| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Francesca Boccafoschi | + 3291 word(s) | 3291 | 2020-11-06 07:59:46 | | | |
| 2 | Lily Guo | -69 word(s) | 3222 | 2020-12-17 10:21:42 | | | | |
| 3 | Lily Guo | Meta information modification | 3222 | 2020-12-21 06:43:13 | | |
Video Upload Options
Hydrogels are three-dimensional (3D) materials able to absorb and retain water in large amounts while maintaining their structural stability. Due to their considerable biocompatibility and similarity with the body’s tissues, hydrogels are one of the most promising groups of biomaterials. The main application of these hydrogels is in regenerative medicine, in which they allow the formation of an environment suitable for cell differentiation and growth. (Draft for definition)
1. Introduction
Angiogenesis is the neo-formation of new blood vessels from pre-existing vessels[1]. As the bloodstream is an essential component for the dispersion of metabolites and nutrients and to remove the excess of toxic products, these processes are fundamental for the proper functioning of the tissues. In fact, the process itself and consequently the capillary structure, can be modified by changing the cells’ metabolic activity. Moreover, the coordination and viability of vessels walls are regulated by oxygen and haemodynamic factors[2]. The angiogenic process consists of sequential steps[3]:
-
vasodilatation of the original vessels, in response to nitrogen monoxide and to the increasing permeability triggered by vascular endothelial growth factor (VEGF);
-
separation of the pericytes from the luminal surface and the break of basement membrane thus allowing the formation of the new vessel:
-
migration and proliferation of the endothelial cells to the damaged tissue;
-
neo-formation of vessel lumen;
-
recruitment of the peri-endothelial cells (pericytes for small capillary and smooth muscle cells for larger vessels);
-
inhibition of endothelial cell proliferation;
-
deposition of the basement membrane and initiating of blood flow.
Many factors are involved in making sure that these processes occur without errors. Among these factors there is the role of extracellular matrix (ECM), a three-dimensional network made of proteoglycans, glycoproteins, and polysaccharides[4][5]. ECM enzymes, such as metalloproteinases, are very important in assuring that the ECM undergoes the conformational and structural changes that allow the correct formation of new vessels. ECM is also involved in the process of cell adhesion through integrins, in this context the integrin receptors are major players in potentiating the signaling events of the early process of angiogenesis[5][6]. These signaling events are triggered by growth factors (GF), which are key players of the angiogenic process. Of these, VEGF, FGF, TGF-β, PDGF and angiopoietin are essential in regulating endhotelial cell proliferation and migration, the migration of other involved cells such as fibroblasts and smooth muscle cells and the production of proteins from the ECM [1][3][7].
Throughout the years, the inhibition and stimulation of angiogenesis have been the focus of numerous studies as it was demonstrated that the alteration of its normal function induces several diseases. However, angiogenesis enhancing can be therapeutic, for instance in ischemic heart disease, wound healing, and peripheral arterial disease[1].
In contrast to the term substitution, regeneration means “to restore the physical integrity of cells, tissues and organs by means of the organisms’ own repair mechanisms”[2]. To pursue this goal, biomaterials, defined as substances with the capability to replace organs or tissues, either cure or expand them [8], are a promising group of materials due to their biodegradability and biocompatibility. Other properties such as the non-toxicity and the ability to sustain a sterilization process also characterize this type of materials. Due to the several applications such as drug delivery, cell encapsulation, tissue engineering scaffolds, wound dressing, soft tissue replacement, contact lenses, and biosensor, hydrogels can be one of the most promising biomaterials in the biomedical field[8][9].
2. Angiogenic Potential in Natural Hydrogels
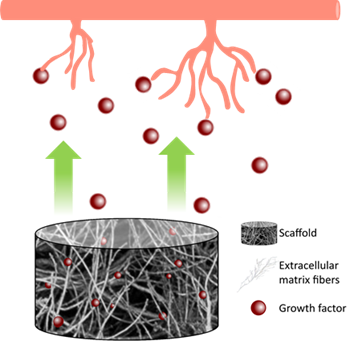
Hydrogels derived from natural sources can be used as basic elements of a proangiogenic system. Firstly, the general advantage in using natural hydrogels is the chemical composition similar to the native ECM and the presence of bioactive molecules that gives the material characteristics as an extraordinary biocompatibility while avoiding immune reactions and promoting cell proliferation and differentiation. Moreover, the capability to release GFs, the porosity of natural hydrogels which allows for new capillary network formation, and in some cases, specific chemical compositions, such as collagen and hyaluronic acid (HA), gives them angiogenic potential. Figure 1 represents this process.
On the other hand, rapid degradation, poor stability, and low mechanical strength represent some of the main limits to a wide use in tissue engineering. These problems can be partially solved using synthetic hydrogels because they have better mechanical and biochemical properties. Some examples of this type of hydrogels are poly(ethylene glycol) (PEG), poly(acrylic acid) (PAA), poly(vinyl alcohol) (PVA), and polyacrylamide (PAAm). However, they are not biocompatible and degradable, indeed usually they are used along with natural hydrogels to obtain a co-polymeric hydrogel presenting adequate characteristics[10]. Examples of novel hybrid hydrogels are: a hydrogel created using decellularized tissues, which represent the bioactive component of the hydrogel, mixed with alginate and PVA[11], another example is crosslinking PEG with hyaluronic acid [12], in both examples the synthetic counterpart confers mechanical stability and strength.
