1000/1000
Hot
Most Recent

Obesity, a complex and multifactorial disease associated with excessive adiposity or body fat, currently affects over a third of the world’s population. Obesity is closely related to a significant increase in the morbidity risk of chronic diseases, such as disability, depression, type 2 diabetes, hypertension, cardiovascular diseases, cancers, and mortality, thus representing a serious public health problem. According to the World Health Organization (WHO), the body mass index (BMI) is used as a tool to assess overweight or obesity; a BMI ≥ 40 kg/m2 is characteristic of severe obesity. Rare genetic obesity disorders are characterized by mutations of genes strongly involved in the central or peripheral regulation of energy balance. These mutations are effective in causing the early onset of severe obesity and insatiable hunger (hyperphagia), suggesting that the genetic component can contribute to 40–70% of obesity.”
According to the World Health Organization (WHO), the body mass index (BMI) is used as a tool to assess overweight or obesity; a BMI ≥ 40 kg/m2 is characteristic of severe obesity. Obesity is a consequence of an energy imbalance between caloric intake and energy expenditure, leading to a positive energy balance with a consequent increase in body weight [1][2]. Factors both hereditary or genetic, family history, socioeconomic and sociocultural conditions are considered risk factors for obesity [3]. Much evidence, through the study of genes strongly involved in the central or peripheral regulation of energy balance, including variants in leptin (LEP), leptin receptor (LEPR), proopiomelanocortin (POMC), neuropeptide Y (NPY), melanocortin receptor (MC4R), and the gene associated with fat mass and obesity (FTO), suggests a genetic component, contributing to 40–70% of obesity. However, the roles of genes in the processes leading to obesity are still unclear [4][5]. Furthermore, the genes that could influence an individual predisposition to gain weight are still unknown. Modern gene technology has improved our understanding of the molecular mechanisms involved in body weight regulation by identifying poorly known genetic aberrations [6][7]. Obesity induced by genetic factors can be classified into monogenic, caused by a single genetic mutation, syndromic, associated with other phenotypes, such as abnormalities of neurological development or organs/systems malformations, and finally, polygenic caused by the mutation of a large number of genes [6][8]. In addition, in the last years, a link between epigenetic modifications and metabolic health in humans has been found; research has been focused on the role of epigenetics and on metabolic disorders associated with obesity [9][10]. This review is aimed to summarize the current knowledge of the genetic causes of obesity, especially monogenic obesity, with the purpose to describe the role of epigenetic mechanisms in obesity and metabolic diseases.
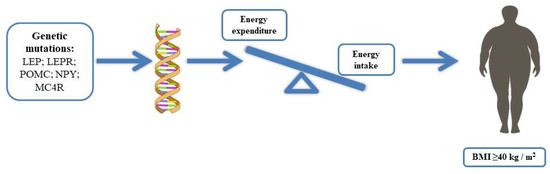
Monogenic obesity is caused by a mutation or deficiency in a single gene [11]. Mutations in genes that have a physiological role in the hypothalamic leptin–melanocortin energy balance system, such as mutations in leptin, leptin receptor, POMC, prohormone 1/3 convertases (PC1/3), MC4R, are related to the currently known monogenic forms of obesity (Figure 1). However, three genes, i.e., SIM1, BDNF, and tropomyosin-related kinase B (TRKB), when mutated, cause obesity, although the exact mechanisms by which these genetic defects lead to obesity are still unknown [12][13][14]. Mutations of the indicated genes are extremely rare, and new studies are essential to investigate these genetic variants with the aim to clarify the mechanisms and promote effective strategies to control and counteract the progression of specific clinical phenotypic features. Furthermore, new techniques, such as mass spectrometry, might reveal new available markers, as in the case of LEPR mutations, useful for early recognition of obesity risk and intervention.
