
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ahmed Zayed | + 3256 word(s) | 3256 | 2020-12-01 07:40:16 | | | |
| 2 | Ahmed Zayed | -27 word(s) | 3229 | 2020-12-16 11:01:58 | | | | |
| 3 | Nicole Yin | Meta information modification | 3229 | 2020-12-17 03:34:28 | | |
Video Upload Options
Since the early life of humankind on the Earth, nature represents the most powerful source for his major needs from food, energy, and therapeutics. Oceans cover more than 70% of Earth’s surface, and therefore, they continue to offer exceptional scaffolds improving the quality of human life. According to the National Oceanic and Atmospheric Administration (United States Department of Commerce), marine microbes represent 98% of ocean biomass. From more than 300,000 described organisms, 12,000 novel compounds have been discovered attracting great interest in the last decades. Marine macroalgae are rich sources of either sulfated or non-sulfated polysaccharides with a wide range of interesting medical applications. Fucoidan is a marine polysaccharide isolated mainly from brown macroalgae with interesting and promising pharmacological activities. Several articles discussed and proved the potential, versatile, and promising pharmacological activities of fucoidans.
1. Introduction
Fucoidans are known as fucose-containing sulfated polysaccharides (FCSPs), where l-fucose always predominates other sugar monomers, such as galactose, mannose, glucose, and uronic acids. l-fucose may exceed 90% of the total sugar composition of fucoidans[1]. Yet, galactose, as in the case of sulfated galactofucans, may possess similar ratios to fucose[2]. Another type of FCSP is isolated from marine invertebrates, called sulfated fucans. In contrast, they are composed of l-fucose only. Hence, the term fucoidans has recently been adopted specifically for the heterogenous marine SPs rich in fucose and derived from the different species of brown algae, including the old names fucoidin and fucoidan, to be consistent with the International Union of Pure and Applied Chemistry (IUPAC) nomenclature system[3]. They are species-specific and, therefore, they do not have a universal chemical structure. Yet, they represent the major component of cell walls and the extracellular matrix (ECM) along with alginate and cellulose in brown seaweeds[4][5]. The physicochemical and chemical heterogeneity of fucoidans was discussed previously, as well as the way in which it affects their application[6]. In addition, fucoidans are characterized by high molecular weights, up to 950 kDa in the native fucoidan of Hizikia fusiforme or Sargassum fusiforme[7]. The presence of sulfate ester groups imparts a negative charge on the macromolecule skeleton responsible for the anionic characteristic of fucoidans[8]. Moreover, chain branching increases the complexity of fucoidans compared to sulfated fucans derived from marine invertebrates[9][10]. Therefore, an investigation of these two groups of fucose-containing biopolymers, i.e., sulfated fucans and fucoidans, requires different investigational approaches. Owing to their complicated chemical structures, enzymatic hydrolysis, mild acid hydrolysis, and autohydrolysis of native fucoidans are always involved in the elucidation of their fine structural features. Such pretreatments enable the production of oligomers or simple fractions that are easily interpreted[11].
2. Chemistry of Fucoidans
The chemistry of fucoidans is highly variable according to their origin, especially regarding their complexity. For instance, fucoidans derived from seaweeds commonly show a branched and more sulfated skeleton with the presence of numerous sugar monomers in addition to α-l-fucose. However, marine invertebrate fucoidans, such as echinoderms (e.g., sea cucumber) and urchins (e.g., Strongylocentrotus droebachiensis), are less complex, consisting of a linear and regular chain of repeating α-l-fucose units[3][9][12][13][14]. These differences make algal fucoidans a more preferable biogenic resource than those from marine invertebrates, in addition to their multiple and interesting biological activities[15].
In the literature, several structural models for seaweed fucoidans have been suggested to describe their important structural features[16][17][18][19] depending on their macroalgal biogenic origin, including species, age, geographical origin, and season of harvesting[2][6][20]. Cao et al. presented several representative fucoidan structures isolated from Fucus evanescens, Fucus vesiculosus, Sargassum mcclurei, Turbinaria ornata, Saccharina cichorioides, and Undaria pinnatifida[2].
Nevertheless, the most widely accepted models are those introduced by Cumashi et al.[21] and Ale et al.[22]. They proposed that seaweed fucoidans are highly heterogenous within brown seaweed species, composed of a linear or branched sulfated l-fucopyranoside backbone linked by not only alternating α-(1→3) and α-(1→4) linkages, but also α-(1→4) and α-(1→3) linkages only. Other sugar monomers can also be found, such as β-d-galactose, β-d-mannose, α-d-glucuronic acid, α-d-glucose, and β-d-xylose, but their positions and binding modes are still not understood[23][24]. However, Bilan et al. studied a fucoidan fraction isolated from Sargassum polycystum (Fucales) and found 2-linked sulfated α-d-galactopyranose residues[19]. Moreover, the l-fucose unit is mono- or disulfated and may be acetylated. These groups are responsible for the anionic characteristic of fucoidans.