Figure 1. Hydrogel’s release of growth factors stimulates new capillary formation.
Because they all have distinctive characteristics to better suit the final application to achieve different purposes it is possible to choose between various types of hydrogels. Natural hydrogels are usually classified in three main categories: i) polysaccharides-based, ii) protein-based and iii) derived from cellularized tissues[13][14][15][16].
2.1. Polysaccharide-Based Hydrogels
Glycosidic bonds link repeated monosaccharide units that create a long carbohydrate molecule called polysaccharide. They are easily available, in fact they represent the most abundant biomolecules in nature which makes them promising biomaterials for several applications including drug delivery, encapsulation of cells, and releasing GFs[17][18].
2.1.1. Glycosaminoglycans
GAGs are long linear polysaccharides. Their structure comprises repeated disaccharide units which can be sulfated, such as heparin, heparan sulfate, keratan sulfate and chondroitin sulfate or nonsulfated, as HA[16]. Depending on the type, the functions of GAGs can change, however they generally affect cell migration, survival and signaling. These functions of GAGs are essential also in the process of angiogenesis indeed, as they can bond VEGF and FGF[19].
Composed by repeated disaccharide units consisting of N-acetylglucosamine and D-glucuronic acid, HA is a linear high molecular weight (about 107 kDa) non-sulfated GAG present in the ECM. Its role consists of a space filler, as a lubricant and helps the processes of wound healing, angiogenesis, and signal transduction[14][16]. HA is characterized by a high biodegradability, biocompatibility, high viscoelasticity and hydrophilia, moreover, it can bind water forming hydrogen bonds with the solvent[20].
HA enzymatic degradation produces fragments of the molecule, called hyaluronan oligosaccharides, of less than 10 disaccharide units, that have shown to promote angiogenesis and wound healing[21][22]. However, since native HA is vulnerable to degradation by hyaluronidase or reactive oxygen species, it is necessary to chemically modify the hydrogel[16], because otherwise the degradation time of the HA scaffold will be faster than the regeneration of the tissue. Modifications can be different: β-cyclodextrin-modified HA (CD-HA)[23][24], adamantane-modified HA (Ad-HA), acrylated HA (AHA)[25][26], dextran-HA (Dex-g-HA)[27], catechol-HA (CA-HA)[18] and methacrylate-modified HA (HAM) [28]. The difference in the modifications gives the hydrogel different abilities, for instance, CA-HA hydrogel used in a mouse model of hindlimb ischemia allows the arousing of capillaries and arterioles[18]. Instead, CD-HA/Ad-HA enhance cell retention at the hypoxic border zone of the ischemic myocardium[28]. Generally, this group of hydrogels stimulates angiogenesis because they can incorporate GFs (PDGF, VEGF and FGF)[15][28][29] and encapsulate cells for creating capillary-like structures[18]. Furthermore, to regulate and prolong the release rate of growth factors, heparin can be included into HA hydrogels through the method of heparinization. In fact, heparin is a sulfated GAG that can covalently bind various angiogenic GFs and has the ability to sequester them in the ECM [15][29]. HA also inhibits platelet adhesion and aggregation, and stimulates angiogenesis which makes it suitable for vascular applications[30]. Lastly, HA hydrogels have another important function: they allow adhesion and degradation of endothelial cells when they are crosslinked with MMP-sensitive peptides[31].
2.1.2. Alginate
Alginate is an anionic copolymer derived from brown seaweed containing blocks of 1,4‐chain mannitol (M) and L-guluronic acid (G) residues. The G block forms a rapid and reversible crosslink in presence of calcium ions that gives the hydrogel stronger mechanical properties compared to other natural derived hydrogels. If the G block content increases, there will be also an improvement of mechanical abilities. For this reason, alginate is one of the most popular and attractive hydrogels used in fiber-based technologies or as biomaterial for the delivery of therapeutic factors[32]. Physical crosslinking ability, good biocompatibility, non-toxicity, and high viscoelasticity are the main properties of alginate hydrogels. Unfortunately, poor stability, poor cell adhesion, and low mechanical strength represent the major limitations[9][14].
Alginate hydrogels are promising delivery system, for instance they can control and deliver VEGF, PDGF and FGF. Similarly, to the HA hydrogels, alginate hydrogels can be heparinized in order to control the release of GFs[15]. Also, pure alginate gels have the ability to deliver GFs, however they showed a low controlled degradability that is an important limitation in studies “in vivo”[31].
