
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Juri Radmilovic | + 1343 word(s) | 1343 | 2020-12-15 11:18:14 | | | |
| 2 | Catherine Yang | Meta information modification | 1343 | 2020-12-28 08:57:08 | | |
Video Upload Options
Among acute coronary syndrome (ACS) patients, 15% have concomitant cancer, especially in the first 6 months after their diagnosis, as well as in advanced metastatic stages. Lung, gastric, and pancreatic cancers are the most frequent malignancies associated with ACS.
1. Introduction
Cardiovascular disease and cancer are responsible for the majority of deaths among persons 65 years of age and older in the Western world [1]. Amidst acute coronary syndrome (ACS) patients, 15% have concomitant cancer at various stages [2]. ACS may occur before or after establishing the diagnosis of cancer, and occasionally the diagnosis is made during hospitalization for ACS. Sometimes double antiplatelet therapy reveals the presence of a lying tumor through unexpected bleeding, such as gastrointestinal bleeding.
The incidence of ACS in patients with newly diagnosed cancer increases in the first 6 months after their diagnosis and in more advanced stages: lung, gastric, or pancreatic cancer are the most recurrent malignancies associated with ACS [3].
It is extremely important to assess the cardiovascular risk in patients with ACS and concomitant cancer: classic risk factors such as hypertension, smoking, diabetes, and obesity are somehow potentiated by several prothrombotic elements secreted by the tumor itself or expressed on its surface. Moreover, chemotherapy and radiotherapy can also exert prothrombotic, vasospastic, and proinflammatory actions [4][5]. Regarding cancer therapy, a highly prothrombotic status has been observed in patients treated with cisplatin, 5-fluorouracil, gemcitabine, erythropoietin, and bevacizumab [2].
Current guidelines for conservative and invasive treatment of ACS cannot be easily applied to all cancer patients. Indeed, prospective studies assessing the efficacy and safety of ACS treatment usually have excluded cases with active cancer. Furthermore, the management of ACS in patients with cancer is quite challenging, this is because it should be effective yet also safe. Thrombocytopenia, coagulopathy, and anemia are often present in these patients, and the probability of cancer recurrence may require interruption of double antiplatelet therapy (DAPT) in case of biopsies, surgery, or re-initiation of cancer therapy [6].
2. Cardiovascular Toxicities Induced by Chemotherapy and Radiotherapy
Different chemotherapeutic agents may damage the cardiovascular system, injuring coronary and peripheral circulation, with acute and long-term consequences.
5-Fluorouracil (5-FU) and its oral pro-drug Capecitabine are associated with angina and ACS: 5-FU is related with endothelial damage and alteration in molecular signaling that controls smooth muscle cell tone, inducing abnormal vasoreactivity [7].
Cisplatin may induce acute coronary thrombosis, determinated by endothelial damage, thromboxane production, and platelet activation and aggregation as the main mechanism [8]. In combination with cisplatin, vinblastine can induce endothelial apoptosis, while bleomicin can worsen endothelial dysfunction, increasing vasotoxic properties of these drugs [9].
Cyclophosphamide may induce Prinzmetal’s angina or hemorrhagic peri-myocarditis [10], while Paclitaxel and Docetaxel are associated with vasospasm, ACS, and cardiac rhythm disturbance such as bradycardia [11].
Vascular endothelial grow factor (VEGF) signaling pathway inhibitors may cause endothelial dysfunction, vascular remodeling, inflammation, and platelet activation, responsible for increased risk of cardiovascular events [12]., Treatment with Sinutinib is related with a reduction in coronary flow reserve and abnormal vasofunctional balance, associated with microvascular impairment and rarefication of microvascular pericytes and capillaries [13]. Sorafenib has been also associated with coronary vasospasm and progression of coronary artery disease (CAD) [14]. An increased bleeding risk has been observed in patients treated with VEGF inhibitors.
Progression of atherosclerosis and ischemic events may be observed during treatment with Nilotinib and Polatenib, two tyrosine kinases inhibitors [15].
Studies [16] reported ACS and Takotsubo cardiomyopathy during Rituximab treatment.
Moreover, increased cardiovascular risk is considered in patients with prostate cancer treated with androgen deprivation therapy (ADT), especially with gonadotropin-releasing hormone (GnRH) agonists [17][18], and in women receiving aromatase inhibitors, as anastrozole and letrozole [19].
Finally, radiation therapy may be related with coronary and peripheral artery disease [20]. Ionizing radiations affect noncancerous cells, and endothelial cells are the most vulnerable: within a short period after radiation exposure, cholesterol plaques and thrombosis can form, and fibrosis may involve all three layers of the vessel wall [21], see Table 1 and Figure 1.
