
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sylvie Lecollinet | + 852 word(s) | 852 | 2020-01-07 02:00:30 | | | |
| 2 | Vivi Li | + 86 word(s) | 938 | 2020-10-29 07:14:10 | | |
Video Upload Options
Neurological disorders represent an important sanitary and economic threat for the equine industry worldwide. Among nervous diseases, viral encephalitis is of growing concern, due to the emergence of arboviruses and to the high contagiosity of herpesvirus-infected horses. The nature, severity and duration of the clinical signs could be different depending on the etiological agent and its virulence. However, definite diagnosis generally requires the implementation of combinations of direct and/or indirect screening assays in specialized laboratories. The equine practitioner, involved in a mission of prevention and surveillance, plays an important role in the clinical diagnosis of viral encephalitis. The general management of the horse is essentially supportive, focused on controlling pain and inflammation within the central nervous system, preventing injuries and providing supportive care. Despite its high medical relevance and economic impact in the equine industry, vaccines are not always available and there is no specific antiviral therapy. In this review, the major virological, clinical and epidemiological features of the main neuropathogenic viruses inducing encephalitis in equids in Europe, including rabies virus (Rhabdoviridae), Equid herpesviruses (Herpesviridae), Borna disease virus (Bornaviridae) and West Nile virus (Flaviviridae), as well as exotic viruses, will be presented.
1. Introduction
Many neurological diseases represent an important sanitary and economic threat to the horse population worldwide. Non-vector-borne equine encephalitis viruses, such as EHV-1, rabies and BoDV, are variably reported in Europe, whereas arthropod-borne infections are usually exotic diseases. Viral encephalitis therapeutic strategies are comparable, whatever the etiologic virus. Horses with neurological conditions must be isolated in a quiet box with limited stimuli (noise, light) and an appropriate bedding providing warmth, comfort, and security. Slings can be used to support paretic horses and avoid long and poor prognosis recumbency (Figure 1). Supportive care will contribute substantially, avoiding complications and improving the prognosis. The use of DMSO (0.4–0.9 g/kg for 5–6 days) has been advocated on the basis of its free radical scavenging properties but its efficacy has not been evaluated scientifically [1]. Nonsteroidal anti-inflammatory drugs may be used to control pyrexia, inflammation and discomfort, while short-term use of glucocorticoids may be beneficial; glucocorticoids proved to be valuable in some EHM horses and it is hypothesized that the treatment reduces the supposed immune-mediated EHV-1 pathogenesis [2]. However, they were also shown to reactivate latent herpesvirus infection and to increase the level and duration of virus shedding [3]. Vaccination is controversial in the face of outbreaks and in particular inactivated vaccines take too long to generate immune responses capable to limit disease spread when outbreaks are seasonal (WNV, other vector-borne viruses in temperate areas).
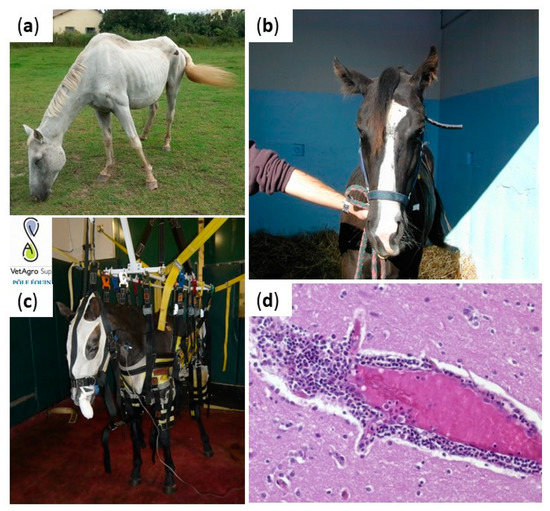
Figure 1. Clinical manifestations and lesions in viral equine encephalitis. Horses infected with equine encephalitis viruses may experience posture deficits (increasing of the lift polygon in (a)), cranial nerve deficits (facial paralysis in (b)), balance deficiencies (slings in (c) can be used to support paretic horses and avoid long and poor prognosis recumbency). Brain lesions are non-specific and include perivascular infiltration of inflammatory cells, observed in (d). Credits: Pr Agnès Leblond, VetAgroSup, and Dr Eve Laloy, French Veterinary School of Alfort.
2. Clinical signs of viral equine encephalitis
Clinical signs of viral equine encephalitis are not specific and overlapping geographical areas can make virus identification very challenging. One recent and striking example of delayed identification of emerging arboviruses due to similarities in clinical presentation and cross-reactive diagnostic tools was given during WNV introduction in the United States, when WNV was initially misdiagnosed with the closely related SLEV [4]. Bearing in mind that three flaviviruses responsible for equine encephalitis are described in Europe, and that serological cross-reactivity is frequently observed in flavivirus indirect diagnosis assays, the development of multiplex approaches that allow the comparison of serological reactions against a wide range of pathogens appear to be valuable options [5][6]. Furthermore, because in about one-half of infectious equine encephalitis, no known pathogen can be evidenced [7], identification of unknown neuropathogenic viruses by classical (electron microscopy) and more recent high-throughput techniques (next generation sequencing for example) is highly desirable [8][9]. In these two recent studies, three viruses, Shuni virus, horse parvovirus-CSF and eqcopivirus, have been identified as potential causes of neurologic disease in horses through unbiased detection from different tissues or body fluids; the demonstration of infectious virus from the brain of sick horses establish Shuni virus as a novel equine neuropathogenic virus [8], while for the other two viruses for which genomic DNA was detected in CSF and/or plasma [9], comparison of virus prevalence in the CSF of healthy horses (case-control study) would be required before a conclusion on the aetiology of equine encephalitis can be reached.