Other molecules that can recruit vascular progenitor cells and induce angiogenesis can be encapsulated in alginate hydrogels. An example is the phospholipid sphingosine-1-phosphate (S1P). For the release of S1P from the alginate hydrogel, a composite alginate-chitosan hydrogel can be used by changing the content of chitosan, the release rate can be controlled. Platelet-rich plasma (PRP) can be incorporated. PRP is a plasma fraction containing several GFs, including VEGF and PDGF which can recruit stem cells and induce angiogenesis. A PRP-alginate-based bioink has been developed for 3D bioprinting scaffolds to elute GFs[32].
A pH-responsive Ca-alginate hydrogel loaded with protamine nanoparticles and hyaluronan oligosaccharides can be used to treat diabetic wounds, which are chronic wounds, and represent a persistent and severe complication of diabetes. These hydrogels regulate antibacterial and neovascularization activities promoting the healing of the wound. In fact, several studies conclude that if the pH becomes more alkaline, there will be an acceleration of the bacterial colonization and biofilm formation, thus prolonging the inflammatory phase and impairing the formation of blood vessels. In addition, because it acts as a cationic antimicrobial peptide, protamine works against a wide range of bacteria causing general disruptions to prokaryotic cells envelope, meanwhile the secretion stimulated by VEGF and the acceleration of the wound healing is a consequence of the addition of hyaluronan oligosaccharides in the hydrogel. Thus, pH responsive Ca-alginate hydrogel loaded with protamine nanoparticles and hyaluronan oligosaccharides enhances endothelial cell capillary-like formation and increases cells in wound healing[33]. Likewise, several studies evidence good results for diabetes mellitus type 1 treatment using alginate hydrogel combined to VEGF for islet encapsulation. This is just another example of how alginate hydrogels can be used as a GFs delivery system[34].
2.1.3. Chitosan
Chitosan is a linear polysaccharide made of N-acetyl-D-glucosamine units, derived from the natural polymer chitin by partial deacetylation. These hydrogels present several advantages such as a good biocompatibility and biodegradability, antibacterial properties, an easy way of controlling degradation, and the possibility of undergoing a sterilization process. On the other hand, inadequate mechanical properties characterize chitosan hydrogels, although it is possible to fix this problem by adding chemical groups or by gelatin crosslinking the hydrogels[14] [15].
Moreover, chitosan is commonly used in the fabrication of hydrogels for application in drug delivery and, particularly in wound healing[35] . In this context chitosan hydrogels have also shown potential in in vivo studies, where their application promoted wound closure, ECM remodeling and angiogenesis[36][37].
As the alginate, chitosan hydrogels release S1P as an angiogenic stimulus[38].A second application consists in using chitosan hydrogels crosslinked with PVA to develop a NO releasing hydrogel, that is another necessary factor for the proliferation and migration of endothelial cells. It has been demonstrated that the hydrogel enhances angiogenesis but the molecular mechanisms behind that need to be further investigated[39].
2.2. Protein-Based Hydrogels
Due to their high biocompatibility and bioactivity, it is common to use proteins-based hydrogels in tissue engineering. Proteins used in hydrogel formation are mainly derived from ECM, such as collagen, or anyhow derived from biological sources, such as fibrin, precisely because these proteins naturally enhance cell adhesion and proliferation[40].
2.2.1. Collagen
The most abundant protein in ECM is collagen, which is widely found in tissues such as skin, cartilage, blood vessels, teeth, bones, and tendons[14]. There are 29 types of collagen but collagen type I, II and III are the most represented in the human body and type I is the most used natural scaffold in tissue engineering research, as it is the major protein component of ECM of connective tissues such as skin, bone, tendons and ligaments, meanwhile type II collagen is mainly found in hyaline cartilage and type III collagen is found in elastic vascular tissue[41][42]. The structure can be divided in four levels of organization; at the beginning it is composed by a tripeptide sequence, until the final structure, which is a three-polypeptide chain, snagged to form a rope structure with three strands. The advantages are extremely numerous, such as biocompatibility, biodegradability, low antigenicity, and low inflammatory response. On the other hand, unmodified collagen hydrogels are weak scaffolds and create degradation products which are composed by amino acids generated by collagenases and metalloproteases. These degradation products activate the coagulation cascade and show a thrombogenic potential[13][16].
The applications are mainly related to the ability to mimic the ECM, to allow cells adhesion and to deliver GFs. It is possible to create microfluidic tubes inside the hydrogel where proangiogenic factors and/or endothelial cells are used to induce angiogenesis. Moreover, collagen hydrogels allow the formation of 3D microcapillary networks by endothelial and perivascular cells[28]. Collagen hydrogels were also used as 3D culture models to study angiogenesis pathways, such as Notch signaling, and new genes interacting with Notch and VEGF signaling[43].
2.2.2. Fibrin
Fibrinogen is a large glycoprotein present in blood plasma. It plays a role in hemostasis, fibrinolysis, inflammatory response, neoplasia, and wound healing. During these processes, fibrinogen is converted into fibrin by the action of an enzyme named thrombin. Fibrin hydrogels are formed through the polymerization of fibrinogen with thrombin and calcium ions through physical interactions. Moreover, the biggest advantage is the opportunity to extract the fibrin from the patient’s blood, in this way the immune and potential inflammatory responses can be overcome. Unluckily, the fast degradation kinesis in vivo, the poor mechanical properties, the narrow ability to control the matrix rigidity and the low elasticity are the main disadvantages[15][28][31][44].
