][46] as well as for the generation of patterned surfaces in different field of applications including bioanalytical science, cell biology, tissue engineering, etc. [47][48][49][50][51]. Moreover, its ability to undergo photolysis by nonresonant two-photon excitation (Figure 3), at high light intensity, facilitates salient properties of this chromophore such as: (a) “phototherapeutic window” between 650 and 950 nm with lower scattering and reduced phototoxic effect [46] and (b) orthogonal protecting group based on wavelength-selective response [51].
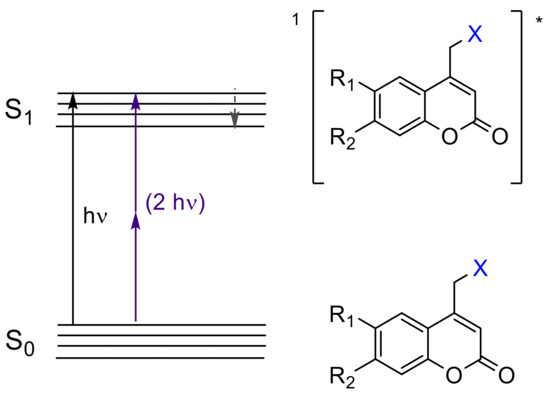
Figure 3. Excited singlet state (S1)* of coumarin-4-yl derivatives by one-photon (UV light) and two-photon transfer (visible and near-IR light). The asterisk (*) denotes the excited state of the molecules.
The caged compounds and photofuses are constituted from a wide range of functional groups: thiol, phosphate, carboxylate, anhydride, sulfate, alcohol as carbonate, amine as carbamate, etc. [46][52] (Figure 4). Recently, the scope of these derivatives has grown, improving their long-wavelength absorption and uncaging efficiency (GM) by extending π-conjugation at 3-position on the coumarin ring [53][54][55][56] (Figure 5).
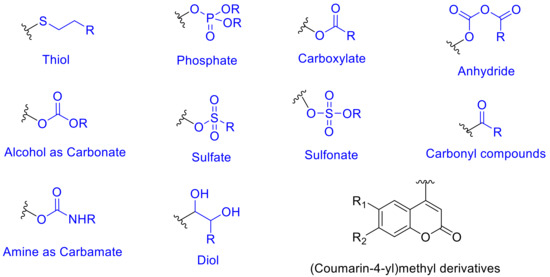
Figure 4. Coumarin-4-yl derivatives employed as caged compounds and photofuses.
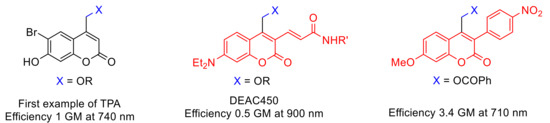
Figure 5. Coumarin-caged compounds undergoing two-photon absorption (TPA).
From the mechanistic point of view, after one-photon absorption (UV light) or two-photon absorption (visible or near-IR light), the relaxation to the lowest level of the excited singlet state (S1) takes place [52] (1[Coum-X]* in Figure 6). This intermediate might progress in one of three ways: (a) fluorescence, (b) nonradiative process, and (c) cleavage of the C-X bond forming a singlet ion pair (Figure 6a–c). The factor that determines the subsequent path is the reaction rate constant (k). When kf (fluorescence rate constant ~108 s−1) [52] and knr (nonradiative rate constant ~108 s−1) [52] are smaller than kcl (cleavage rate constant ~109 s−1) [52], the photolysis predominates. In this case, any possible photophysical deactivation (a) or (b) is hampered due to the higher stability of the excited state and its subsequent separated species. These pathways have been well studied by Bendig et al. in ester and amide derivatives [52][57][58][59][60].
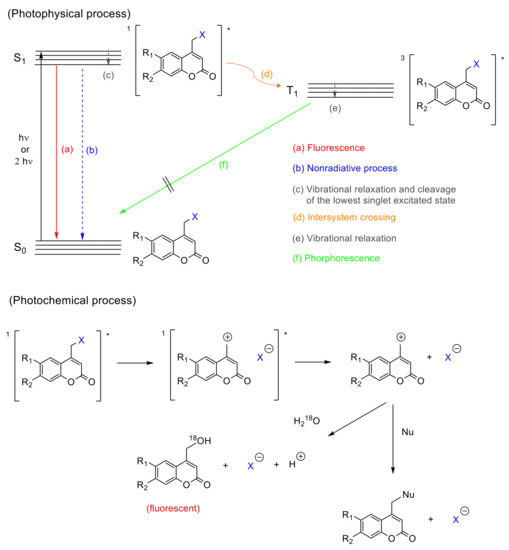
Figure 6. Mechanism of the photocleavage reaction of coumarin derivatives. The asterisk (*) denotes the excited state of the molecules.
There is evidence that the cleavage of the C-X bond in coumarin derivatives follows a heterolytic route. A study carried out with 18O-labeled water, also by Bendig et al., pointed the generation of ionic species by SN1 mechanism, after isolating 4-(18O-hydroxymethyl)coumarin out. This mechanism is evidenced by the higher efficiencies obtained using polar protic solvents and good leaving groups (X with low pka values) in the coumarin derivatives [58] (solvent-assisted photoheterolysis, Figure 6). Additionally, 4-methylcoumarin is only detected in trace amounts, which is expected to be the main product in a homolytic cleavage and favors the heterolytic hypothesis [58]. However, the possibility of a homolytic route should not be entirely excluded, since a singlet electron transfer from the homolytic bond cleavage could drive to the ion pair as well [58].
There is also a controversy regarding a possible intersystem crossing (Figure 6d). Studies by Arai et al., carried out with 7-aminocoumarins, showed a weak transient band in the range of 500–700 nm (Figure 6e) that was ascribed to the triplet state. This result suggests that the photolysis of caged compounds bearing this chromophore partially proceeds from the triplet excited state, which is convenient since the deactivation by recombination of the separated species is not allowed from a quantum mechanics point of view [61]. On the contrary, Bendig et al. postulated that this process proceeds only from the excited singlet state, based on the lack of phosphorescence (Figure 6f) characteristic for triplet states, and the trace amount of 4-methylcoumarin, which should be formed from the radical species generated in the triplet state after the intersystem crossing (Figure 6d) [58].
Once the singlet ion pair is formed after photolysis, according to Bendig et al., the ions can escape from the solvent cage and the (coumarin)methylium cation reacts with any nucleophile found in the medium.