Table 1. Chemotherapeutic agents associated with acute coronary syndrome. ACS: acute coronary syndrome; CAD: coronary artery disease.
|
Incidence |
Presentations |
Cancer Therapy |
|
|
Antimetabolites |
|||
|
5-Fluorouracil [7] |
0.1–18% |
Angina, Vasospasm, ACS |
Colorectal, pancreas, gastric breast |
|
Alkylating agents |
|||
|
Cisplatin [8] |
0.2–12% |
Angina, coronary thrombosis, ACS, progression of CAD |
Ovarian, Testicular, Bladder, squamous cell of head and neck, mesothelioma |
|
Antitumor antibiotics |
|||
|
Bleomycine [9] |
<2% |
Angina, vasospasm, ACS |
Testicular, cervix, Hodgkin’s and non-Hodgkins lymphoma |
|
Anti-microtubule agents |
|||
|
Vinblastine [9] |
<5% |
Angina, ACS |
Testicular, lymphoma, breast cancer |
|
Monoclonal antibodies |
|||
|
Rituximab [16] |
<1% |
Vasospasm, ACS, Takotsubo cardiomyopathy |
Non-Hodgkin’s lymphoma, Chronic Lymphocytic Leukemia |
|
Tyrosine kinase inhibitors |
|||
|
Sorafenib [14] |
1% |
Angina, ACS |
Liver, renal cell, thyroid cancer |
|
5–8% |
ACS, Takotsubo cardiomyopathy, progression of CAD |
Pancreas, renal cell, gastrointestinal stromal tumor |
|
|
Nilotinib [15] |
8–12% |
ACS, progression of CAD, peripheral artery disease |
Chronic myeloid Leukemia |
|
Hormone therapy |
|||
|
Aromatase inhibitors (anastrozole) [19] |
2% |
Angina, ACS |
Breast cancer |
|
Anti-androgens (bicalbutide) [17] |
1–33% |
ACS, progression of CAD |
Prostate cancer |
|
Gonadotropin-releasing hormone agonist (goserelin) [18] |
1–2% |
Angina, ACS |
Prostate cancer |
|
Gonadothropin-releasing hormone antagonist (degarelix) [18] |
<1% |
ACS |
Prostate cancer |
Figure 1. Pathogenesis of acute coronary syndrome in cancer patients. ACS: acute coronary syndrome.
3. Clinical Presentation of ACS in Cancer Patients
The clinical presentation of ACS in cancer patients may be different from that of noncancer patients. Dyspnea is one of the most incessant symptoms (44%), followed by chest pain (30%) and hypotension (23%) [22]. Additionally, in a significant percentage of cases, ACS may begin in the form of silent ischemia, probably as a consequence of the altered perception of angina due to the neurotoxicity of some chemotherapies. Ambiguous abnormalities on electrocardiograms and increased cardiac troponin levels do not necessarily indicate ACS [2].
It was also found that about 85% of all ACS in cancer patients are non-ST segment elevation myocardial infarction (NSTEMI), whilst in women there are not infrequent cases of myocardial infarction with nonobstructive coronary arteries (MINOCA) and, in particular, Takotsubo syndrome [23].
4. Medical Therapy
4.1. Antiplatelet Therapy
Antiplatelet therapy represents a big challenge in cancer patients with ACS. These cases have a high risk of stent thrombosis and bleeding complications, which are often worsened by the presence of thrombocytopenia.
4.2. Triple Therapy
Atrial Fibrillation and ACS in Cancer Patients
One of the most complex management strategies is the treatment of the patients with cancer, atrial fibrillation, and ACS.
Cancer is present in about 2.5% of patients with nonvalvular atrial fibrillation (NVAF), and this arrhythmia is associated with a poor prognosis and in-hospital mortality. Moreover, cancer and its treatments contribute to an increased prevalence of NVAF: anticancer treatment and cancer-associated factors, such as inflammation and dehydration, augment the risk of thromboembolic complications [24].
There is little evidence regarding the use of direct-acting oral anticoagulants (DOACs) in patients with cancer and atrial fibrillation. Anticancer drugs used in clinical practice may potentiate or decrease the anticoagulant effect. For example, vinblastine, doxorubicin, sunitinib, and enzalutamide may decrease the anticoagulant effect whilst increasing the risk of embolism. Conversely, crizotinib, abiraterone, and cyclosporine tend to increase the anticoagulant effect and consequently the risk of bleeding [25].
Furthermore, in addition to the type of anticancer therapy, it is essential to pay attention to the tumor site. As previously described, the use of edoxaban and rivaroxaban is associated with an increased risk of gastrointestinal bleeding [26].