Fibrin hydrogels have been studied for many reasons mainly because of the intrinsic angiogenic abilities, secondly because of the cells/GFs delivery applications and lastly for being an artificial microenvironment that greatly mimics the native ECMs [26][44].
GFs such as VEGF and FGF can be released by fibrin hydrogels, however, the result is usually uncontrollable and lasts at maximum 24 h. The techniques to overcome this problem are numerous and they can depend on the type of growth factor. Concerning VEGF, a covalent VEGF-modified fibrin gel can be created with an engineered variant of the factor that can covalently bind fibrin via trans glutamination. Using this modified hydrogel in vivo, the nearby cells can repopulate and degrade the matrix, inducing the hydrogel carrier degradation that allows the release of VEGF. Another method to control the releasing time is to bind heparin into the gels. In HA, alginate and gelatin hydrogels, heparin has the ability to bind VEGF and FGF to reduce and control the release rate[45][46]. Furthermore, fibrin hydrogels are perfect materials for 3D creation of blood vessel capillaries because they are capable of accommodating endothelial cells and mesenchymal cells[28][29] and also thanks to hydrogel’s ability to release VEGF and PDGF[46].
2.2.3. Gelatin
Gelatin is a polymer obtained from the hydrolysis of collagen. Containing many arginine-glycine-aspartic acid sequences, gelatin hydrogels allow cell adhesion and improve matrix metalloproteinases abilities. Moreover, they possess a good biocompatibility, biodegradability, low immunogenicity, and cell affinity. On the other hand, low thermal stability and poor mechanical strength are the two main limitations[14][47].
As other hydrogels, gelatin based ones can release different molecules, such as the stromal derived factor-1 (SDF-1)[48]. Because it is able to mobilize endothelial cells and pro-angiogenic bone marrow derived cells, this factor acts in the process of angiogenic healing. The release of SDF-1 from the hydrogel is controlled by the degradation rate and by cells invasion[29]. In fact, gelatin hydrogels can be enzymatically degraded and it is possible to control the range of degradation that can vary from a few days to several months[49].
Moreover, gelatin-based hydrogels also guarantee the release and the bioactivity of VEGF[50] To achieve a better binding between VEGF and the hydrogel, heparin can be added[51]. Moreover, several studies demonstrate that gelatin hydrogels influence the secretion of VEGF and MMPs from the endothelial cells. For instance, by applying endogenous electrical fields on the gels, the cells are stimulated and release the enzymes and the GF[52].
Likewise, methacrylate gelatin (GelMA) which is typically created by modified extracellular matrix comprising of methacrylate groups added to the amine-containing side groups of the natural gelatin, is a potential scaffold for the release of GFs thanks to a low antigenicity and a better solubility [47]. For instance, VEGF can be delivered by GelMA hydrogel to promote the growth of the endothelium[53]. In addition, this hydrogel can also be used for 3D printing of tube-like structures [13].
Another important application of gelatin hydrogel indicates the ability to induce a local hypoxic environment or stimulate hypoxia-inducible factor-1 (HIF-1). A hypoxic microenvironment promotes the formation of vascular system and the angiogenesis process[29][49]. A way to create these conditions consists in using laccase, which is an enzyme with the ability to fully consume oxygen[29]. Another method involves the use of deferoxamine (DFO). In fact, with DFO the level of hypoxia-inducible factor-1 (HIF-1) and VEGF significantly increases compared to the gel without DFO. For this reason, the expression of angiogenesis-related genes increases with DFO addition[49].
2.3. Hydrogels Derived from Decellularized Tissues
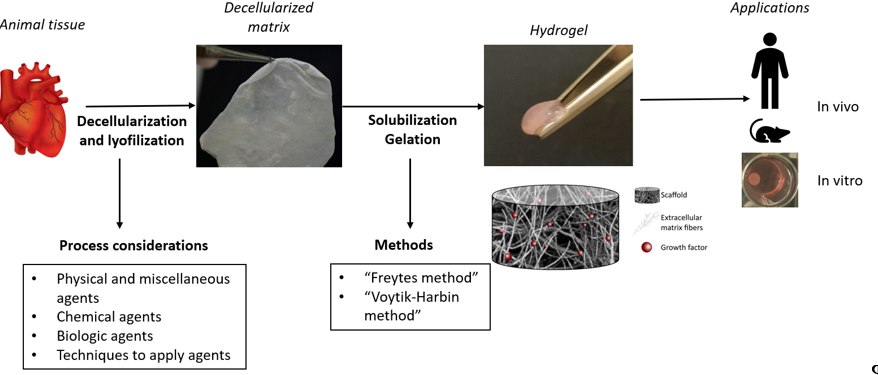
It is well known that the role of the ECM is essential for a tissue because it can maintain the homeostasis and it can influence many processes, such as the regulation of angiogenesis and cell adhesion. For this reason, the goal of the decellularization consists in achieving sufficient cells and nucleic acids removal from the source tissue while preserving the ECM structure and composition[54][55].