According to the models proposed by Cumashi et al. and Ale et al., the chemical structures of fucoidans are represented on the basis of their origin, as shown in Figure 1. For examples, in Fucales, fucoidans show l-fucopyranoside chains linked with alternating α-(1→4) and α-(1→3) glycosidic linkages. C-2 and/or C-4 (rarely at C-3) are usually substituted with sulfate ester groups (–SO3−), according to the type of glycosidic linkages[2]. Moreover, side branching was detected at C-4, alternating with sulfate groups in F. serratus L. in α-(1→3) l-fucopyranoside units. On the other hand, in Laminariales and Chordariales, fucoidan subunits are mainly linked by α-(1→3) glycosidic linkages. Additionally, at C-2, other sugar monomers can be detected as a side branch, whereas sulfate ester groups are common at C-4. The chemical structures may also vary within the same organism on the basis of the applied extraction methods[25].
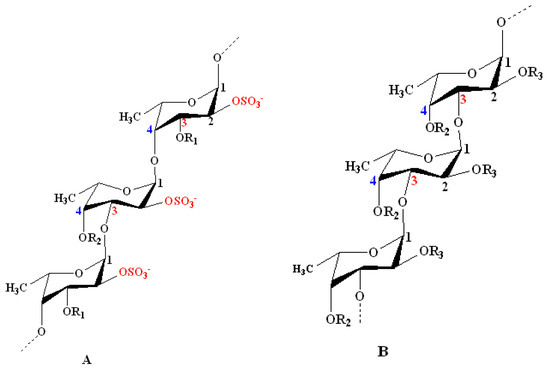
Figure 1. Structural models for the chemical structure of fucoidans derived from some species of seaweeds as proposed by Cumashi et al. and Ale et al.[22][23]. Model A: Model representing fucoidans from some species of Fucales. It shows repeating l-fucopyranoside units linked with alternating α-(1→4) and α-(1→3) glycosidic linkages. C-2 is always substituted with a sulfate ester group. Examples include Fucus vesiculosus and Ascophyllum nodosum: R1 = SO3−, R2 = H; F. serratus L.: R1 = H, R2 = side chain or SO3−; and Fucus evanescens C. Ag: R1 = H, R2 = SO3− or H. Model B: Model representing some species of Laminariales and Chordariales. Both orders show a repeated α-(1→3)-linked branched l-fucopyranoside backbone at C-2. Sulfate ester groups mainly substitute C-4 and sometimes C-2. Examples include Laminaria saccharina (Laminariales): R2 = OSO3−, R3 = H alternating with OSO3− and l-fucose; and Cladosiphon okamuranus (Chordariales): R2 = OSO3− alternating with H, R3 = OSO3− alternating with H and uronic acid. Other minor sugar units (e.g., mannose and galactose) and acetyl groups occur in fucoidan structures at certain unknown positions[23].
Recently, Usoltseva et al. revealed other models in Laminariales members, i.e., Saccharina or Laminaria cichorioides and Laminaria longipes. They detected unusual fucoidans with α-(1→3) linkages that also contain α-(1→4)- and α-(1→2)-linked fucopyranoside residues[26]. Additionally, Wang et al. showed that α-(1→4) linkages may be present in the fucoidan backbone of Laminaria japonica[27]. As a consequence of these complex characteristics and heterogeneity, it is always difficult to characterize the chemical structure of the whole polymer using a single technique. Spectrometric methods (e.g., Fourier-transform infrared (FT-IR), NMR and MS) are used to elucidate the structural features, especially the position of sulfate ester groups and glycosidic bonds. In addition, chromatographic methods, such as gel permeation (GPC) also known as size-exclusion chromatography (SEC), are applied for the determination of molecular-weight parameters or averages. Currently, advanced hyphenated spectrometric techniques, such as HPLC–MS/MS, are applied[28][29].
3. Characterization of Fucoidan Quality
3.1. Fucoidan Characteristics
3.1.1. Sugar Content
The Dubois or phenol–sulfuric acid assay is a simple acid-catalyzed condensation reaction, which is commonly employed for the determination of total sugar concentration in carbohydrates[30]. Fucoidan and 5% (w/v) aqueous phenol solutions are mixed, and then concentrated sulfuric acid is carefully added. Afterward, the sample is mixed vigorously, and the absorbance is recorded at 490 nm. The reaction mechanism is based on color development upon the dehydration of sugars to furfural derivatives with sulfuric acid. The furfural product is then condensed with phenol to produce stable colored compounds.