Patients with cancer who are already on an oral anticoagulant, vitamin-K antagonist (VKA), or DOACs for NVAF and who develop ACS should receive low-molecular-weight heparin (LMWH) for the first six weeks. This should be followed by, if the bleeding risk (HASBLED score <3) is low, VKA or DOAC; if the risk of bleeding is high (HASBLED score ≥3) or the prognosis is poor, it is advisable to continue with LMWH, while monitoring the blood count and renal function [27]. The anticoagulant therapy needs to be associated with the antiplatelet therapy, preferably with ASA and Clopidogrel. According to recent NSTEMI ESC Guidelines [22], after considering the ischemic and bleeding risk, the triple therapy should be maintained for 1 week to 1 month depending on the patient risk profile. After that, one of the two antiplatelet drugs is suspended (preferably ASA); from the sixth/twelfth month it is possible to continue only with anticoagulant therapy [22][25][26]. During triple therapy in cancer patients, VKA should be titrated to obtain international normalized ratio (INR) of less than 2.5 or DOAC at reduced doses [28], see Figure 1.
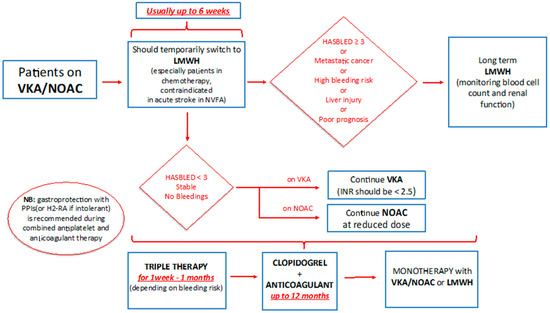
Figure 1. Management of anticoagulant therapy in cancer patients. VKA: vitamin K antagonist; NOAC: new oral anticoagulant; LMWH: low-molecular-weight heparin.
References
- Yusuf, S.W.; Cipolla, C.; Durand, J.-B.; Lenihan, D.J. Cancer and cardiovascular disease. Cardiol. Res. Pract. 2011, 2011, 1.
- Goldberg, R.J.; Chen, H.-Y.; Saczynski, J.S.; McManus, D.D.; Lessard, D.; Yarzebski, J.; Lapane, K.L.; Gore, J.M. The impact of cardiac and noncardiac comorbidities on the short-term outcomes of patients hospitalized with acute myocardial infarction: A population-based perspective. Clin. Epidemiol. 2013, 5, 439-48.
- Yeh, E.T.; Chang, H.-M. Cancer and Clot. J. Am. Coll. Cardiol. 2017, 70, 939–941.
- Hasin, T.; Iakobishvili, Z.; Weisz, G. Associated risk of malignancy in patients with cardiovascular disease: Evidence and possible mechanism. Am. J. Med. 2017, 130, 780–785.
- Navi, B.B.; Reiner, A.S.; Kamel, H.; Iadecola, C.; Okin, P.M.; Elkind, M.S.; Panageas, K.S.; DeAngelis, L.M. Risk of arterial thromboembolism in patients with cancer. J. Am. Coll. Cardiol. 2017, 70, 926–938.
- Yusuf, S.W.; Daraban, N.; Abbasi, N.; Lei, X.; Durand, J.-B.; Daher, I.N. Treatment and outcomes of acute coronary syndrome in the cancer population. Clin. Cardiol. 2012, 35, 443–450.
- Polk, A.; Vaage-Nilsen, M.; Vistisen, K.; Nielsen, D.L. Cardiotoxicity in cancer patients treated with 5-fluorouracil or capecitabine: A systematic review of incidence, manifestations and predisposing factors. Cancer Treat. Rev. 2013, 39, 974–984.
- Karabay, K.O.; Yildiz, O.; Aytekin, V. Multiple coronary thrombi with cisplatin. J. Invasive Cardiol. 2014, 26, E18–E20.
- Jafri, M.; Protheroe, A. Cisplatin-associated thrombosis. Anti-Cancer Drugs 2008, 19, 927–929.
- Katayama, M.; Imai, Y.; Hashimoto, H.; Kurata, M.; Nagai, K.; Tamita, K.; Morioka, S.; Furukawa, Y. Fulminant fatal cardiotoxicity following cyclophosphamide therapy. J. Cardiol. 2009, 54, 330–334.
- Gupta, S.; Ghosh, J.; Bajpai, J.; Maheshwari, A.; Shah, K. Acute non-ST elevation myocardial infarction following paclitaxel administration for ovarian carcinoma: A case report and review of literature. J. Cancer Res. Ther. 2012, 8, 442-4.