Figure 2. Decellularized ECM hydrogel’s process obtention.
Due to the ability to maintain the major components of the ECM such as the collagen, the GAGs, and the elastin fibers, the ECM scaffolds can promote cell growth, differentiation, proliferation, migration, and angiogenesis[2]. Indeed, in comparison to the other types of hydrogels, this one also manages to keep the physiological ratio between all the ECM components. Moreover, these hydrogels are used as a 3D culturing model that allow the cell adhesion, becoming a better system to simulate a real tissue[55]. Additionally, Wassenaar et al. suggest the effect of the ECM hydrogel in the blood vessel formation and the macrophages’ attraction through endothelial progenitor cells recruitment[56].
3. Conclusion
Angiogenesis a complex process that involves many factors, for this reason the research involving the angiogenic potential of hydrogels mainly focuses on the biological interactions of GFs and cells in the hydrogels. The intrinsic potential of the different components used to make the hydrogel is evaluated and, also, the possibility of enriching the scaffold with molecules that can improve its potential, such as the addition of GFs involved in angiogenesis.
The different materials used show different potentials, and several studies show that cell to cell and GFs interactions, along with the chemical composition and porosity of natural hydrogels, allow for capillary-like structure formation, making natural hydrogels worth of further investigation.
References
- Adair, T.H.; Montani, J.-P. Angiogenesis. Morgan & Claypool Life Sciences. 2010. Available online: http://www.ncbi.nlm.nih.gov/books/NBK53242/ (accessed on 28 July 2020).
- Jung, S.; Kleinheinz, J. Angiogenesis—The Key to Regeneration. In Regenerative Medicine and Tissue Engineering; Andrades, J.A., Ed.; InTech, London, UK: 2013, doi:10.5772/55542.
- Kumar, V.; Abbas, A.K.; Nelson, F. Robbins e Cotran, Le Basi Patologiche Delle Malattie; Elsevier Milano: Amsterdam, The Netherlands, 2006.
- Mongiat, M.; Andreuzzi, E.; Tarticchio, G.; Paulitti, A. Extracellular Matrix, a Hard Player in Angiogenesis. Int. J. Mol. Sci. 2016, 17, 1822, doi:10.3390/ijms17111822.
- Kusindarta, D.L.; Wihadmadyatami, H. The Role of Extracellular Matrix in Tissue Regeneration. In Tissue Regeneration; Kaoud, H.A., Hay, E.-S., Ed.; InTech, London, UK: 2018, doi:10.5772/intechopen.75728.
- Stupack, D.G.; Cheresh, D.A. ECM Remodeling Regulates Angiogenesis: Endothelial Integrins Look for New Ligands. Sci. Signal. 2002, 2002, pe7, doi:10.1126/stke.2002.119.pe7.
- Eskander, R.N.; Tewari, K.S. Beyond angiogenesis blockade: Targeted therapy for advanced cervical cancer. J. Gynecol. Oncol. 2014, 25, 249, doi:10.3802/jgo.2014.25.3.249.
- Patel, A.; Mequanint, K. Hydrogel Biomaterials. In Biomedical Engineering—Frontiers and Challenges; Fazel, R., Ed.; InTech, London, UK: 2011, doi:10.5772/24856.
- Gibbs, D.M.R.; Black, C.R.M.; Dawson, J.I. Oreffo ROC. A review of hydrogel use in fracture healing and bone regeneration: Hydrogel use in fracture healing and bone regeneration. J. Tissue Eng. Regen. Med. 2016, 10, 187–198, doi:10.1002/term.1968.
- Guvendiren, M.; Burdick, J.A. Engineering synthetic hydrogel microenvironments to instruct stem cells. Curr. Opin. Biotechnol. 2013, 24, 841–846, doi:10.1016/j.copbio.2013.03.009.
- Francis, L.; Greco, K.V.; Boccaccini, A.R.; Roether, J.J.; English, N.R.; Huang, H.; Ploeg, R.; Ansari, T. Development of a novel hybrid bioactive hydrogel for future clinical applications. J. Biomater. Appl. 2018, 33, 447–465, doi:10.1177/0885328218794163.
- Vallmajo‐Martin, Q.; Broguiere, N.; Millan, C.; Zenobi‐Wong, M.; Ehrbar, M. PEG/HA Hybrid Hydrogels for Biologically and Mechanically Tailorable Bone Marrow Organoids. Adv. Funct. Mater. 2020, 1910282, doi:10.1002/adfm.201910282.
- Catoira, M.C.; Fusaro, L.; Di Francesco, D.; Ramella, M.; Boccafoschi, F. Overview of natural hydrogels for regenerative medicine applications. J. Mater. Sci. Mater Med. 2019, 30, 115, doi:10.1007/s10856-019-6318-7.
- Bao, W.; Li, M.; Yang, Y. Advancements and Frontiers in the High Performance of Natural Hydrogels for Cartilage Tissue Engineering. Front. Chem. 2020, 8, 53, doi:10.3389/fchem.2020.00053.
