| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hiroki Kuniyasu | + 1890 word(s) | 1890 | 2020-12-18 08:46:37 | | | |
| 2 | Rita Xu | -760 word(s) | 1130 | 2020-12-29 07:48:23 | | | | |
| 3 | Rita Xu | -1 word(s) | 1129 | 2020-12-30 03:07:21 | | |
Video Upload Options
Pterostilbene (PTE) is a natural sterbenoid contained in blueberries that has an antioxidant effect. In contrast, PTE also generates oxidative stress in cancer cells and provides an antitumor effect.
1. Introduction
Pterostilbene (PTE), a known nutritional ingredient in blueberries, is a natural dimethylated analog of resveratrol (RES) [1]. Compared to RES, which is the same stilbenoid, PTE has a higher bioavailability and a high blood retention for a long duration, which results in stronger effects than RES [2][3]. Due to its antioxidant effects, PTE is expected to be useful for the prevention of carcinogenesis, neurodegenerative diseases, inflammatory diseases, hyperlipidemia, and vascular disorders, as well as for improving diabetes [1][2]. Extensive research has been conducted on the application of PTE to cancer treatment. PTE inhibits cancer growth, anti-apoptotic survival, metastasis, and cancer cell stemness; however, the mechanism has not been completely clarified [4][5].
The mechanism of action of PTE revealed so far is diverse. Its actions in cancer include arrest of cell cycle in S and G2/M phase, induction of apoptosis by reactive oxygen species (ROS) and autophagy, suppression of matrix metalloproteinase-2/-9 expression and sphere formation, and inhibition of cell surface fibronectin polymerization, which diminishes the metastatic potential [6][7][8]. The cancer-related factors and signal transduction pathways that PTE suppresses are also diverse, and include Janus kinase-2/signal transduction and activator of transcription-3, human telomerase reverse transcriptase, c-Myc, mutant epithelial growth factor receptor, protein kinase B (also known as AKT), multiple drug resistance-1, sirtuin-1, DNA methyltransferase, extracellular signal-regulated kinase, cyclin D1, mammalian target of rapamycin, metastasis-associated protein, and nuclear factor-κB [9][10][11][12][13][14][15][16][17]. In addition, PTE activates autophagy, mutated in the Ataxia telangiectasia mutated/Ataxia telangiectasia and Rad3-related/Checkpoint kinase-1/p53 pathway, and sirtuin-1 pathway [13][18][19][20]. Moreover, PTE affects promoter DNA methylation, histone modifications, and microRNAs, resulting in alteration of epigenetic gene expression [20].
2. Effect of Pterostilbene (PTE) on Cell Proliferation and Stemness in Gastrointestinal Cancer Cells
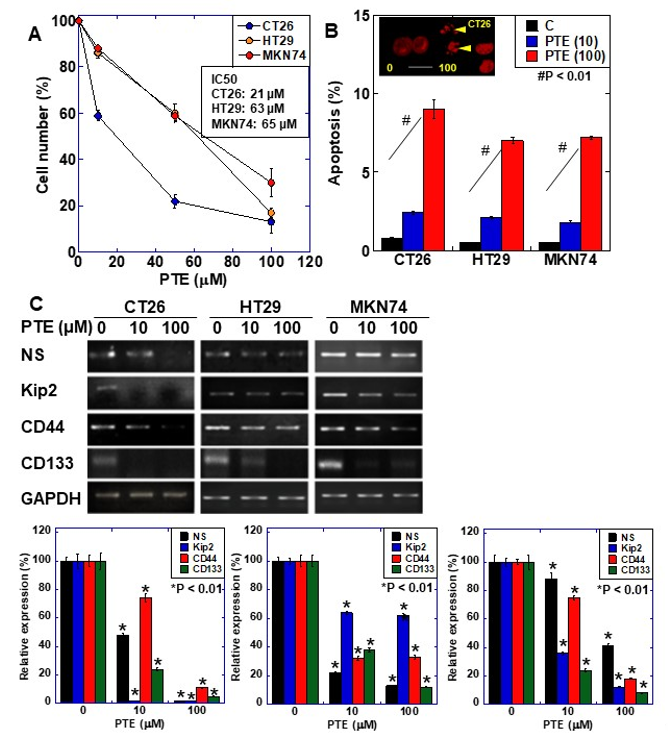
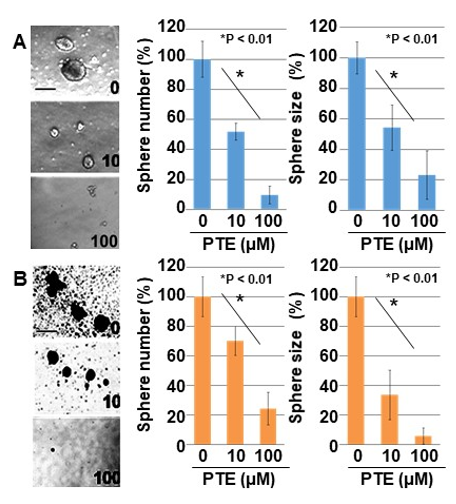