The Somogyi–Nelson test is also used for the determination of reducing sugars, where copper and fucoidan solutions are mixed carefully and incubated in a boiling water bath. Afterward, the arsenic molybdate reagent is added. The reaction mixture is then incubated at room temperature and analyzed at 500 nm. The mechanism is based on a redox reaction, where reducing sugars are oxidized by the weakly alkaline copper reagent to a sugar acid, while Cu2+ is reduced to Cu+. Then, the arsenic molybdate reagent is used to regenerate Cu2+ ions, thereby reducing arsenic molybdate and producing a characteristic blue color[31][32].
3.1.2. Fucose Content
The Dische or cysteine–sulfuric acid assay is carried out to quantify l-fucose content in hydrolyzed fucoidan solutions[33]. The test consists of mixing the fucoidan solution with diluted sulfuric acid (1:6). Then, the reaction mixture is incubated at 100 °C for a period, and the reaction is stopped by cooling in an ice bath. Thereafter, an aqueous l-cysteine solution is added, and the absorbance is measured at two wavelengths, namely, 396 and 430 nm. According to the difference of those two measurements, the possible interference of hexoses can be excluded[34]. However, algal polyphenols may interfere to a great extent in colorimetric fucose determination. Alternatively, as fucose is a neutral sugar, it can be determined using more sensitive methods, such as HPLC and GC after derivatization[35]. Details are provided in Section 4.3 with regard to the investigation of fucoidan monomeric composition.
3.1.3. Fucoidan Content
The usual problem in the quantitative determination of fucoidan content is the absence of an appropriate standard. Commercial preparations may be insufficiently purified and may be structurally different from analytical samples. Nevertheless, on the basis of the anionic characteristic of fucoidans, thiazine dyes, such as in the toluidine blue (TB) assay according to Hahn et al.[36] and the Heparin Red® Ultra assay according to Warttinger et al.[37][38], can be applied. The TB assay is based on the formation of a charge-transfer complex between the thiazine dye and the polysaccharide[39]. It consists of mixing fucoidan-containing solutions with TB at pH 1 for better reaction sensitivity. The absorbance is then measured at 632 nm using an aqueous solution of commercially purified fucoidan as a reference standard in a concentration range of 0–2.5 g·L−1. The color changes are demonstrated in Figure 2, whereby Figure 2A shows the metachromatic effect of fucoidan on the polycationic thiazine dye toluidine blue. A hypochromic effect is shown with a hypsochromic shift of the toluidine blue ultraviolet/visible light (UV/Vis) spectrum following the addition of polyanionic molecules (e.g., fucoidan). On the other hand, the Heparin Red® Ultra assay is based on the fluorescence-quenching ability of fucoidans after incubation with Heparin Red® reagent, as depicted in Figure 2C. It may be carried out using excitation and emission wavelengths of 570 and 605 nm, respectively. The reaction shows potential selectivity for fucoidan even in the presence of sodium alginate salt, as demonstrated in Figure 2D[38]. The Heparin Red® Ultra assay also demonstrates great sensitivity in a linear range of 0.0–8.0 μg·mL−1. The results of such investigations indicate the relative quality of fucoidans and their degree of purity.
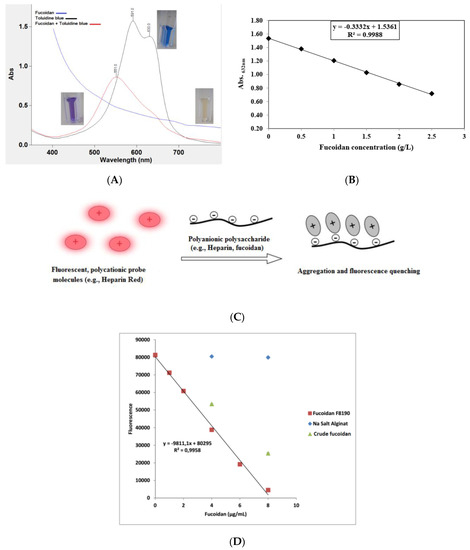
Figure 2. (A) Metachromatic effect of fucoidan on the polycationic thiazine dye toluidine blue (TB). A hypsochromic shift and hypochromic effect are observed after the reaction of TB with fucoidans. (B) Calibration curve of TB assay showing the reaction linearity in a specified fucoidan concentration, i.e., 0–2.5 g·L−1. (C) Representation of polyanionic polysaccharide reaction with fluorescent perylene diimide molecules (e.g., Heparin Red®). The reaction electrostatically produces aggregates, followed by fluorescence quenching (modified according to[40]). (D) Calibration curve of Heparin Red® assay showing crude fucoidan samples deviating from the linear range of the reference sample with no interference from alginate[41]. The ultraviolet/visible light (UV/Vis) measurement was conducted using a UV/Vis spectrometer (Cary 60 UV/Vis, Agilent Technologies, USA), while the fluorescence was recorded using a spectrofluorometer (FP-8300, JASCO Deutschland GmbH, Germany).