- Chen, X.-L.; Lei, Y.-H.; Liu, C.-F.; Yang, Q.-F.; Zuo, P.-Y.; Liu, C.-Y.; Chen, C.-Z.; Liu, Y.-W. Angiogenesis inhibitor bevacizumab increases the risk of ischemic heart disease associated with chemotherapy: A meta-analysis. PLoS ONE 2013, 8, e66721.
- Kappers, M.H.W.; Smedts, F.M.; Horn, T.; Van Esch, J.H.; Sleijfer, S.; Leijten, F.; Wesseling, S.; Strevens, H.; Danser, A.H.J.; Meiracker, A.H.V.D. The Vascular endothelial growth factor receptor inhibitor sunitinib causes a preeclampsia-like syndrome with activation of the endothelin system. Hypertension 2011, 58, 295–302.
- Naib, T.; Steingart, R.M.; Chen, C.L. Sorafenib-associated multivessel coronary artery vasospasm. Herz 2011, 36, 348–351.
- Quintás-Cardama, A.; Kantarjian, H.; Cortes, J. Nilotinib-associated vascular events. Clin. Lymphoma Myeloma Leuk. 2012, 12, 337–340.
- Gogia, A.; Khurana, S.; Paramanik, R. Acute myocardial infarction after first dose of rituximab infusion. Turk. J. Hematol. 2014, 31, 95–96.
- Ziehr, D.R.; Chen, M.-H.; Zhang, D.; Braccioforte, M.H.; Moran, B.J.; Mahal, B.A.; Hyatt, A.S.; Basaria, S.; Beard, C.J.; Beckman, J.A.; et al. Association of androgen-deprivation therapy with excess cardiac-specific mortality in men with prostate cancer. BJU Int. 2014, 116, 358–365.
- Nanda, A.; Chen, M.-H.; Braccioforte, M.H.; Moran, B.J.; D’Amico, A.V. Hormonal therapy use for prostate cancer and mortality in men with coronary artery disease–induced congestive heart failure or myocardial infarction. JAMA 2009, 302, 866–873.
- Amir, E.; Seruga, B.; Niraula, S.; Carlsson, L.; Ocaña, A. Toxicity of adjuvant endocrine therapy in postmenopausal breast cancer patients: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2011, 103, 1299–1309.
- Stewart, F.A.; Heeneman, S.; Poele, J.T.; Kruse, J.; Russell, N.S.; Gijbels, M.; Daemen, M. Ionizing radiation accelerates the development of atherosclerotic lesions in ApoE—Mice and predisposes to an inflammatory plaque phenotype prone to hemorrhage. Am. J. Pathol. 2006, 168, 649–658.
- Lee, M.S.; Finch, W.; Mahmud, E. Cardiovascular complications of radiotherapy. Am. J. Cardiol. 2013, 112, 1688–1696.
- Collet, J.-P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2020, 37, 267–315.
- Munoz, E.; Iliescu, G.; Vejpongsa, P.; Charitakis, K.; Karimzad, K.; Lopez-Mattei, J.; Yusuf, S.W.; Marmagkiolis, K.; Iliescu, C. Takotsubo stress cardiomyopathy. J. Am. Coll. Cardiol. 2016, 68, 1143–1144.
- Steffel, J.; Verhamme, P.; Potpara, T.S.; Albaladejo, P.; Antz, M.; Desteghe, L.; Haeusler, K.G.; Oldgren, J.; Reinecke, H.; Roldan-Schilling, V.; et al. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur. Heart J. 2018, 39, 1330–1393.
- Ording, A.G.; Horváth-Puhó, E.; Adelborg, K.; Pedersen, L.; Prandoni, P.; Sørensen, H.T. Thromboembolic and bleeding complications during oral anticoagulation therapy in cancer patients with atrial fibrillation: A Danish nationwide population-based cohort study. Cancer Med. 2017, 6, 1165–1172.
- Shah, S.; Norby, F.L.; Datta, Y.H.; Lutsey, P.L.; MacLehose, R.F.; Chen, L.Y.; Alonso, A. Comparative effectiveness of direct oral anticoagulants and warfarin in patients with cancer and atrial fibrillation. Blood Adv. 2018, 2, 200–209.
- Farmakis, D.; Parissis, J.; Filippatos, G. Insights into onco-cardiology: Atrial fibrillation in cancer. JACC 2014, 63, 945–953.
- Banasiak, W.; Zymliński, R.; Undas, A. Optimal management of cancer patients with acute coronary syndrome. Pol. Arch. Intern. Med. 2018, 128.