The effect of PTE on cell proliferation was examined using three gastrointestinal cancer cell lines, namely HT29, MKN74, and CT26 (Figure 1A). Dose-dependent growth inhibition was observed in all three cell lines, including at the low concentration of 10 μM of PTE. Next, we examined the gene expression of four types of stem cell markers to evaluate the effect of PTE on stemness (Figure 1B). Expression of the four markers decreased in a dose-dependent manner in all cell lines. The effect of PTE on stemness was also examined by sphere assay (Figure 2). In both CT26 cells (Figure 2A) and HT29 cells (Figure 2B), sphere formation was suppressed in terms of the number and size in a dose-dependent manner.
Figure 1. Effect of pterostilbene (PTE) on cell proliferation and expression of stem cell markers in cancer cell lines. (A) Cell proliferation was assessed by 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium,inner salt (MTS) assay. Cells were treated with PTE for 48 h. (B) Apoptosis was assessed by ethidium bromide (EtBr) staining. Insert, CT26 cells treated with PTE for 48 h were stained with EtBr. Arrow head, apoptosis body. Scale bar, 20 µM. (C) mRNA expression of stem cell markers; nucleostemin (NS), CD44, Kip2, and CD133 were examined by RT-PCR. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was amplified for loading standard. Lower panels were semi-quantification of stem cell marker expression examined by RT-PCR. Error bar, standard deviation from three independent examinations. Statistical difference was calculated by ordinary analysis of variance. * Statistical difference from PTE (0 µM). PTE, pterostilbene; IC50, 50% inhibitory concentration.
Figure 2. Effect of PTE on sphere formation in cancer cell lines. Sphere formation was examined in 10,000 cells with or without PTE treatment for 7 days. (A) CT26 cells. (B) HT29 cells. Pictures were images of phase-contrast microscopy. Error bar, standard deviation from three independent examinations. Statistical difference was calculated by ordinary analysis of variance. Scale bar, 50 μm. TE, pterostilbene.
3. Effect of PTE on Generation of Reactive Oxygen Species (ROS)
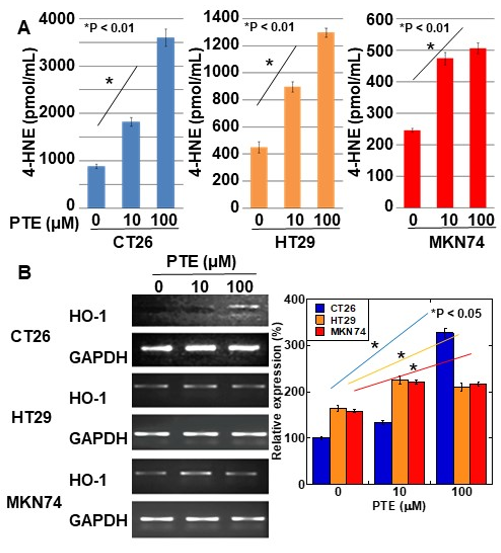
Intracellular levels of 4-hydroxynonenal (4-HNE) were determined for evaluating ROS generation by PTE treatment (Figure 3A). In all three cell lines, 4-HNE concentration increased in a PTE dose-dependent manner. Furthermore, we investigated the gene expression of heme oxygenase (HO)-1, which is known to be a rescue protein against oxidative stress [21][22] (Figure 3B). In CT26 cells, a high concentration of PTE (100 μM) induced heme oxygenase-1 (HO-1) expression; however, in HT29 and MKN74 cells, low- and high-concentrations of PTE (10 and 100 μM, respectively) induced HO-1 expression.