2.2. [2πs + 2πs] Photocycloaddition Reaction of Coumarin Groups
The [2πs + 2πs] photocycloaddition of coumarin derivatives upon irradiation with UV light (>300 nm), to generate cyclobutane derivatives with itself or even with another double bond in the reaction medium, has been widely employed in the synthesis of polymeric networks [41][62][63][64][65][66][67][68]. The advantages of this method are the creation of a network without extra monomers or photoinitiators [66] and the possibility to reverse the process (by regenerating the double bonds using radiation wavelength <290 nm). This reversible process provides networks with diverse properties such as self-healing [68][69][70][71], reversible wettability [72], reversible thickness [73], and reversible assembly in supramolecular architectures [74][75], which makes the photodimerization of coumarin a useful reaction pathway in the field of polymers.
Apart from a few exceptions [76][77], thermal [2πs + 2πs] cycloaddition processes are forbidden due to conservation of orbital symmetry [77][78]. Therefore, the photo-induced reaction (exposure with wavelengths in the UVA spectral region) takes place between the excited antibonding orbital π of one double bond (excited HOMO = SOMO = π*) and the antibonding orbital π (LUMO = π*) of another double bond (Figure 7). This way, the cyclobutane ring/the two new sigma bonds is/are formed from two π bonds (Figure 8).
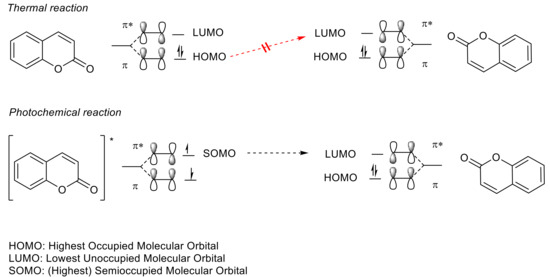
Figure 7. Conservation of orbital symmetry for [2πs + 2πs] cycloaddition of coumarin. The asterisk (*) denotes the excited state of the molecules.
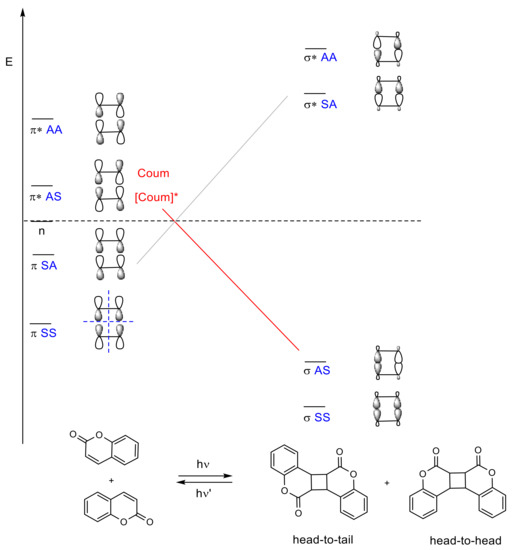
Figure 8. Energy and correlation diagram of the [2πs + 2πs] photodimerization of coumarin. The asterisk (*) denotes the excited state of the molecules.
The mechanistic approach of the [2πs + 2πs] photocycloaddition of coumarin can be rationalized by comparing the hypothetical mechanism between enones and alkenes [79], since coumarin derivatives are formally enones. After one- or two-photon absorption of the ground state of coumarin (0Coum) [80], the excited singlet state 1[Coum]* is produced by either n → π* or π → π* transition (Figure 9) [77][78]. This intermediate might progress in one of the following four pathways: (a) fluorescence, (b) nonradiative process, (c) intersystem crossing to an excited triplet state 3[Coum]*, and (d) the formation of a singlet exciplex 1[1Coum-0Coum]* (Figure 6). Since the intersystem crossing in six-membered cyclic enones is an efficient process, path (c) is most common [77][78][81].
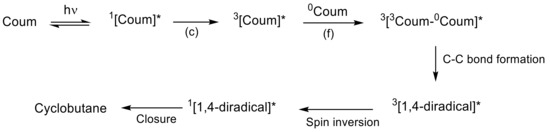
Figure 9. Possible mechanism of cyclobutane formation from [2πs + 2πs] photodimerization of coumarin. The asterisk (*) denotes the excited state of the molecules.
In turn, the triplet state 3[Coum]* can either (c) decay back to the ground state or (f) combine with the ground state of coumarin (0Coum) to generate a triplet exciplex 3[3Coum-0Coum]*. The exciplex can form a carbon–carbon bond and produce a triplet 1,4-diradical, 3[1,4-diradical]*, which must undergo spin inversion to the singlet diradical, 1[1,4-diradical]*, before a closure to the cyclobutane ring occurs (Figure 9).
In a more recent work, Bach et al. studied the role of a Lewis acid in the intramolecular [2πs + 2πs] photocycloaddition of coumarin derivatives and dihydropyridones [82]. In this study, it was pointed out that the uncatalyzed [2πs + 2πs] photocycloaddition of coumarin derivatives goes through its singlet state, while the use of a Lewis acid seems to stabilize the singlet state and facilitates the intersystem crossing (ISC), and therefore, the coumarin photocycloaddition in this case occurs via the triple state.
In [2πs + 2πs] photocycloadditions, four possible products could be obtained: syn head-to-head, syn head-to-tail, anti head-to-head, and anti head-to-tail (Figure 8). Their stereoselectivity and regioselectivity depends on several factors (polarity of solvents, addition of a sensitizer, crystal packing, and distances between double bonds) and is related to the reaction medium (in solution, in solid, or even in inclusion complex). A review published by Trenor et al. details the effects of radiation doses, solvent, and concentration over the [2πs + 2πs] photocycloadditions in coumarin derivatives in different media [29].
Reversible [2πs + 2πs] photocycloaddition, leading to the cleavage of the cyclobutane rings, can be symmetric (the same double bonds are generated) or asymmetric (two different types of doubles bonds). The photocleavage of cyclobutane coumarin dimers was studied by Hasegawa et al., concluding that the photocleavage, in this case, is symmetric [29] since the cleavage of cyclobutanes attached to five- or six-member ring takes place and maintains the more stable ring. On the other hand, Görner et al. proposed, on the basis of their studies in presence and absence of triplet state sensitizers, that the photocleavage occurs via a nonfluorescent and short-lived singlet state [83]. In addition, in turn, Motzkus et al. corroborated Görner’s results and proposed a ring scission by steps with the formation of intermediates [84].