The principle behind the reaction of fucoidans with basic or cationic dyes was successfully applied using Alcian blue stain for the detection of fucoidans and its fragments after degradation experiments with fucoidanases in carbohydrate polyacrylamide gel electrophoresis (C-PAGE)[42]. Moreover, other similar anionic polysaccharides from carrageenan could be detected using the same principle[43]. Currently, several commercial highly purified fucoidans are marketed by well-known companies, such as Sigma-Aldrich® and Marinova®, derived from F. vesiculosus and other brown algae species[10][44].
A more sensitive and selective electrochemical method for the detection of fucoidan was developed by Kim et al. in biological fluids and nutritional supplements. The method is based on potentiometric sensors using polyion-sensitive membrane electrodes. Examples of compounds acting as ion exchangers were tridodecyl methylammonium (TDMA) and dinonylnaphthalene sulfonate (DNNS)[10][45].
3.1.4. Sulfate Content
As developed by Dodgson and Price, sulfate content can be analyzed on the basis of barium sulfate (BaSO4) precipitation after the addition of barium chloride (BaCl2) in gelatin using sodium sulfate (Na2SO4) or potassium sulfate (K2SO4)[46][47]. The sulfate amount is determined by turbidimetry at 500 nm[48]. Since sulfate ester groups are susceptible to hydrolysis, turbidimetric analysis requires preliminary liberation of the sulfate groups via acid hydrolysis using 4 M HCl at 100 °C for 6 h[49] or 2 M trifluoroacetic acid (TFA) at 100 °C for 8 h[50].
Using inductively coupled plasma mass spectrometry (ICP-MS), the sulfate content of fucoidan isolated from L. hyperborean was determined. Sulfur contents were determined by dissolving the dried fucoidan (70 °C for 90 min) in 1 M HNO3. The sulfation degree was determined by utilizing a mass balance equation, assuming that every sulfate group was associated with a sodium counterion[51].
3.1.5. Uronic Acid Content
A colorimetric determination of uronic acids is usually performed using meta-phenylphenol according to the procedures presented by Filisetti-Cozzi and Carpita[8][52] or Blumenkrantz and Asboe-Hansen[53]. The same principle can be applied with m-phenylphenol to form a colored condensation product, where the sugar is firstly dehydrated by heating with sulfuric acid before the addition of m-phenylphenol and incubation at room temperature. The absorbance is then recorded at 525 nm. A modified uronic acid carbazole reaction is sometimes also applied[44][54].
Moreover, specific HPLC techniques based on monomer derivatization were reported. They include high-performance anion-exchange chromatography (HPAEC) coupled with pulsed amperometry detection (PAD). This method is commonly known as Dionex HPAEC–PAD, i.e., implementing a Dionex ICS-2500 system equipped with CarboPac™ PA20 analytical and guard columns. It depends on the fact that uronic acids are weak acids that can be derivatized to oxyanions at alkaline pH values[55][56].
In Section 3.2.3, alginate is discussed as a potential contaminant of fucoidans, leading to an increase in uronic acid content in fucoidan products if not properly removed. Hence, the identification of uronic acids is necessary to distinguish the components of fucoidan from the components of alginic acids. The uronic acids of fucoidans mainly constitute α-d-glucuronic acid[57][58], while those in alginate constitute α-l-guluronic acid (G-block) and β-d-mannuronic acid (M-block) linked via α-(1→4) bonds[59], as shown in Figure 3. These blocks produce a characteristic NMR pattern, from which the M/G ratio can be calculated[60][61].
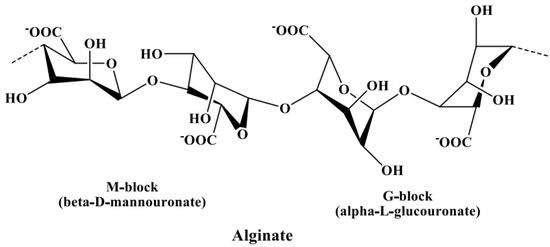
Figure 3. Chemical structure of alginate composed of α-l-guluronic acid (G-block) and β-d-mannuronic acid (M-block) linked via α-(1→4) glycosidic bonds.