Figure 3. Effect of PTE on oxidative stress in cancer cell lines. (A) 4-hydroxynonenal (4-HNE) levels were measured by enzyme-linked immunosorbent assay (ELISA). Cells were treated with PTE for 48 h. (B) mRNA expression of heme oxygenase-1 (HO-1) was examined by RT-PCR. GAPDH was amplified as a loading standard. Right panel was semi-quantification of HO-1 expression examined by RT-PCR. Error bar, standard deviation from three independent examinations. Statistical difference was calculated by ordinary analysis of variance. PTE, pterostilbene; HNE. Hydroxynonenal; HO, heme oxygenase.
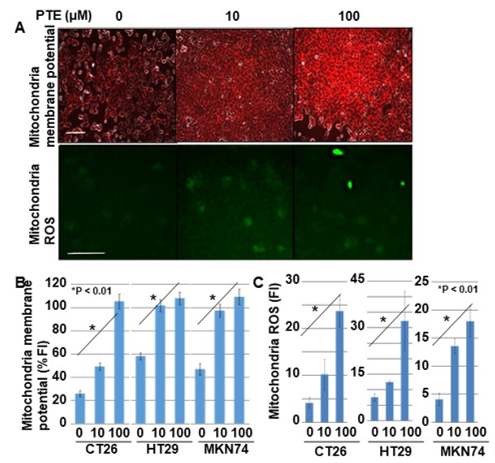
Next, we examined the effect of PTE on mitochondrial membrane potential and mitochondrial ROS generation (Figure 4). The mitochondrial membrane potential of all cell lines increased in a PTE-dose-dependent manner (Figure 4A,B). Furthermore, ROS derived from mitochondria also increased in a PTE-dose-dependent manner (Figure 4A,C).
Figure 4. Effect of PTE on mitochondrial function in cancer cells. Mitochondrial membrane voltage and mitochondrial reactive oxidative species (ROS) were examined by tetramethyl rhodamine (TMRE) and dihydrorhodamine (DHR), respectively. Cells were treated with PTE for 48 h. (A) Fluorescence images of TMRE and DHR. TMRE image was merged with phase-contrasted image. Scale bar, 25 μm. (B,C) Semi-quantification of mitochondrial membrane potential (TMRE) and mitochondrial ROS (DHR), respectively. Error bar, standard deviation from three independent examinations. Statistical difference was calculated by ordinary analysis of variance. PTE, pterostilbene; TMRE, tetramethyl rhodamine; ROS, reactive oxidative species; DHR, dihydrorhodamine 123; FI, fluorescence intensity.
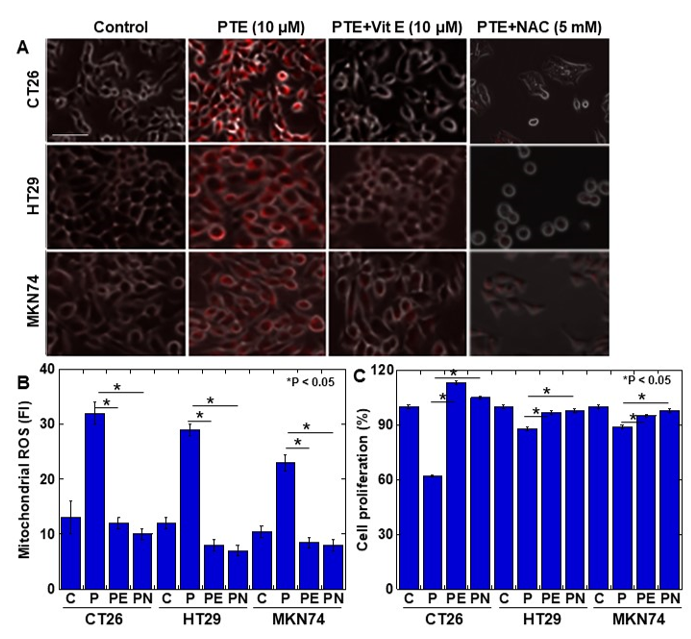
Finally, to examine whether PTE-induced ROS generation causes cytotoxicity, cells were treated with vitamin E or N-acetyl-L-cysteine (NAC) to suppress ROS generation (Figure 5). The generation of mitochondrial ROS was almost completely suppressed to the control levels by vitamin E and NAC (Figure 5A,B). The concurrent treatment with PTE and vitamin E or NAC showed that the PTE-mediated inhibition of cell proliferation was rescued by vitamin E and NAC (Figure 5C).