Joy et al., reported two different mechanisms for coumarin chromophores in photoresponsive and biodegradable functional polyesters [85]. In this case, irradiation (350 nm) of the coumarin chromophores bearing good leaving groups (ester or phosphate group) in the 4-methyl position leads to the cross-linked network, while the scission of the coumarin photodimers is obtained upon irradiation at 254 nm.
2.3. Coumarin Derivatives Serving as Photoinitiators
In pursuit of photopolymerization reactions [86] involving novel photoinitiators (PIs), which possess strong two-photon absorption (TPA) in the visible/near-IR region [87][88], and with the aim of providing better features for 3D printing [87][88][89], three-dimensional optical data storage [90][91], and microfabrication [90][92][93], research into coumarin and its derivatives has been growing up to now. These applications, which use TPA, benefit from the localized excitation of the photocurable resin near to the focal volume of the laser, due to its probability of being proportional to the square of light intensity [90][93].
Different types of coumarin-based two-photon photoinitiators (2PIs) have been developed: (a) unimolecular, (b) bimolecular, and (c) multicomponent systems [88].
-
(a) Unimolecular system (photocleavable PI, Type I): upon absorption, the excited state of the PI undergoes a homolytic cleavage to produce free radicals. Subsequently, an electron transfer from one of these radicals to a monomer generates the radical anion species responsible for the polymerization [86][90]. In contrast to the photocleavage mechanism in (coumarin-4-yl)methyl derivatives, the radical species are generated from the triplet state after the intersystem crossing [87][89][92][94]. This type of coumarin-based 2PIs is generally constituted by conjugated carbonyl groups (photocleavage of a double bond in α-carbonyl position) [92][94] or by oxime-ester (photocleavage of an N-O bond) [87][95][96][97] (Figure 10).
-
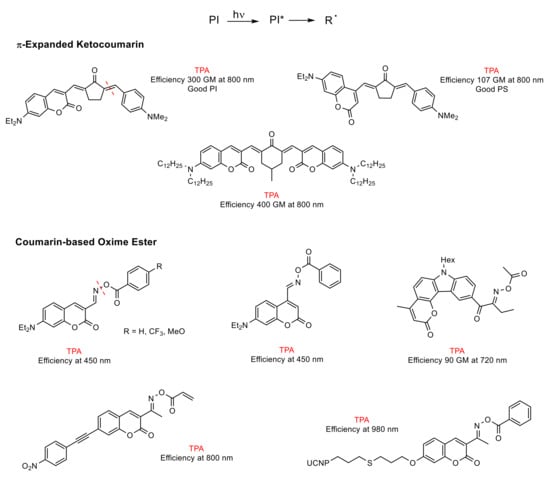
Figure 10. Unimolecular coumarin-based photoinitiators (PIs). The asterisk (*) denotes the excited state of the molecules.
-
(b) Bimolecular system (PI/coI (co-initiator) or PI/PS (photosensitizer), Type II) [86]: Once the PI is excited in the PI/coI system, a transfer of an electron/proton takes place between both compounds (see Figure 11a), thus resulting in radicals or ions that initiate the polymerization reaction. Some examples of typical coIs, employed in combination with ketocoumarins as PIs, are bis-(4-tert-butylphenyl)iodonium hexafluorophosphate (Iod or SpeedCure 938), N-phenylglycine (NPG), and ethyl 4-(dimethylamino)benzoate (EDB) [89]. The triplet state pathway is also possible in the mechanism of bimolecular system (KC/Iod), since free energy change for an electron transfer (∆Get) from the aforementioned state is favorable [88][89].
-
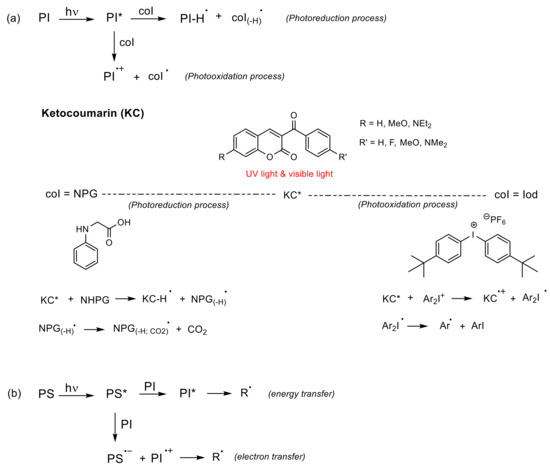
Figure 11. Bimolecular coumarin-based PIs. (a) Photoreduction process where the coI transfers an electron and a proton into the excited state of the PI, and photooxidation process where the excited state of PI transfers an electron into the coI; (b) The excited PS might transfer energy or an electron into the PI. The asterisk (*) denotes the excited state of the molecules.
In contrast, in the PI/PS system, a transfer of energy or an electron occurs from the excited PS to the PI after irradiation, thereby generating radical or ions [86], as shown in Figure 11b. To the best of our knowledge, there are cases of coumarin derivatives used as photosensitizers, albeit only in multicomponent systems [98].
-
(c) Multicomponent system (three or more compounds): this system involves combinations that allow an improvement in the performance of PIs under the conditions required by the applications [86]. Thus, its mechanism is rather complex.
Lalevée et al. studied in depth the mechanism of trimolecular systems [99], concluding that its advantage over bimolecular systems is its ability to convert terminating species (PHI● radicals) into initiating species (coI(-H)● and A●), which increases the yield of the initiating species (see Figure 12). Another valuable aspect of this system, highlighted by Lalevée, is the regeneration of the PI. The performance of the PI as a photocatalyst (PC) allows the use of low light intensity as well as low amounts of PI.
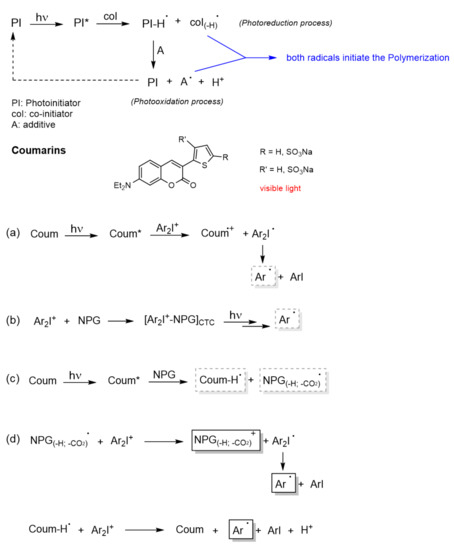
Figure 12. Multicomponent coumarin-based PIs. Mechanistic pathway of the systems: (a) Coum/Iod, (b) NPG/Iod, (c) Coum/NPG, and (d) Coum/Iod/NPG. The asterisk (*) denotes the excited state of the molecules.