3.2. Potential Coextracted Impurities
Since fucoidans are found in a highly complicated cell-wall matrix in addition to other polymers, such as cellulose, alginate, and protein, as well as polyphenols[62], several investigations should be carried out to detect and quantify such components. Moreover, other components may be also coextracted and present in crude fucoidans such as laminaran, mannitol, lipids, and pigments[15][63][64]. Hence, comprehensive downstream processes should be applied to remove all of these compounds as best as possible[65]. However, for reproducible and trusted biological activities, potential contaminants, such as proteins, alginate, laminaran, and total phenolic content should be quantified to determine the quality grade of fucoidans.
3.2.1. Protein
The Folin–phenol[66] and Bradford assays are applied to determine protein content in fucoidan products, using bovine serum albumin as a reference standard for calibration[8][67]. The Lowry and Bradford assays are based on colorimetric determination, where they produce colored solutions recorded at 750 and 595 nm, respectively, in response to protein and/or amino acids. The Folin–phenol reagent consists of phosphomolybdic–phosphotungstic acid, which is reduced to a blue-colored solution by protein in an alkaline Cu2+ tartrate solution[66][68], whereas the color in the Bradford assay is formed due to complex formation between the protein and the Coomassie blue G-250 dye. Under acidic conditions, the protonated red dye is transformed to an anionic blue form through a dye–protein electrostatic and hydrophobic interaction[69][70].
Both assays show variable results, due to variations in protein composition, pH, and sample concentration[68], whereby only the tyrosine, tryptophan, and cysteine amino acids can react[71]. In addition, the Lowry method is not specific enough since the results are highly affected by the presence of interfering compounds that can also chelate Cu2+ (e.g., nitrogenous and phenolic compounds)[71][72].
3.2.2. Phenolic Compounds
Phenolic compounds in brown algae vary structurally from simple molecules (e.g., hydroxybenzoic acid derivatives, such as gallic, phenolic, and cinnamic acids) or flavonoids (e.g., flavan-3-ol derivatives, such as epicatechin or epigallocatechin) to more complex phlorotannin polymeric structures (e.g., phlorethols, fuhalols, fucols, fucophlorethols, and eckol)[73].
As previously discussed, polyphenols are tightly noncovalently bound to fucoidans in the cell wall, which contribute along with fucoxanthin to the brown color of the crude fucoidan extract[65]. The total phenolic content can be quantified using the Folin–Ciocalteu method, especially for crude fucoidan products[74][75]. Additionally, the 2,4-dimethoxybenzaldehyde (DMBA) assay may be applied for phlorotannin content[76]. The Folin–Ciocalteu method is similar to the Folin–phenol applied for protein determination; however, the absorbance is recorded at 620 nm[75]. Nonetheless, interference from sugar monomers is common and may lead to false results. Gallic acid is commonly used as a reference standard and, therefore, the results are expressed as gallic acid equivalents[77].
3.2.3. Alginate
Precipitation of alginate by divalent ions (e.g., Ca2+ or Ba2+) is a common pretreatment step during fucoidan extraction[22][78]. An acidic medium, i.e., below the pKa of carboxylic groups, also helps in the precipitation of alginate as alginic acid[79]. Therefore, for the efficient removal of alginate during fucoidan extraction, both conditions are usually applied[80]. Nevertheless, traces of alginate are frequently detected in crude fucoidan extracts from brown algae[56]. Even the application of enzyme-assisted extraction employing an alginate lyase from Sphingomonas sp. (SALy) resulted in the crude fucoidan product containing substantial alginate, thus requiring a further purification step[81][82].
Since alginate is composed of β-d-mannuronic (M-block) and α-l-guluronic (G-block) acids as building blocks[83], it may interfere with the determination of uronic acids during fucoidan chemical characterization. Therefore, alginate can instead be determined as a function of the metachromatic change induced upon binding to cationic dyes, such as 1,9-dimethyl methylene blue (DMMB)[84], or using the TB assay. However, due to the different pKa values of the sulfate ester group in fucoidans and carboxylic group in alginate, the different measurements at pH 1.0 and pH 7.0 can be used to quantify alginate content, where, at pH 1.0, fucoidan is ionized and interacts only with TB, while, at pH 7.0, both are ionized and induce color changes[36]. Dionex HPAEC–PAD can potentially be applied for the specific determination of alginate building blocks, thereby excluding interference from the uronic acids of fucoidans[56].
3.2.4. Laminaran
Laminaran is a neutral water-soluble glucan found in brown algae functioning as a reserve food[63][64]. Its presence in crude fucoidan preparations is highly possible, owing to its precipitation with fucoidan after the addition of high volumes of ethanol (e.g., 70% v/v). Enzyme-assisted fucoidan extraction conducted using commercial enzyme mixtures, i.e., carbohydrase mixtures, can target the degradation of laminarin, leading to its removal[81]. Fortunately, laminarin cannot interact with cationic dyes during the determination of fucoidan content using TB and perylene diimide derivative (PDD) assays. The same principle is applied in the purification of fucoidan using anion exchange chromatography (e.g., DEAE–cellulose) in the presence of laminaran[65]. Therefore, laminaran is easily separated from fucoidan after the first step of purification.