Figure 5. Effect of vitamin E on PTE-induced oxidative stress in cancer cell lines. Cells were treated with or without PTE (10 μM) and/or vitamin E (10 μM) and/or N-acetyl-L-cysteine (NAC) (5 mM) for 48 h. (A) Mitochondrial ROS was assessed by MitoROS (red color). MitoROS image was merged with phase-contrasted image. (B) Cell proliferation was assessed by MTS. (C) Semi-quantification of mitochondrial ROS. C, control; P, PTE; PE, PTE+vitamin E; PN, PTE+NAC. Error bar, standard deviation from three independent examinations. Statistical difference was calculated by ordinary analysis of variance. Scale bar, 25 μm. PTE, pterostilbene; ROS, reactive oxidative species; FI, fluorescence intensity; NAC, N-acetyl-L-cysteine; MTS, 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium.
References
- McCormack, D.; McFadden, D. A Review of Pterostilbene Antioxidant Activity and Disease Modification. Oxidative Med. Cell. Longev. 2013, 2013, 1–15, doi:10.1155/2013/575482.
- Kosuru, R.; Rai, U.; Prakash, S.; Singh, A.; Singh, S. Promising therapeutic potential of pterostilbene and its mechanistic insight based on preclinical evidence. J. Pharmacol. 2016, 789, 229–243, doi:10.1016/j.ejphar.2016.07.046.
- Ma, Z.; Zhang, X.; Xu, L.; Liu, D.; Di, S.; Li, W.; Zhang, J.; Zhang, H.; Li, X.; Han, J.; Yan, X. Pterostilbene: Mechanisms of its action as onc ostatic agent in cell models and in vivo studies. Res. 2019, 145, 104265.
- McCormack, D.; McFadden, D. Pterostilbene and Cancer: Current Review. Surg. Res. 2012, 173, e53–e61, doi:10.1016/j.jss.2011.09.054.
- Dandawate, P.R.; Subramaniam, D.; A Jensen, R.; Anant, S. Targeting cancer stem cells and signaling pathways by phytochemicals: Novel approach for breast cancer therapy. Cancer Biol. 2016, 192–208, doi:10.1016/j.semcancer.2016.09.001.
- Shin, H.J.; Han, J.M.; Choi, Y.S.; Jung, H.J. Pterostilbene Suppresses both Cancer Cells and Cancer Stem-Like Cells in Cervical Cancer with Superior Bioavailability to Resveratrol. Molecules 2020, 25, 228, doi:10.3390/molecules25010228.
- Tsai, H.-Y.; Ho, C.-T.; Chen, Y.-K. Biological actions and molecular effects of resveratrol, pterostilbene, and 3′-hydroxypterostilbene. Food Drug Anal. 2017, 25, 134–147, doi:10.1016/j.jfda.2016.07.004.
- Wang, Y.-J.; Lin, J.-F.; Cheng, L.-H.; Chang, W.-T.; Kao, Y.-H.; Chang, M.-M.; Wang, B.-J.; Cheng, H.-C. Pterostilbene prevents AKT-ERK axis-mediated polymerization of surface fibronectin on suspended lung cancer cells independently of apoptosis and suppresses metastasis. Hematol. Oncol. 2017, 10, 1–14, doi:10.1186/s13045-017-0441-z.
- Wen, W.; Lowe, G.; Roberts, C.M.; Finlay, J.; Han, E.S.; Glackin, C.A.; Dellinger, T.H. Pterostilbene Suppresses Ovarian Cancer Growth via Induction of Apoptosis and Blockade of Cell Cycle Progression Involving Inhibition of the STAT3 Pathway. J. Mol. Sci. 2018, 19, 1983, doi:10.3390/ijms19071983.
- Liu, Y.; Wang, L.; Wu, Y.; Lv, C.; Li, X.; Cao, X.; Yang, M.; Feng, D.; Luo, Z. Pterostilbene exerts antitumor activity against human osteosarcoma cells by inhibiting the JAK2/STAT3 signaling pathway. Toxicology 2013, 304, 120–131, doi:10.1016/j.tox.2012.12.018.