The first mechanistic pathway (Figure 12a) is exactly the same as in the bimolecular system, i.e., upon absorption, the excited coumarin (Coum*) transfers an electron to the Iod (the acceptor agent). This transfer is allowed from the singlet state as well as from the triplet state. Then, the iodonium salt (Ar2I+) decomposes into an aryl radical (Ar●) and aryl iodine. The formation of this radical (Ar●) was confirmed by detection of the radical adduct Ar●/NPG (N-phenylglycine) by ESR-ST (electron spin resonance spin trapping) experiments.
In the second mechanistic pathway (Figure 12b), the formation of a Charge Transfer Complex (CTC) [88][100] between NPG (donor component) and iodine (acceptor component) was proposed. This complex might also release the aryl radicals, since some photopolymerizations were carried out with high conversions using this bimolecular system.
The third mechanistic pathway (Figure 12c), also follows a bimolecular system. The interaction of the excited coumarin (Coum*) with NPG leads to the generation of a radical. This radical is obtained following the next sequences: (a) PNG transfers an electron to Coum*, (b) then a proton, and (c) in the last, step it undergoes decarboxylation. Since the decarboxylation step is irreversible, this radical might be considered as the initiating species in the trimolecular system.
After this analysis and doing a recombination of the active species, the mechanism postulated by Lavelée et al. for the trimolecular system is described in the fourth mechanistic pathway (Figure 12d). The first initiating radical, generated from Coum/NPG system, reacts with the iodonium salt to generate a cation (NPG(-H;-CO2)+) and the aryl radical (Ar●). On the other hand, the protonated coumarin radical also reacts with the iodonium salt regenerating the PI (Coum) and yielding an aryl radical (Ar●) and a proton. Therefore, this trimolecular systems is able to catalyze free radical polymerizations as well as cationic polymerizations.
References
- Borges, F.; Roleira, F.; Milhazes, N.; Santana, L.; Uriarte, E. Simple Coumarins and Analogues in Medicinal Chemistry: Occurrence, Synthesis and Biological Activity. Curr. Med. Chem. 2005, 12, 887–916. [Google Scholar] [CrossRef] [PubMed]
- Ajay Kumar, K.; Renuka, N.; Pavithra, G.; Vasanth Kumar, G. Comprehensive review on coumarins: Molecules of potential chemical and pharmacological interest. J. Chem. Pharm. Res. 2015, 7, 67–81. [Google Scholar]
- Venugopala, K.N.; Rashmi, V.; Odhav, B. Review on natural coumarin lead compounds for their pharmacological activity. BioMed Res. Int. 2013, 2013, 963248. [Google Scholar] [CrossRef]
- Lei, L.; Xue, Y.-B.; Liu, Z.; Peng, S.-S.; He, Y.; Zhang, Y.; Fang, R.; Wang, J.-P.; Luo, Z.-W.; Yao, G.-M.; et al. Coumarin derivatives from Ainsliaea fragrans and their anticoagulant activity. Sci. Rep. 2015, 5, 13544. [Google Scholar] [CrossRef]
- Lacy, A.; O’Kennedy, R. Studies on Coumarins and Coumarin-Related Compounds to Determine their Therapeutic Role in the Treatment of Cancer. Curr. Pharm. Des. 2004, 10, 3797–3811. [Google Scholar] [CrossRef] [PubMed]
- O’Kennedy, R.; Thornes, R.D. Coumarins: Biology, Applications and Mode of Action; Wiley: Hoboken, NJ, USA, 1997; ISBN 978-0-471-96997-6. [Google Scholar]
- Cooke, D.; Fitzpatrick, B.; O’Kennedy, R.; Mc Cormack, T.; Egan, D. Coumarins—Multifaceted Molecules with Many Analytical and Other Applications. Coumarins: Biology, Applications and Mode of Action; John Wiley & Sons: Hoboken, NJ, USA, 1997. [Google Scholar]
- Cooke, D. Studies on the Mode of Action of Coumarins (Coumarin, 6-hydroxycoumarin, 7-hydroxycoumarin and Esculetin) at a Cellular Level. Ph.D. Thesis, Dublin City University, Dublin, Ireland, 1999. [Google Scholar]
- Huawei, C.; Walsh, C.T. Coumarin formation in novobiocin biosynthesis: V-hydroxylation of the aminoacyl enzyme tyrosyl-S-NovH by a cytochrome P450 NovI. Chem. Biol. 2001, 8, 301–312. [Google Scholar]
- Chamsaz, E.A.; Mankoci, S.; Barton, H.A.; Joy, A. Nontoxic Cationic Coumarin Polyester Coatings Prevent Pseudomonas aeruginosa Biofilm Formation. ACS Appl. Mater. Interfaces 2017, 9, 6704–6711. [Google Scholar] [CrossRef]
- Brahmbhatt, D.I.; Singh, S.; Patel, K.C. Synthesis, characterization and biological activity of some poly(coumarin ethylene)s. Eur. Polym. J. 1999, 35, 317–324. [Google Scholar] [CrossRef]
- Erol, I.; Sanli, G.; Dİlek, M.; Ozcan, L. Synthesis and characterization of novel methacrylate copolymers based on sulfonamide and coumarine: Monomer reactivity ratios, biological activity, thermal stability, and optical properties. J. Polym. Sci. A Polym. Chem. 2010, 48, 4323–4334. [Google Scholar] [CrossRef]
- Chitra, R.; Kayalvizhy, E.; Jeyanthi, P.; Pazhanisamy, P. Synthesis, Characterization and Antimicrobial Screening of Coumarin Copolymers. Chem. Sci. Trans. 2014, 3, 722–730. [Google Scholar] [CrossRef]