References
- Georg Kopplin; Anne Mari Rokstad; Hugo Mélida; Vincent Bulone; Gudmund Skjåk-Bræk; Finn Lillelund Aachmann; Structural Characterization of Fucoidan fromLaminaria hyperborea: Assessment of Coagulation and Inflammatory Properties and Their Structure–Function Relationship. ACS Applied Bio Materials 2018, 1, 1880-1892, 10.1021/acsabm.8b00436.
- Hang Thi Thuy Cao; Maria Dalgaard Mikkelsen; Mateusz Jakub Lezyk; Ly M. Bui; Van T. T. Tran; A.S. Silchenko; M.I. Kusaykin; Pham Duc Thinh; Bang H. Truong; Jesper Holck; et al.Anne S. Meyer Novel Enzyme Actions for Sulphated Galactofucan Depolymerisation and a New Engineering Strategy for Molecular Stabilisation of Fucoidan Degrading Enzymes. Marine Drugs 2018, 16, 422, 10.3390/md16110422.
- Estelle Deniaud-Bouët; Kevin Hardouin; Philippe Potin; Bernard Kloareg; Cécile Hervé; A review about brown algal cell walls and fucose-containing sulfated polysaccharides: Cell wall context, biomedical properties and key research challenges. Carbohydrate Polymers 2017, 175, 395-408, 10.1016/j.carbpol.2017.07.082.
- Wang, Y.; Xing, M.; Cao, Q.; Ji, A.; Liang, H.; Song, S. Biological activities of fucoidan and the factors mediating its therapeutic effects: A review of recent studies. Mar. Drugs 2019, 17, 183.
- Michel, G.; Tonon, T.; Scornet, D.; Cock, J.M.; Kloareg, B. The cell wall polysaccharide metabolism of the brown alga Ectocarpus siliculosus. Insights into the evolution of extracellular matrix polysaccharides in eukaryotes. New Phytol. 2010, 188, 82–97.
- Ahmed Zayed; Roland Ulber; Fucoidan production: Approval key challenges and opportunities. Carbohydrate Polymers 2019, 211, 289-297, 10.1016/j.carbpol.2019.01.105.
- Andrea Désirée Holtkamp; Svenja Kelly; Roland Ulber; Siegmund Lang; Fucoidans and fucoidanases—focus on techniques for molecular structure elucidation and modification of marine polysaccharides. Applied Microbiology and Biotechnology 2009, 82, 1-11, 10.1007/s00253-008-1790-x.
- Niloofar Jokar Borazjani; Mehdi Tabarsa; SangGuan You; Masoud Rezaei; Improved immunomodulatory and antioxidant properties of unrefined fucoidans from Sargassum angustifolium by hydrolysis. Journal of Food Science and Technology 2017, 54, 4016-4025, 10.1007/s13197-017-2867-2.
- Silchenko, A.S.; Rasin, A.B.; Kusaykin, M.I.; Kalinovsky, A.I.; Miansong, Z.; Changheng, L.; Malyarenko, O.S.; Zueva, A.O.; Zvyagintseva, T.N.; Ermakova, S.P. Structure, enzymatic transformation, anticancer activity of fucoidan and sulphated fucooligosaccharides from Sargassum horneri. Carbohydr. Polym. 2017, 175, 654–660.
- Fitton, J.H.; Stringer, D.N.; Karpiniec, S.S. Therapies from fucoidan: An update. Mar. Drugs 2015, 13, 5920–5946.
- Roza V. Menshova; Natalia M. Shevchenko; Tatiana I. Imbs; Tatiana N. Zvyagintseva; Olesya S. Malyarenko; Tatyana S. Zaporoshets; Natalia N. Besednova; S.P. Ermakova; Fucoidans from Brown Alga Fucus evanescens: Structure and Biological Activity. Frontiers in Marine Science 2016, 3, 129, 10.3389/fmars.2016.00129.
- Mourão, P.A.; Pereira, M.S. Searching for alternatives to heparin: Sulfated fucans from marine invertebrates. Trends Cardiovasc. Med. 1999, 9, 225–232.
- Vilela-Silva, A.C.; Castro, M.O.; Valente, A.P.; Biermann, C.H.; Mourao, P.A. Sulfated fucans from the egg jellies of the closely related sea urchins Strongylocentrotus droebachiensis and Strongylocentrotus pallidus ensure species-specific fertilization. J. Biol. Chem. 2002, 277, 379–387.