- Rufaihah, A.J.; Seliktar, D. Hydrogels for therapeutic cardiovascular angiogenesis. Adv. Drug Deliv. Rev. 2016, 96, 31–39, doi:10.1016/j.addr.2015.07.003.
- Vieira, S.; da Silva Morais, A.; Silva-Correia, J.; Oliveira, J.M.; Reis, R.L. Natural-Based Hydrogels: From Processing to Applications: Natural-based hydrogels. In Encyclopedia of Polymer Science and Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; pp. 1–27, doi:10.1002/0471440264.pst652.
- Silva, A.K.A.; Juenet, M.; Meddahi-Pellé, A.; Letourneur, D. Polysaccharide-based strategies for heart tissue engineering. Carbohydr. Polym. 2015, 116, 267–277, doi:10.1016/j.carbpol.2014.06.010.
- Coviello, T.; Matricardi, P.; Marianecci, C.; Alhaique, F. Polysaccharide hydrogels for modified release formulations. J. Control. Release 2007, 119, 5–24, doi:10.1016/j.jconrel.2007.01.004.
- Chua, J. A Glycan Approach to Angiogenesis. PloS ONE 2017, 12, e0182301, doi:10.17605/OSF.IO/UMCW4.
- Burdick, J.A.; Prestwich, G.D. Hyaluronic Acid Hydrogels for Biomedical Applications. Adv. Mater. 2011, 23, H41–H56, doi:10.1002/adma.201003963.
- Gao, F.; Yang, C.X.; Mo, W.; Liu, Y.W.; He, Y.Q. Hyaluronan oligosaccharides are potential stimulators to angiogenesis via RHAMM mediated signal pathway in wound healing. CIM 2008, 31, 106, doi:10.25011/cim.v31i3.3467.
- Wang, N.; Liu, C.; Wang, X.; He, T.; Li, L.; Liang, X.; Wang, L.; Song, L.; Wei, Y.; Wu, Q.; et al. Hyaluronic Acid Oligosaccharides Improve Myocardial Function Reconstruction and Angiogenesis against Myocardial Infarction by Regulation of Macrophages. Theranostics 2019, 9, 1980–1992, doi:10.7150/thno.31073.
- Chen, C.W.; Wang, L.L.; Zaman, S.; Gordon, J.; Arisi, M.F.; Venkataraman, C.M.; Chung, J.J.; Hung, G.; Gaffey, A.C.; Spruce, L.A.; et al. Sustained release of endothelial progenitor cell-derived extracellular vesicles from shear-thinning hydrogels improves angiogenesis and promotes function after myocardial infarction. Cardiovasc. Res. 2018, 114, 1029–1040, doi:10.1093/cvr/cvy067.
- Gaffey, A.C.; Chen, M.H.; Venkataraman, C.M.; Trubelja, A.; Rodel, C.B.; Dinh, P.V.; Hung, G.; MacArthur, J.W.; Soopan, R.V.; Burdick, J.A.; et al. Injectable shear-thinning hydrogels used to deliver endothelial progenitor cells, enhance cell engraftment, and improve ischemic myocardium. J. Thorac. Cardiovasc. Surg. 2015, 150, 1268–1277, doi:10.1016/j.jtcvs.2015.07.035.
- Hanjaya-Putra, D.; Wong, K.T.; Hirotsu, K.; Khetan, S.; Burdick, J.A.; Gerecht, S. Spatial control of cell-mediated degradation to regulate vasculogenesis and angiogenesis in hyaluronan hydrogels. Biomaterials 2012, 33, 6123–6131, doi:10.1016/j.biomaterials.2012.05.027.
- Song, M.; Jang, H.; Lee, J.; Kim, J.H.; Kim, S.H.; Sun, K.; Park, Y. Regeneration of chronic myocardial infarction by injectable hydrogels containing stem cell homing factor SDF-1 and angiogenic peptide Ac-SDKP. Biomaterials 2014, 35, 2436–2445, doi:10.1016/j.biomaterials.2013.12.011.
- Portalska, K.J.; Teixeira, L.M.; Leijten, J.C.H. Boosting Angiogenesis and Functional Vascularization in Injectable Dextran–Hyaluronic Acid Hydrogels by Endothelial-Like Mesenchymal Stromal Cells. Tissue Eng. Part A 2014, 20, 819–829, doi:10.1089/ten.tea.2013.0280.
- Wenz, A.; Tjoeng, I.; Schneider, I.; Kluger, P.J.; Borchers, K. Improved vasculogenesis and bone matrix formation through coculture of endothelial cells and stem cells in tissue‐specific methacryloyl gelatin‐based hydrogels. Biotechnol. Bioeng. 2018, 115, 2643–2653, doi:10.1002/bit.26792.
- Blatchley, M.R.; Gerecht, S. Acellular implantable and injectable hydrogels for vascular regeneration. Biomed. Mater. 2015, 10, 034001, doi:10.1088/1748-6041/10/3/034001.