- Hamdi, N.; Saoud, M.; Romerosa, A.; Hassen, R.B. Synthesis, spectroscopic and antibacterial investigations of new hydroxy ethers and heterocyclic coumarin derivatives. J. Heterocycl. Chem. 2008, 45, 1835–1842. [Google Scholar] [CrossRef]
- Srivastava, A.; Mishra, V.; Singh, P.; Kumar, R. Coumarin-based polymer and its silver nanocomposite as advanced antibacterial agents: Synthetic path, kinetics of polymerization, and applications. J. Appl. Polym. Sci. 2012, 126, 395–407. [Google Scholar] [CrossRef]
- Venkatesan, S.; Ranjithkumar, B.; Rajeshkumar, S.; Anver Basha, K. Synthesis, characterization, thermal stability and antibacterial activity of coumarin based methacrylate copolymers. Chin. J. Polym. Sci. 2014, 32, 1373–1380. [Google Scholar] [CrossRef]
- Katsori, A.M.; Hadjipavlou-Litina, D. Coumarin derivatives: An updated patent review (2012–2014). Expert Opin. Pat. 2014, 24, 1323–1347. [Google Scholar] [CrossRef] [PubMed]
- Lončarić, M.; Gašo-Sokač, D.; Jokić, S.; Molnar, M. Recent Advances in the Synthesis of Coumarin Derivatives from Different Starting Materials. Biomolecules 2020, 10, 151. [Google Scholar] [CrossRef]
- Patel, H.J.; Patel, M.G.; Patel, R.J.; Patel, K.H.; Patel, R.M. Synthesis, Characterization, Thermal Studies, and Antimicrobial Screening of Poly (acrylate)s Bearing 4-Methyl Coumarin Side Groups. Iran. Polym. J. 2008, 17, 635–644. [Google Scholar]
- Chebil, L.; Rhouma, G.B.; Chekir-Ghedira, L.; Ghoul, M. Enzymatic Polymerization of Rutin and Esculin and Evaluation of the Antioxidant Capacity of Polyrutin and Polyesculin. In Biotechnology; Ekinci, D., Ed.; InTech: London, UK, 2015; ISBN 978-953-51-2040-7. [Google Scholar]
- Li, Q.; Wei, L.; Zhang, J.; Gu, G.; Guo, Z. Significantly enhanced antioxidant activity of chitosan through chemical modification with coumarins. Polym. Chem. 2019, 10, 1480–1488. [Google Scholar] [CrossRef]
- Iranshahi, M.; Askari, M.; Sahebkar, A.; Hadjipavlou-Litina, D. Evaluation of antioxidant, anti-inflammatory and lipoxygenase inhibitory activities of the prenylated coumarin umbelliprenin. DARU 2009, 99–104. [Google Scholar]
- Liu, X.; Zhang, R.; Li, T.; Zhu, P.; Zhuang, Q. Novel Fully Biobased Benzoxazines from Rosin: Synthesis and Properties. ACS Sustain. Chem. Eng. 2017, 5, 10682–10692. [Google Scholar] [CrossRef]
- Stefanachi, A.; Leonetti, F.; Pisani, L.; Catto, M.; Carotti, A. Coumarin: A Natural, Privileged and Versatile Scaffold for Bioactive Compounds. Molecules 2018, 23, 250. [Google Scholar] [CrossRef]
- Olmedo, D.; Sancho, R.; Bedoya, L.M.; López-Pérez, J.L.; Del Olmo, E.; Muñoz, E.; Alcamí, J.; Gupta, M.P.; San Feliciano, A. 3-Phenylcoumarins as inhibitors of HIV-1 replication. Molecules 2012, 17, 9245–9257. [Google Scholar] [CrossRef] [PubMed]
- Bairagi, S.H.; Salaskar, P.P.; Loke, S.D.; Surve, N.N.; Tandel, D.V. Medicinal Significance of Coumarins: A Review. Int. J. Pharm. Res. 2012, 4, 16–19. [Google Scholar]
- Ciamician, G.; Silber, P. Chemische Lichtwirkungen. Chem. Ber 1902, 35, 4128–4131. [Google Scholar] [CrossRef]
- Chen, Y.; Chou, C.-F. Reversible photodimerization of coumarin derivatives dispersed in poly(vinyl acetate). J. Polym. Sci. Part A Polym. Chem. 1995, 33, 2705–2714. [Google Scholar] [CrossRef]
- Trenor, S.R.; Shultz, A.R.; Love, B.J.; Long, T.E. Coumarins in polymers: From light harvesting to photo-cross-linkable tissue scaffolds. Chem. Rev. 2004, 104, 3059–3077. [Google Scholar] [CrossRef]
- Wheelock, C.E. The Fluoreszence of Some Coumarins. J. Chem. Ed. 1950, 81, 1348–1352. [Google Scholar]
- Kotchapradist, P.; Prachumrak, N.; Sunonnam, T.; Tarsang, R.; Namuangruk, S.; Sudyoadsuk, T.; Keawin, T.; Jungsuttiwong, S.; Promarak, V. N-coumarin derivatives as hole-transporting emitters for high efficiency solution-processed pure green electroluminescent devices. Dyes Pigment. 2015, 112, 227–235. [Google Scholar] [CrossRef]
- Prachumrak, N.; Pojanasopa, S.; Tarsang, R.; Namuangruk, S.; Jungsuttiwong, S.; Keawin, T.; Sudyoadsuk, T.; Promarak, V. Synthesis and characterization of carbazole dendronized coumarin derivatives as solution-processed non-doped emitters and hole-transporters for electroluminescent devices. New J. Chem. 2014, 38, 3282. [Google Scholar] [CrossRef]
- Prachumrak, N.; Potjanasopa, S.; Rattanawan, R.; Namuangruk, S.; Jungsuttiwong, S.; Keawin, T.; Sudyoadsuk, T.; Promarak, V. Coumarin-cored carbazole dendrimers as solution-processed non-doped green emitters for electroluminescent devices. Tetrahedron 2014, 70, 6249–6257. [Google Scholar] [CrossRef]
- Teixeira, E.; Lima, J.C.; Parola, A.J.; Branco, P.S. Incorporation of Coumarin-Based Fluorescent Monomers into Co-Oligomeric Molecules. Polymers 2018, 10, 396. [Google Scholar] [CrossRef]
- Gindre, D.; Iliopoulos, K.; Krupka, O.; Evrard, M.; Champigny, E.; Sallé, M. Coumarin-Containing Polymers for High Density Non-Linear Optical Data Storage. Molecules 2016, 21, 147. [Google Scholar] [CrossRef] [PubMed]