- Daniel, M.; Tollefsbol, T.O. Pterostilbene down‐regulates hTERT at physiological concentrations in breast cancer cells: Potentially through the inhibition of cMyc. Cell. Biochem. 2018, 119, 3326–3337, doi:10.1002/jcb.26495.
- Bracht, J.W.P.; Karachaliou, N.; Berenguer, J.; Pedraz-Valdunciel, C.; Filipska, M.; Codony-Servat, C.; Codony-Servat, J.; Rosell, R. Osimertinib and pterostilbene in EGFR-mutation-positive non-small cell lung cancer (NSCLC). J. Biol. Sci. 2019, 15, 2607–2614, doi:10.7150/ijbs.32889.
- Chang, H.P.; Lu, C.C.; Chiang, J.H.; Tsai, F.J.; Juan, Y.N.; Tsao, J.W.; Chiu, H.Y.; Yang, J.S. Pterostilbene modulates the suppression of multidrug resistance protein 1 and triggers autophagic and apoptotic mechanisms in cisplatin-resistant human oral cancer CAR cells via AKT signaling. J. Oncol. 2018, 52, 1504–1514.
- Bin, W.H.; Da, L.H.; Xue, Y.; Jing, B. Pterostilbene (3’,5’-dimethoxy-resveratrol) exerts potent antitumor effects in HeLa human cervical cancer cells via disruption of mitochondrial membrane potential, apoptosis induction and targeting m-TOR/PI3K/Akt signalling pathway. Buon 2018, 23, 1384–1389.
- Kala, R.; Shah, H.N.; Martin, S.L.; Tollefsbol, T.O. Epigenetic-based combinatorial resveratrol and pterostilbene alters DNA damage response by affecting SIRT1 and DNMT enzyme expression, including SIRT1-dependent γ-H2AX and telomerase regulation in triple-negative breast cancer. BMC Cancer 2015, 15, 1–18, doi:10.1186/s12885-015-1693-z.
- Wakimoto, R.; Ono, M.; Takeshima, M.; Higuchi, T.; Nakano, S. Differential Anticancer Activity of Pterostilbene Against Three Subtypes of Human Breast Cancer Cells. Res. 2017, 37, 6153–6159, doi:10.21873/anticanres.12064.
- Dhar, S.; Kumar, A.; Zhang, L.; Rimando, A.M.; Lage, J.M.; Lewin, J.R.; Atfi, A.; Zhang, X.; Levenson, A.S. Dietary pterostilbene is a novel MTA1-targeted chemopreventive and therapeutic agent in prostate cancer. Oncotarget 2016, 7, 18469–18484, doi:10.18632/oncotarget.7841.
- Lee, H.; Kim, Y.; Jeong, J.H.; Ryu, J.-H.; Kim, W. ATM/CHK/p53 Pathway Dependent Chemopreventive and Therapeutic Activity on Lung Cancer by Pterostilbene. PLoS ONE 2016, 11, e0162335, doi:10.1371/journal.pone.0162335.
- Cheng, Y.; Di, S.; Fan, C.; Cai, L.; Gao, C.; Jiang, P.; Hu, W.; Ma, Z.; Jiang, S.; Dong, Y.; et al. SIRT1 activation by pterostilbene attenuates the skeletal muscle oxidative stress injury and mitochondrial dysfunction induced by ischemia reperfusion injury. Apoptosis 2016, 21, 905–916, doi:10.1007/s10495-016-1258-x.
- Seo, E.J.; Fischer, N.; Efferth, T. Phytochemicals as inhibitors of NF-κB for treatment of Alzheimer’s disease. Res. 2018, 129, 262–273.
- Waza, A.A.; Hamid, Z.; Ali, S.; Bhat, S.A.; Bhat, M.A. A review on heme oxygenase-1 induction: is it a necessary evil. Res. 2018, 67, 579–588, doi:10.1007/s00011-018-1151-x.
- Sasaki, T.; Yoshida, K.; Kondo, H.; Ohmori, H.; Kuniyasu, H. Heme oxygenase-1 accelerates protumoral effects of nitric oxide in cancer cells. Archiv. 2005, 446, 525–531, doi:10.1007/s00428-005-1247-x.