Syndromic obesity includes Bardet–Biedl, Prader–Willi, Alstrom, and Smith–Magenis syndromes, characterized by obesity as the predominant phenotype and associated with disorders such as mental retardation, congenital defects in organs, dysmorphism of the limbs, or endocrine and facial dysfunction. Although numerous studies have revealed the genes or chromosomal regions involved in the etiology of many of these syndromes, their relationship with the development of obesity is still unknown[6][11][15]. Bardet–Biedel syndrome (BBS) is a rare autosomal recessive ciliopathy, characterized by retinal dystrophy, obesity, post-axial polydactyly, renal dysfunction, mental retardation, and hypogonadism. Currently, mutations in 18 different genes causing BBS have been identified [16][17]. Prader–Willi Syndrome (PWS) is the most common cause of syndromic obesity worldwide. Patients with this syndrome have severe neonatal hypotonia, eating disorders, such as anorexia and hypophagia, global cognitive impairment, behavioral abnormalities, hypotonia, delayed motor development, and hormonal deficiencies, such as growth hormone deficiency, hypothyroidism, hypogonadism, and ghrelin abnormalities. The genetic defect in PWS is the inactivation of the Prader–Willi critical region (PWCR) located in the 15q11–13 region of the paternal chromosome, while the PWCR on the maternal chromosome is epigenetically silenced through methylation, which leads to the monoallelic expression of paternal genes. The genes localized in the PWCR and expressed in the hypothalamus, involved in the syndrome, are MKRN3 (makorin 3), MAGEL2 (similar to MAGE 2), NDN (necdin), NPAP1 (nuclear pore-associated protein 1), SNURF–SNRPN (SNRPN upstream reading frame–small nuclear ribosomal protein 1)[18]. Alstrom syndrome is another rare autosomal recessive syndrome caused by mutations in the ALMS1 gene, which shows retinal degeneration, early-onset obesity, type 2 diabetes mellitus, and perceptive hearing loss, as observed in the case of BBS. In addition, this syndrome exhibits primary cilia dysfunction, suggesting that the ALMS1 protein located in the centrosome and ciliary basal body plays a role in the formation or maintenance of primary cilia[13][19]. Smith–Magenis syndrome (SMS) is a neurodevelopmental disorder associated with intellectual disability, sleep disturbance, early-onset obesity, and extensive behavioral deficits, caused by a heterozygous microdeletion containing retinoic acid-induced gene 1 (RAI1) or by a mutation within RAI1. Like PWS, it shows early weight gain during development and reduced satiety [20][21]. The relationships between the development of obesity and the genes or chromosomal regions involved in the development of these syndromes require to be fully clarified. Moreover, there are several symptoms in common among the various syndromes; therefore, further studies are fundamental to differentiate the various syndromic forms for early diagnosis of and specific treatment for each disease.
Polygenic obesity is due to the simultaneous presence of DNA variation in multiple genes and interaction with environmental factors; previous studies have shown that the specific set of polygenic variants relevant to obesity in one individual will hardly correspond to polygenic variants in another obese subject, because of inter-individual heterogeneity [8][22].
Currently, two gene variants, i.e., of MC4R, already known as the most common cause of monogenic obesity, and FTO-associated gene, have been identified, with small but replicable effects on body weight. Further studies are fundamental to confirm the involvement in the regulation of body weight of a third gene, i.e., insulin-induced gene 2 (INSIG2)[23][24][25][26][27].
In conclusion, further investigations are crucial to disclose the genes involved in the pathogenesis of obesity, as well as all the molecular pathways involved in genetic obesity. Moreover, new forthcoming data might permit the design of drugs effective in preventing and counteracting this complex pathology.