- Iliopoulos, K.; Krupka, O.; Gindre, D.; Sallé, M. Reversible two-photon optical data storage in coumarin-based copolymers. J. Am. Chem. Soc. 2010, 132, 14343–14345. [Google Scholar] [CrossRef] [PubMed]
- Miluski, P.; Kochanowicz, M.; Zmojda, J.; Dorosz, D. Energy conversion in 7-(Diethylamino)coumarin doped PMMA fluorescent fibre. Opt. Quant. Electron. 2017, 49. [Google Scholar] [CrossRef]
- Albertazzi, L.; Storti, B.; Marchetti, L.; Beltram, F. Delivery and subcellular targeting of dendrimer-based fluorescent pH sensors in living cells. J. Am. Chem. Soc. 2010, 132, 18158–18167. [Google Scholar] [CrossRef]
- Chung, J.W.; Lee, K.; Neikirk, C.; Nelson, C.M.; Priestley, R.D. Photoresponsive coumarin-stabilized polymeric nanoparticles as a detectable drug carrier. Small 2012, 8, 1693–1700. [Google Scholar] [CrossRef] [PubMed]
- Samanta, P.; Kapat, K.; Maiti, S.; Biswas, G.; Dhara, S.; Dhara, D. pH-labile and photochemically cross-linkable polymer vesicles from coumarin based random copolymer for cancer therapy. J. Colloid Interface Sci. 2019, 555, 132–144. [Google Scholar] [CrossRef]
- Rahimi, S.; Khoee, S.; Ghandi, M. Development of photo and pH dual crosslinked coumarin-containing chitosan nanoparticles for controlled drug release. Carbohydr. Polym. 2018, 201, 236–245. [Google Scholar] [CrossRef]
- Lin, H.-M.; Wang, W.-K.; Hsiung, P.-A.; Shyu, S.-G. Light-sensitive intelligent drug delivery systems of coumarin-modified mesoporous bioactive glass. Acta Biomater. 2010, 6, 3256–3263. [Google Scholar] [CrossRef]
- Huang, Y.; Dong, R.; Zhu, X.; Yan, D. Photo-responsive polymeric micelles. Soft Matter 2014, 10, 6121–6138. [Google Scholar] [CrossRef]
- Wang, H.; Miao, W.; Wang, F.; Cheng, Y. A Self-Assembled Coumarin-Anchored Dendrimer for Efficient Gene Delivery and Light-Responsive Drug Delivery. Biomacromolecules 2018, 19, 2194–2201. [Google Scholar] [CrossRef]
- Pelliccioli, A.P.; Wirz, J. Photoremovable protecting groups: Reaction mechanisms and applications. Photochem. Photobiol. Sci. 2002, 1, 441–458. [Google Scholar] [CrossRef] [PubMed]
- Klán, P.; Šolomek, T.; Bochet, C.G.; Blanc, A.; Givens, R.; Rubina, M.; Popik, V.; Kostikov, A.; Wirz, J. Photoremovable protecting groups in chemistry and biology: Reaction mechanisms and efficacy. Chem. Rev. 2013, 113, 119–191. [Google Scholar] [CrossRef] [PubMed]
- Stegmaier, P.; Alonso, J.M.; Campo, A.D. Photoresponsive surfaces with two independent wavelength-selective functional levels. Langmuir 2008, 24, 11872–11879. [Google Scholar] [CrossRef] [PubMed]
- Wylie, R.G.; Shoichet, M.S. Two-photon micropatterning of amines within an agarose hydrogel. J. Mater. Chem. 2008, 18, 2716–2721. [Google Scholar] [CrossRef]
- San Miguel, V.; Bochet, C.G.; del Campo, A. Wavelength-selective caged surfaces: How many functional levels are possible? J. Am. Chem. Soc. 2011, 133, 5380–5388. [Google Scholar] [CrossRef]
- Bowen, A.M.; Ritchey, J.A.; Moore, J.S.; Nuzzo, R.G. Programmable chemical gradient patterns by soft grayscale lithography. Small 2011, 7, 3350–3362. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Miguel, V.S.; del Campo, A. Light-triggered multifunctionality at surfaces mediated by photolabile protecting groups. Macromol. Rapid Commun. 2013, 34, 310–329. [Google Scholar] [CrossRef]
- Schmidt, R.; Geissler, D.; Hagen, V.; Bendig, J. Mechanism of photocleavage of (coumarin-4-yl)methyl esters. J. Phys. Chem. A 2007, 111, 5768–5774. [Google Scholar] [CrossRef]
- Furuta, T.; Wang, S.S.-H.; Dantzker, J.L.; Dore, T.M.; Bybee, W.J.; Callaway, E.M.; Denk, W.; Tsien, R.Y. Brominated 7-hydroxycoumarin-4-ylmethyls: Photolabile protecting groups with biologically useful cross-sections for two photon photolysis. Proc. Natl. Acad. Sci. USA 1999, 96, 1193–1200. [Google Scholar] [CrossRef]
- Olson, J.P.; Kwon, H.-B.; Takasaki, K.T.; Chiu, C.Q.; Higley, M.J.; Sabatini, B.L.; Ellis-Davies, G.C.R. Optically selective two-photon uncaging of glutamate at 900 nm. J. Am. Chem. Soc. 2013, 135, 5954–5957. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Boinapally, S.; Katan, C.; Abe, M. Synthesis and photochemical reactivity of caged glutamates with a π-extended coumarin chromophore as a photolabile protecting group. Tetrahedron Lett. 2013, 54, 7171–7174. [Google Scholar] [CrossRef]
- Chitose, Y.; Abe, M.; Furukawa, K.; Katan, C. Design, Synthesis, and Reaction of π-Extended Coumarin-based New Caged Compounds with Two-photon Absorption Character in the Near-IR Region. Chem. Lett. 2016, 45, 1186–1188. [Google Scholar] [CrossRef]
- Bendig, J.; Helm, S.; Hagen, V. (Coumarin-4-yl)Methyl Ester of cGMP and 8-Br-cGMP: Photochemical Fluorescence Enhancement. J. Fluoresc. 1997, 7, 357–361. [Google Scholar] [CrossRef]
- Schade, B.; Hagen, V.; Schmidt, R.; Herbrich, R.; Krause, E.; Eckardt, T.; Bendig, J. Deactivation Behavior and Excited-State Properties of (Coumarin-4-yl)methyl Derivatives. 1. Photocleavage of (7-Methoxycoumarin-4-yl)methyl-Caged Acids with Fluorescence Enhancement. J. Org. Chem. 1999, 64, 9109–9117. [Google Scholar] [CrossRef]