- West, D.; Hampson, I.; Arnold, F.; Kumar, S. Angiogenesis induced by degradation products of hyaluronic acid. Science 1985, 228, 1324–1326, doi:10.1126/science.2408340.
- Wei, Z.; Volkova, E.; Blatchley, M.R.; Gerecht, S. Hydrogel vehicles for sequential delivery of protein drugs to promote vascular regeneration. Adv. Drug Delivery
- Faramarzi, N.; Yazdi, I.K.; Nabavinia, M.; Gemma, A.; Fanelli, A.; Caizzone, A.; Ptaszek, L.M.; Sinha, I.; Khademhosseini, A.; Ruskin, J.N.; et al. Patient-Specific Bioinks for 3D Bioprinting of Tissue Engineering Scaffolds. Adv. Healthc. Mater. 2018, 7, 1701347, doi:10.1002/adhm.201701347.
- Wang, T.; Zheng, Y.; Shi, Y.; Zhao, L. pH-responsive calcium alginate hydrogel laden with protamine nanoparticles and hyaluronan oligosaccharide promotes diabetic wound healing by enhancing angiogenesis and antibacterial activity. Drug Deliv. Transl. Res. 2019, 9, 227–239, doi:10.1007/s13346-018-00609-8.
- Yin, N.; Han, Y.; Xu, H.; Gao, Y.; Yi, T.; Yao, J.; Dong, L.; Cheng, D.; Chen, Z. VEGF-conjugated alginate hydrogel prompt angiogenesis and improve pancreatic islet engraftment and function in type 1 diabetes. Mater. Sci. Eng. C 2016, 59, 958–964, doi:10.1016/j.msec.2015.11.009.
- Hamedi, H.; Moradi, S.; Hudson, S.M.; Tonelli, A.E. Chitosan based hydrogels and their applications for drug delivery in wound dressings: A review. Carbohydr. Polym. 2018, 199, 445–460, doi:10.1016/j.carbpol.2018.06.114.
- Lin, Z.; Li, R.; Liu, Y.; Zhao, Y.; Ao, N.; Wang, J.; Li, L.; Wu, G. Histatin1-modified thiolated chitosan hydrogels enhance wound healing by accelerating cell adhesion, migration and angiogenesis. Carbohydr. Polym. 2020, 230, 115710, doi:10.1016/j.carbpol.2019.115710.
- Li, Q.; Cui, J.; Huang, H.; Yue, Z.; Chang, Y.; Li, N.; Han, Z.; Han, Z.; Guo, Z.; Li, Z. IGF-1C domain-modified chitosan hydrogel accelerates cutaneous wound healing by promoting angiogenesis. Future Med. Chem. 2020, 12, 1239–1251, doi:10.4155/fmc-2020-0071.
- Williams, P.A.; Campbell, K.T.; Gharaviram, H.; Madrigal, J.L.; Silva, E.A. Alginate-Chitosan Hydrogels Provide a Sustained Gradient of Sphingosine-1-Phosphate for Therapeutic Angiogenesis. Ann. Biomed. Eng. 2017, 45, 1003–1014, doi:10.1007/s10439-016-1768-2.
- Zahid, A.A.; Ahmed, R.; Raza ur Rehman, S.; Augustine, R.; Tariq, M.; Hasan, A. Nitric oxide releasing chitosan-poly (vinyl alcohol) hydrogel promotes angiogenesis in chick embryo model. Int. J. Biol. Macromol. 2019, 136, 901–910, doi:10.1016/j.ijbiomac.2019.06.136.
- Cooke, M.J.; Phillips, S.R.; Shah, D.S.H.; Athey, D.; Lakey, J.H.; Przyborski, S.A. Enhanced cell attachment using a novel cell culture surface presenting functional domains from extracellular matrix proteins. Cytotechnology 2008, 56, 71–79, doi:10.1007/s10616-007-9119-7.
- Pang, Y.; Greisler, H.P. Using a type I collagen based system to understand cell-scaffold interactions and to deliver chimeric collagen binding growth factors for vascular tissue engineering. BMJ 2011, doi:10.2310/JIM.0b013e3181ee81f7.
- Balasubramanian, P.; Prabhakaran, M.P.; Sireesha, M.; Ramakrishna, S. Collagen in Human Tissues: Structure, Function, and Biomedical Implications from a Tissue Engineering Perspective. In Polymer Composites—Polyolefin Fractionation—Polymeric Peptidomimetics—Collagens; Abe, A., Kausch, H.-H., Möller, M., Pasch, H., Eds.; Springer: Berlin/ Heidelberg, Germany, 2012; Volume 251, pp. 173–206, doi:10.1007/12_2012_176.
- Bai, J. Angiogenic responses in a 3D micro-engineered environment of primary endothelial cells and pericytes. Angiogenesis 2020, doi:10.1007/s10456-020-09746-6.
- Atluri, P.; Miller, J.S.; Emery, R.J.; Hung, G.; Trubelja, A.; Cohen, J.E.; Lloyd, K.; Han, J.; Gaffey, A.C.; MacArthur, J.W.; et al. Tissue-engineered, hydrogel-based endothelial progenitor cell therapy robustly revascularizes ischemic myocardium and preserves ventricular function. J. Thorac. Cardiovasc. Surg. 2014, 148, 1090–1098, doi:10.1016/j.jtcvs.2014.06.038.