- Vilela-Silva, A.C.; Alves, A.P.; Valente, A.P.; Vacquier, V.D.; Mourão, P.A. Structure of the sulfated alpha-L-fucan from the egg jelly coat of the sea urchin Strongylocentrotus franciscanus: Patterns of preferential 2-O- and 4-O-sulfation determine sperm cell recognition. Glycobiology 1999, 9, 927–933.
- Thomas Hahn; Siegmund Lang; Roland Ulber; Kai Muffler; Novel procedures for the extraction of fucoidan from brown algae. Process Biochemistry 2012, 47, 1691-1698, 10.1016/j.procbio.2012.06.016.
- Bo Li; Fei Lu; Xinjun Wei; Ruixiang Zhao; Fucoidan: Structure and Bioactivity. Molecules 2008, 13, 1671-1695, 10.3390/molecules13081671.
- Jiao, G.; Yu, G.; Zhang, J.; Ewart, H.S. Chemical Structures and Bioactivities of Sulfated Polysaccharides from Marine Algae. Mar. Drugs 2011, 9, 196–223.
- Patankar, M.S.; Oehninger, S.; Barnett, T.; Williams, R.L.; Clark, G.F. A revised structure for fucoidan may explain some of its biological activities. J. Biol. Chem. 1993, 268, 21770–21776.
- Bilan, M.I.; Grachev, A.A.; Shashkov, A.S.; Thuy, T.T.T.; Van, T.T.T.; Ly, B.M.; Nifantiev, N.E.; Usov, A.I. Preliminary investigation of a highly sulfated galactofucan fraction isolated from the brown alga Sargassum polycystum. Carbohydr. Res. 2013, 377, 48–57.
- H. R. Fletcher; P. Biller; Andrew B. Ross; Jessica Adams; The seasonal variation of fucoidan within three species of brown macroalgae. Algal Research 2017, 22, 79-86, 10.1016/j.algal.2016.10.015.
- Albana Cumashi; Natalia A. Ushakova; Marina E. Preobrazhenskaya; Armida D'incecco; Antonio Piccoli; Licia Totani; Nicola Tinari; Galina E. Morozevich; Albert E. Berman; Maria I. Bilan; et al.Anatolii I. UsovNadezhda E. UstyuzhaninaAlexey A. GrachevCraig J. SandersonMaeve KellyGabriel A. RabinovichStefano IacobelliNikolay E. Nifantiev A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology 2007, 17, 541-552, 10.1093/glycob/cwm014.
- Marcel T. Ale; Jørn Dalgaard Mikkelsen; Anne S. Meyer; Important Determinants for Fucoidan Bioactivity: A Critical Review of Structure-Function Relations and Extraction Methods for Fucose-Containing Sulfated Polysaccharides from Brown Seaweeds. Marine Drugs 2011, 9, 2106-2130, 10.3390/md9102106.
- Cumashi, A.; Ushakova, N.A.; Preobrazhenskaya, M.E.; D’Incecco, A.; Piccoli, A.; Totani, L.; Tinari, N.; Morozevich, G.E.; Berman, A.E.; Bilan, M.I.; et al. A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology 2007, 17, 541–552.
- Duarte, M.E.; Cardoso, M.A.; Noseda, M.D.; Cerezo, A.S. Structural studies on fucoidans from the brown seaweed Sargassum stenophyllum. Carbohydr. Res. 2001, 333, 281–293.
- Marcel Tutor Ale; Anne S. Meyer; Fucoidans from brown seaweeds: an update on structures, extraction techniques and use of enzymes as tools for structural elucidation. RSC Advances 2013, 3, 8131-8141, 10.1039/c3ra23373a.
- Roza V. Usoltseva; Natalia M. Shevchenko; Olesya S. Malyarenko; Stanislav Anastyuk; Anna E. Kasprik; Nikolay V. Zvyagintsev; Svetlana P. Ermakova; Fucoidans from brown algae Laminaria longipes and Saccharina cichorioides: Structural characteristics, anticancer and radiosensitizing activity in vitro. Carbohydrate Polymers 2019, 221, 157-165, 10.1016/j.carbpol.2019.05.079.
- Jing Wang; Quanbin Zhang; Zhongshan Zhang; Hong Zhang; Xizhen Niu; Structural studies on a novel fucogalactan sulfate extracted from the brown seaweed Laminaria japonica. International Journal of Biological Macromolecules 2010, 47, 126-131, 10.1016/j.ijbiomac.2010.05.010.
- Zhu, Z.; Zhu, B.; Ai, C.; Lu, J.; Wu, S.L.; Liu, Y.; Wang, L.; Yang, J.; Song, S.; Liu, X. Development and application of a HPLC-MS/MS method for quantitation of fucosylated chondroitin sulfate and fucoidan in sea cucumbers. Carbohydr. Res. 2018, 466, 11–17.