Epigenetics is one of the promising areas of future medical research that can influence the way people develop and manage diseases[28]. Epigenetics is the study of heritable changes in gene expression that result in phenotype variations without changes in the DNA sequence. Factors such as age, diet, environment, and disease status can influence epigenetic changes [29][30]. Epigenetics represents a control layer to determine which genes are down-regulated and which genes are up-regulated in specific cells of the body [31][32]. The epigenetic mechanisms currently identified are DNA methylation, posttranslational modification of histone proteins, and non-coding RNA (ncRNA)-associated gene silencing [31][32]. The risk of developing obesity or diseases such as type 2 diabetes can be influenced by diet and environmental factors. Therefore, numerous scientists are interested in studying the implication of different epigenetic processes in the pathogenesis of these diseases [33][34][35]. Although it is known that external factors can cause cell-type dependent epigenetic changes, the regulation of these processes, as well as the extent of the changes, the types of cells in which they occur, and the most predisposed individuals, remain unknown. Several studies showed that a healthy diet can positively influence the individual epigenetic profile; several data, indeed, indicate that normal-weight and non-diabetic people have epigenetic profiles different from those of obese and diabetic subjects [36]. Non-nutritional risk factors associated with obesity such as hyperglycemia, inflammation, endocrine disruptors, hypoxia, and oxidative stress, as well as nutritional factors, appear to be involved in epigenetic modifications that influence adipogenesis and insulin sensitivity [34]. The emergence of epigenetic profiles that will contribute to the creation of individual microbiomes and susceptibility to the development of obesity, inflammation, and a cluster of other disorders, is already observed in fetal age and is influenced by maternal diet during pregnancy. Indeed, recent evidence has shown that maternal nutrition is closely associated with obesity in the offspring, indicating that obesity has an evolutionary origin [37][38]. Currently, studies on animal models demonstrate an effect on the epigenome of early childhood nutritional exposure and long-term metabolic health of newborns; on the contrary, human data are still limited, although several studies have provided clear evidence that exposure to unhealthy nutrition during pregnancy is associated with methylation changes in newborns, resulting in modification of the adult phenotype [39][40]. In 2012, Begum et al. studied the implication of malnutrition in pregnancy on the development of obesity and type II diabetes in a sheep model. The authors found that sheep that had suffered from moderate maternal malnutrition showed a decrease in the methylation of the promoters of the proopiomelanocortin receptor (POMCR) and glucocorticoid receptor (GR) in the fetal hypothalamus, which could lead to a long-term altered regulation of energy balance in the newborn [41]. In addition, epigenetic changes induced by unhealthy feeding can directly affect adulthood. Animal model studies, indeed, have shown that a diet rich in fats and carbohydrates, with a consequent increase in body weight, can be associated with changes in DNA methylation patterns, which affect the promoter region of several genes involved in energetic homeostasis and obesity, such as LEP, NADH dehydrogenase (ubiquinone) 1 β subcomplex subunit 6 (NDUFB6), and FASN. Recent evidence has suggested that nutrients involved in the methyl group metabolism can significantly influence epigenetics [42][43]. The genetic and epigenetic machinery regulates body weight homeostasis; it appears, indeed, that the epigenetic regulation of gene expression represents an important factor in the inflammatory process, with the consequent secretion of adipokines, such as leptin, and cytokines, such as TNF, caused by the increase in adipose tissue associated with obesity. In 2009, Campion et al. assessed whether epigenetic regulation of the human TNF promoter by cytosine methylation could be involved in the predisposition to lose weight after a balanced low-calorie diet, constituting the first step towards a personalized diet based on epigenetic criteria. Their data showed that TNF promoter methylation was a good biomarker for predicting diet-induced weight loss [44]. These results were confirmed in 2011 by Cordero et al., who suggested that the methylation levels of leptin and TNF-alpha could be used as epigenetic biomarkers with regard to the response to a low-calorie diet. Moreover, it is possible to predict the susceptibility to weight loss, as well as some comorbidities, such as hypertension or type 2 diabetes [45]. Further studies are required to identify and understand the role of epigenetic signals that could be used as early predictors of metabolic risk. At the same time, these data will permit the development of drugs or dietary treatments to improve the prevention and therapy of obesity.
The expression of genes, in particular, those related to the leptin–melanocortin system, fundamental for the regulation of energy homeostasis, involved in the development of obesity, is modulated by genetic factors and environmental factors. It is important to clarify the pathways involved in the pathogenesis of obesity induced by genetic mutations and to design specific therapeutic strategies. Furthermore, new scientific advancements are essential to identify molecules able to interfere with molecular pathways and control the progression of symptoms. It will be useful to manage molecules able to cross the blood–brain barrier and exert their therapeutic effects on central neurons. Currently, because genetic mutations do not fully clarify the inheritance of obesity, other forms of variation, such as that due to epigenetics, must be taken into the account. Complex interactions between food components and epigenetic mechanisms, such as histone modifications, DNA methylation, non-coding RNA expression, and chromatin remodeling factors, lead to the dynamic regulation of gene expression that controls cell phenotype. In particular, predisposition to obesity and weight loss outcomes have been repeatedly associated with changes in epigenetic patterns. Nutritional influence on epigenetic regulation can occur at all ages, although the perinatal period is the moment of maximum phenotypic plasticity.