- Eckardt, T.; Hagen, V.; Schade, B.; Schmidt, R.; Schweitzer, C.; Bendig, J. Deactivation behavior and excited-state properties of (coumarin-4-yl)methyl derivatives. 2. Photocleavage of selected (coumarin-4-yl)methyl-caged adenosine cyclic 3’,5’-monophosphates with fluorescence enhancement. J. Org. Chem. 2002; 67, 703–710. [Google Scholar] [CrossRef]
- Schmidt, R.; Geissler, D.; Hagen, V.; Bendig, J. Kinetics study of the photocleavage of (coumarin-4-yl)methyl esters. J. Phys. Chem. A 2005, 109, 5000–5004. [Google Scholar] [CrossRef]
- Senda, N.; Momotake, A.; Nishimura, Y.; Arai, T. Synthesis and Photochemical Properties of a New Water-Soluble Coumarin, Designed as a Chromophore for Highly Water-Soluble and Photolabile Protecting Group. BCSJ 2006, 79, 1753–1757. [Google Scholar] [CrossRef]
- Sinkel, C.; Greiner, A.; Agarwal, S. A Polymeric Drug Depot Based on 7-(2′-Methacryloyloxyethoxy)-4-methylcoumarin Copolymers for Photoinduced Release of 5-Fluorouracil Designed for the Treatment of Secondary Cataracts. Macromol. Chem. Phys. 2010, 211, 1857–1867. [Google Scholar] [CrossRef]
- López-Vilanova, L.; Martinez, I.; Corrales, T.; Catalina, F. Photoreversible crosslinking of poly-(ethylene-butyl-acrylate) copolymers functionalized with coumarin chromophores using microwave methodology. React. Funct. Polym. 2014, 85, 28–35. [Google Scholar] [CrossRef]
- Jellali, R.; Alexandre, M.; Jérôme, C. Photosensitive polydimethylsiloxane networks for adjustable-patterned films. Polym. Chem. 2017, 8, 2499–2508. [Google Scholar] [CrossRef]
- Jellali, R.; Bertrand, V.; Alexandre, M.; Rosière, N.; Grauwels, M.; de Pauw-Gillet, M.-C.; Jérôme, C. Photoreversibility and Biocompatibility of Polydimethylsiloxane-Coumarin as Adjustable Intraocular Lens Material. Macromol. Biosci. 2017, 17. [Google Scholar] [CrossRef]
- Govindarajan, S.R.; Jain, T.; Choi, J.-W.; Joy, A.; Isayeva, I.; Vorvolakos, K. A hydrophilic coumarin-based polyester for ambient-temperature initiator-free 3D printing: Chemistry, rheology and interface formation. Polymer 2018, 152, 9–17. [Google Scholar] [CrossRef]
- Hughes, T.; Simon, G.P.; Saito, K. Photocuring of 4-arm coumarin-functionalised monomers to form highly photoreversible crosslinked epoxy coatings. Polym. Chem. 2019, 10, 2134–2142. [Google Scholar] [CrossRef]
- Abdollahi, A.; Roghani-Mamaqani, H.; Herizchi, A.; Alidaei-Sharif, H.; Enayati, A.; Sajedi-Amin, S. Light-induced spherical to dumbbell-like morphology transition of coumarin-functionalized latex nanoparticles by a [2π + 2π] cycloaddition reaction: A fast and facile strategy to anisotropic geometry. Polym. Chem. 2020, 11, 2053–2069. [Google Scholar] [CrossRef]
- Ling, J.; Rong, M.Z.; Zhang, M.Q. Coumarin imparts repeated photochemical remendability to polyurethane. J. Mater. Chem. 2011, 21, 18373. [Google Scholar] [CrossRef]
- Ling, J.; Rong, M.Z.; Zhang, M.Q. Photo-stimulated self-healing polyurethane containing dihydroxyl coumarin derivatives. Polymer 2012, 53, 2691–2698. [Google Scholar] [CrossRef]
- Aguirresarobe, R.H.; Martin, L.; Aramburu, N.; Irusta, L.; Fernandez-Berridi, M.J. Coumarin based light responsive healable waterborne polyurethanes. Prog. Org. Coat. 2016, 99, 314–321. [Google Scholar] [CrossRef]
- Wagner, N.; Theato, P. Light-induced wettability changes on polymer surfaces. Polymer 2014, 55, 3436–3453. [Google Scholar] [CrossRef]
- Sato, E.; Nagai, S.; Matsumoto, A. Reversible thickness control of polymer thin films containing photoreactive coumarin derivative units. Prog. Org. Coat. 2013, 76, 1747–1751. [Google Scholar] [CrossRef]
- He, J.; Zhao, Y. Light-responsive polymer micelles, nano- and microgels based on the reversible photodimerization of coumarin. Dye. Pigment. 2011, 89, 278–283. [Google Scholar] [CrossRef]
- Li, J.; Jiang, H.; Hu, W.; Xia, H.; Zou, G.; Zhang, Q. Photo-controlled hierarchical assembly and fusion of coumarin-containing polydiacetylene vesicles. Macromol. Rapid Commun. 2013, 34, 274–279. [Google Scholar] [CrossRef]
- Wen, Y.; Song, Y.; Zhao, D.; Ding, K.; Bian, J.; Zhang, X.; Wang, J.; Liu, Y.; Jiang, L.; Zhu, D. Highly regio- and enantioselective thermal 2 + 2 cycloaddition of coumarin in a crystalline inclusion complex under high vacuum. Chem. Commun. 2005, 2732–2734. [Google Scholar] [CrossRef] [PubMed]
- Goodall, G.W.; Hayes, W. Advances in cycloaddition polymerizations. Chem. Soc. Rev. 2006, 35, 280–312. [Google Scholar] [CrossRef] [PubMed]
- Woodward, R.B.; Hoffmann, R. The Conservation of Orbital Symmetry. Angew. Chem. Int. Ed. 1969, 8, 781–932. [Google Scholar] [CrossRef]
- Crimmins, M.T.; Reinhold, T.L. Organic Reactions: Enone olefin [2 + 2] photochemical cycloadditions. Org. React. 2004, 44, 297–588. [Google Scholar]
- Belfield, K.D.; Bondar, M.V.; Liu, Y.; Przhonska, O.V. Photophysical and photochemical properties of 5,7-dimethoxycoumarin under one- and two-photon excitation. J. Phys. Org. Chem. 2003, 16, 69–78. [Google Scholar] [CrossRef]