- Wei, Z.; Volkova, E.; Blatchley, M.R.; Gerecht, S. Hydrogel vehicles for sequential delivery of protein drugs to promote vascular regeneration. Adv. Drug Delivery Rev. 2019, 149–150, 95–106, doi:10.1016/j.addr.2019.08.005.
- Zhao, N.; Suzuki, A.; Zhang, X.; Peng Shi, P.; Abune, L.; Coyne, J.; Jia, H.; Xiong, N.; Zhang, G.; Wang, Y. Dual Aptamer-Functionalized in Situ Injectable Fibrin Hydrogel for Promotion of Angiogenesis via Codelivery of Vascular Endothelial Growth Factor and Platelet-Derived Growth Factor-BB. Acs Appl. Mater. Interfaces 2019, 11, 18123–18132, doi:10.1021/acsami.9b02462.
- Modaresifar, K.; Hadjizadeh, A.; Niknejad, H. Design and fabrication of GelMA/chitosan nanoparticles composite hydrogel for angiogenic growth factor delivery. Artif. Cells Nanomed. Biotechnol. 2017, 46, 1799–1808, doi:10.1080/21691401.2017.1392970.
- Saito, T.; Tabata, Y. Hypoxia-induced angiogenesis is increased by the controlled release of deferoxiamine from gelatin hydrogels. Acta Biomater. 2014, 10, 3641–3649, doi:10.1016/j.actbio.2014.04.021.
- Kimura, Y.; Tabata, Y. Controlled Release of Stromal-Cell-Derived Factor-1 from Gelatin Hydrogels Enhances Angiogenesis. J. Biomater. Sci. Polym. Ed. 2010, 21, 37–51, doi:10.1163/156856209X410193.
- Gnavi, S.; di Blasio, L.; Tonda-Turo, C.; Mancardi, A.; Primo, L.; Ciardelli, G.; Gambarotta, G.; Geuna, S.; Perroteau, I. Gelatin-based hydrogel for vascular endothelial growth factor release in peripheral nerve tissue engineering: VEGF-releasing hydrogel. J. Tissue Eng. Regen. Med. 2017, 11, 459–470, doi:10.1002/term.1936.
- Li, Z.; Qu, T.; Ding, C.; Ma, C.; Sun, H.; Li, S.; Liu, X. Injectable gelatin derivative hydrogels with sustained vascular endothelial growth factor release for induced angiogenesis. Acta Biomater. 2015, 13, 88–100, doi:10.1016/j.actbio.2014.11.002.
- Tzoneva, R.; Uzunova, V.; Apostolova, S. Angiogenic potential of endothelial and tumor cells seeded on gelatin–based hydrogels in response to electrical stimulations. Clin. Hemorheol. Microcirc. 2017, 64, 941–949, doi:10.3233/CH-168040.
- Ngo, M.T.; Harley, B.A. The Influence of Hyaluronic Acid and Glioblastoma Cell Coculture on the Formation of Endothelial Cell Networks in Gelatin Hydrogels. Adv. Healthc. Mater. 2017, 6, 1700687, doi:10.1002/adhm.201700687.
- Seo, Y.; Jung, Y.; Kim, S.H. Decellularized heart ECM hydrogel using supercritical carbon dioxide for improved angiogenesis. Acta Biomater. 2018, 67, 270–281, doi:10.1016/j.actbio.2017.11.046.
- Getova, V.E.; van Dongen, J.A.; Brouwer, L.A.; Harmsen, M.C. Adipose tissue-derived ECM hydrogels and their use as 3D culture scaffold. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1693–1701, doi:10.1080/21691401.2019.1608215.
- Wassenaar, J.W.; Gaetani, R.; Garcia, J.J.; Braden, R.L.; Luo, C.G.; Huang, D.; DeMaria, A.N.; Omens, J.H.; Christman, K.L. Evidence for Mechanisms Underlying the Functional Benefits of a Myocardial Matrix Hydrogel for Post-MI Treatment. J. Am. Coll. Cardiol. 2016, 67, 1074–1086, doi:10.1016/j.jacc.2015.12.035.
- Wassenaar, J.W.; Gaetani, R.; Garcia, J.J.; Braden, R.L.; Luo, C.G.; Huang, D.; DeMaria, A.N.; Omens, J.H.; Christman, K.L. Evidence for Mechanisms Underlying the Functional Benefits of a Myocardial Matrix Hydrogel for Post-MI Treatment. J. Am. Coll. Cardiol. 2016, 67, 1074–1086, doi:10.1016/j.jacc.2015.12.035.
- Ngo, M.T.; Harley, B.A. The Influence of Hyaluronic Acid and Glioblastoma Cell Coculture on the Formation of Endothelial Cell Networks in Gelatin Hydrogels. Adv. Healthc. Mater. 2017, 6, 1700687, doi:10.1002/adhm.201700687.