3. Arboviruses
Arboviruses are the most important cause of encephalitis in horses and many of these viruses are also significant human pathogens. Some of these arboviruses have recently emerged or resurged, such as WNV, JEV, SLEV, EEEV or EEV and an increased rate of emergence of vector-borne diseases can be inferred from recent studies [10]. A high diversity of mosquito species have been reported in Europe (mainly from Aedes, Culex and Culiseta genera), and highly invasive Aedes albopictus and Ae. japonicus have rapidly established in several European countries over the last decade [11][12]. Vector competence of native and invasive European mosquito species for equine encephalitis viruses, other than WNV and JEV, has been unfrequently evaluated [13][14][15] Consequently, identification of European regions at risk for the spread of exotic equine encephalitis viruses is difficult and mainly relies on information on mosquito and animal hosts density and on records of opportunistic mosquito species [16]. On-time control of vector-borne infections relies on the use of sentinel systems, including horses or sentinel chicken flocks for example, to provide warning of virus activity and initiate mosquito control measures [17].
References
- Pellegrini-Masini, A.; Livesey, L.C. Meningitis and encephalomyelitis in horses. Vet. Clin. N. Am. Equine Pract. 2006, 22, 553–589.
- Reed, S.M.; Toribio, R.E. Equine herpesvirus 1 and 4. Vet. Clin. N. Am. Equine Pract. 2004, 20, 631–642.
- Slater, J.D.; Borchers, K.; Thackray, A.M.; Field, H.J. The trigeminal ganglion is a location for equine herpesvirus 1 latency and reactivation in the horse. J. Gen. Virol. 1994, 75, 2007–2016.
- Roehrig, J.T. West nile virus in the United States—A historical perspective. Viruses 2013, 5, 3088–3108.
- Beck, C.; Despres, P.; Paulous, S.; Vanhomwegen, J.; Lowenski, S.; Nowotny, N.; Durand, B.; Garnier, A.; Blaise-Boisseau, S.; Guitton, E.; et al. A High-Performance Multiplex Immunoassay for Serodiagnosis of Flavivirus-Associated Neurological Diseases in Horses. BioMed Res. Int. 2015, 2015, 678084.
- Cleton, N.B.; van Maanen, K.; Bergervoet, S.A.; Bon, N.; Beck, C.; Godeke, G.J.; Lecollinet, S.; Bowen, R.; Lelli, D.; Nowotny, N.; et al. A Serological Protein Microarray for Detection of Multiple Cross-Reactive Flavivirus Infections in Horses for Veterinary and Public Health Surveillance. Transbound. Emerg. Dis. 2017, 64, 1801–1812.
- Laugier, C.T.; Tapprest, J. Fréquence de la pathologie nerveuse et de ses différentes causes dans un effectif de 4319 chevaux autopsiés. Bull. Epidémiologique St. Anim. Et Aliment. Spécial Équidé 2012, 19, 9.
- Van Eeden, C.; Williams, J.H.; Gerdes, T.G.; van Wilpe, E.; Viljoen, A.; Swanepoel, R.; Venter, M. Shuni virus as cause of neurologic disease in horses. Emerg. Infect. Dis. 2012, 18, 318–321.
- Altan, E.; Li, Y.; Sabino-Santos, G., Jr.; Sawaswong, V.; Barnum, S.; Pusterla, N.; Deng, X.; Delwart, E. Viruses in Horses with Neurologic and Respiratory Diseases. Viruses 2019, 11, 942.
- Cohen, M.L. Changing patterns of infectious disease. Nature 2000, 406, 762–767.
- Martinet, J.P.; Ferte, H.; Failloux, A.B.; Schaffner, F.; Depaquit, J. Mosquitoes of North-Western Europe as Potential Vectors of Arboviruses: A Review. Viruses 2019, 11, 1059.
- Cunze, S.; Koch, L.K.; Kochmann, J.; Klimpel, S. Aedes albopictus and Aedes japonicus—Two invasive mosquito species with different temperature niches in Europe. Parasites Vectors 2016, 9, 573.
- Vogels, C.B.; Goertz, G.P.; Pijlman, G.P.; Koenraadt, C.J. Vector competence of European mosquitoes for West Nile virus. Emerg. Microbes Infect. 2017, 6, e96.
- De Wispelaere, M.; Despres, P.; Choumet, V. European Aedes albopictus and Culex pipiens Are Competent Vectors for Japanese Encephalitis Virus. PLoS Negl. Trop. Dis. 2017, 11, e0005294.
- Huber, K.; Jansen, S.; Leggewie, M.; Badusche, M.; Schmidt-Chanasit, J.; Becker, N.; Tannich, E.; Becker, S.C. Aedes japonicus japonicus (Diptera: Culicidae) from Germany have vector competence for Japan encephalitis virus but are refractory to infection with West Nile virus. Parasitol. Res. 2014, 113, 3195–3199.
- Durand, B.; Lecollinet, S.; Beck, C.; Martinez-Lopez, B.; Balenghien, T.; Chevalier, V. Identification of hotspots in the European union for the introduction of four zoonotic arboviroses by live animal trade. PLoS ONE 2013, 8, e70000.
- Gossner, C.M.; Marrama, L.; Carson, M.; Allerberger, F.; Calistri, P.; Dilaveris, D.; Lecollinet, S.; Morgan, D.; Nowotny, N.; Paty, M.C.; et al. West Nile virus surveillance in Europe: Moving towards an integrated animal-human-vector approach. Euro Surveill. 2017, 22.