- Schuster, D.I.; Lem, G.; Kaprinidis, N.A. New insights into an old mechanism: [2 + 2] photocycloaddition of enones to alkenes. Chem. Rev. 1993, 93, 3–22. [Google Scholar] [CrossRef]
- Brimioulle, R.; Bauer, A.; Bach, T. Enantioselective Lewis Acid Catalysis in Intramolecular 2 + 2 Photocycloaddition Reactions: A Mechanistic Comparison between Representative Coumarin and Enone Substrates. J. Am. Chem. Soc. 2015, 137, 5170–5176. [Google Scholar] [CrossRef]
- Wolff, T.; Görner, H. Photocleavage of dimers of coumarin and 6-alkylcoumarins. J. Photochem. Photobiol. A 2010, 209, 219–223. [Google Scholar] [CrossRef]
- Jiang, M.; Paul, N.; Bieniek, N.; Buckup, T.; Hampp, N.; Motzkus, M. Photocleavage of coumarin dimers studied by femtosecond UV transient absorption spectroscopy. Phys. Chem. Chem. Phys. 2017, 19, 4597–4606. [Google Scholar] [CrossRef]
- Maddipatla, M.V.S.N.; Wehrung, D.; Tang, C.; Fan, W.; Oyewumi, M.O.; Miyoshi, T.; Joy, A. Photoresponsive Coumarin Polyesters That Exhibit Cross-Linking and Chain Scission Properties. Macromolecules 2013, 46, 5133–5140. [Google Scholar] [CrossRef]
- Fouassier, J.-P.; Morlet-Savary, F.; Lalevée, J.; Allonas, X.; Ley, C. Dyes as Photoinitiators or Photosensitizers of Polymerization Reactions. Materials 2010, 3, 5130–5142. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Hu, P.; Zhu, J.; Liu, R.; Li, Z.; Hu, Z.; Chen, Q.; Dietliker, K.; Liska, R. Cleavable Unimolecular Photoinitiators Based on Oxime-Ester Chemistry for Two-Photon Three-Dimensional Printing. ChemPhotoChem 2019, 3, 1090–1094. [Google Scholar] [CrossRef]
- Abdallah, M.; Hijazi, A.; Graff, B.; Fouassier, J.-P.; Rodeghiero, G.; Gualandi, A.; Dumur, F.; Cozzi, P.G.; Lalevée, J. Coumarin derivatives as versatile photoinitiators for 3D printing, polymerization in water and photocomposite synthesis. Polym. Chem. 2019, 10, 872–884. [Google Scholar] [CrossRef]
- Abdallah, M.; Dumur, F.; Hijazi, A.; Rodeghiero, G.; Gualandi, A.; Cozzi, P.G.; Lalevée, J. Keto-coumarin scaffold for photoinitiators for 3D printing and photocomposites. J. Polym. Sci. 2020, 58, 1115–1129. [Google Scholar] [CrossRef]
- Cumpston, B.H.; Ananthavel, S.P.; Barlow, S.; Dyer, D.L.; Ehrlich, J.E.; Erskine, L.L.; Heikal, A.A.; Kuebler, S.M.; Lee, I.Y.S.; McCord-Maughon, D.; et al. Two-photon polymerization initiators for three-dimensional optical data storage and microfabrication. Nature 1999, 398, 51–54. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, Y.; Li, Y. Photoexcitation mechanisms of new D-π-A coumarin derivatives in linear and nonlinear optical processes. J. Mater. Sci. 2016, 27, 7132–7140. [Google Scholar] [CrossRef]
- Xue, J.; Zhao, Y.; Wu, F.; Fang, D.-C. Effect of bridging position on the two-photon polymerization initiating efficiencies of novel coumarin/benzylidene cyclopentanone dyes. J. Phys. Chem. A 2010, 114, 5171–5179. [Google Scholar] [CrossRef]
- Whitby, R.; Kay, A.; Simpson, M.C. Triphenylamine two-photon photoinitiators for 3D laser microfabrication. In Three-Dimensional Microfabrication Using Two-Photon Polymerization; Elsevier: Amsterdam, The Netherlands, 2020; pp. 101–141. ISBN 9780128178270. [Google Scholar]
- Nazir, R.; Danilevicius, P.; Ciuciu, A.I.; Chatzinikolaidou, M.; Gray, D.; Flamigni, L.; Farsari, M.; Gryko, D.T. π-Expanded Ketocoumarins as Efficient, Biocompatible Initiators for Two-Photon-Induced Polymerization. Chem. Mater. 2014, 26, 3175–3184. [Google Scholar] [CrossRef]
- Li, Z.; Zou, X.; Zhu, G.; Liu, X.; Liu, R. Coumarin-Based Oxime Esters: Photobleachable and Versatile Unimolecular Initiators for Acrylate and Thiol-Based Click Photopolymerization under Visible Light-Emitting Diode Light Irradiation. ACS Appl. Mater. Interfaces 2018, 10, 16113–16123. [Google Scholar] [CrossRef]
- Zhou, R.; Malval, J.-P.; Jin, M.; Spangenberg, A.; Pan, H.; Wan, D.; Morlet-Savary, F.; Knopf, S. A two-photon active chevron-shaped type I photoinitiator designed for 3D stereolithography. Chem. Commun. 2019, 55, 6233–6236. [Google Scholar] [CrossRef]
- Li, Z.; Zou, X.; Shi, F.; Liu, R.; Yagci, Y. Highly efficient dandelion-like near-infrared light photoinitiator for free radical and thiol-ene photopolymerizations. Nat. Commun. 2019, 10, 3560. [Google Scholar] [CrossRef] [PubMed]
- Senyurt, A.F.; Hoyle, C.E. Three component ketocoumarin, amine, maleimide photoinitiator II. Eur. Polym. J. 2006, 42, 3133–3139. [Google Scholar] [CrossRef]
- Zivic, N.; Bouzrati-Zerelli, M.; Kermagoret, A.; Dumur, F.; Fouassier, J.-P.; Gigmes, D.; Lalevée, J. Photocatalysts in Polymerization Reactions. ChemCatChem 2016, 8, 1617–1631. [Google Scholar] [CrossRef]
- Garra, P.; Graff, B.; Morlet-Savary, F.; Dietlin, C.; Becht, J.-M.; Fouassier, J.-P.; Lalevée, J. Charge Transfer Complexes as Pan-Scaled Photoinitiating Systems: From 50 μm 3D Printed Polymers at 405 nm to Extremely Deep Photopolymerization (31 cm). Macromolecules 2018, 51, 57–70. [Google Scholar] [CrossRef]