- Yu, L.; Xue, C.; Chang, Y.; Xu, X.; Ge, L.; Liu, G.; Wang, Y. Structure elucidation of fucoidan composed of a novel tetrafucose repeating unit from sea cucumber Thelenota ananas. Food Chem. 2014, 146, 113–119
- Michel Y Dubois; K A Gilles; J. K. Hamilton; P. A. Rebers; Fang Gao Smith; A Colorimetric Method for the Determination of Sugars. Nature 2006, 168, 167-167, 10.1038/168167a0.
- Smogyi, M. Notes on sugar determination. J. Biol. Chem. 1952, 195, 19–23.
- Shao, Y.; Lin, A.H. Improvement in the quantification of reducing sugars by miniaturizing the Somogyi-Nelson assay using a microtiter plate. Food Chem. 2018, 240, 898–903.
- Z Dische; L B Shettles; A specific color reaction of methylpentoses and a spectrophotometric micromethod for their determination.. Journal of Biological Chemistry 1948, 175, 595–603.
- V. Djurdjić; Ljuba Mandic; A spectrophotometric method for simultaneous determination of protein-bound hexoses and fucose with a mixture of l-cysteine and phenol. Analytical Biochemistry 1990, 188, 222-227, 10.1016/0003-2697(90)90556-o.
- Hee Joon Yoo; Dong-Ju You; Kwang-Won Lee; Characterization and Immunomodulatory Effects of High Molecular Weight Fucoidan Fraction from the Sporophyll of Undaria pinnatifida in Cyclophosphamide-Induced Immunosuppressed Mice. Marine Drugs 2019, 17, 447, 10.3390/md17080447.
- Thomas Hahn; Mariya Schulz; Ralf Stadtmüller; Ahmed Zayed; Kai Muffler; Siegmund Lang; Roland Ulber; A Cationic Dye for the Specific Determination of Sulfated Polysaccharides. Analytical Letters 2016, 49, 1948-1962, 10.1080/00032719.2015.1126839.
- Warttinger, U.; Giese, C.; Harenberg, J.; Krämer, R. Direct quantification of brown algae-derived fucoidans in human plasma by a fluorescent probe assay. arXiv 2016, arXiv:1608.00108.
- Zayed, A.; Dienemann, C.; Giese, C.; Krämer, R.; Ulber, R. An immobilized perylene diimide derivative for fucoidan purification from a crude brown algae extract. Process Biochem. 2018, 65, 233–238
- Qingcai Jiao; Qian Liu; Simple Spectrophotometric Method for the Estimation of Algal Polysaccharide Concentrations. Journal of Agricultural and Food Chemistry 1999, 47, 996-998, 10.1021/jf980815f.
- Melissa Rappold; Ulrich Warttinger; Roland Krämer; A Fluorescent Probe for Glycosaminoglycans Applied to the Detection of Dermatan Sulfate by a Mix-and-Read Assay. Molecules 2017, 22, 768, 10.3390/molecules22050768.
- Zayed, A. Bioactive Compounds from Marine Sources; TU Kaiserslautern: Kaiserslautern, Germany, 2018.
- Valérie Descamps; Sébastien Colin; Marc Lahaye; Murielle Jam; Christophe Richard; Philippe Potin; Tristan Barbeyron; Jean-Claude Yvin; Bernard Kloareg; Isolation and Culture of a Marine Bacterium Degrading the Sulfated Fucans from Marine Brown Algae. Marine Biotechnology 2005, 8, 27-39, 10.1007/s10126-005-5107-0.
- Dorota Ziółkowska; Agnieszka Kaniewska; Jan Lamkiewicz; Alexander Shyichuk; Determination of carrageenan by means of photometric titration with Methylene Blue and Toluidine Blue dyes. Carbohydrate Polymers 2017, 165, 1-6, 10.1016/j.carbpol.2017.02.029.
- Wilfred Mak; Sheng Kelvin Wang; Tingting Liu; Nazimah Hamid; Yan Li; Jun Lu; William Lindsey White; Anti-Proliferation Potential and Content of Fucoidan Extracted from Sporophyll of New Zealand Undaria pinnatifida. Frontiers in Nutrition 2014, 1, 9, 10.3389/fnut.2014.00009.
- Ji Min Kim; Loc Nguyen; Mary Frances Barr; Michael Morabito; Damien Stringer; J. Helen Fitton; Kelly A. Mowery; Quantitative determination of fucoidan using polyion-sensitive membrane electrodes. Analytica Chimica Acta 2015, 877, 1-8, 10.1016/j.aca.2015.04.020.
- Dodgson, K.S.; Price, R.G. A note on the determination of the ester sulphate content of sulphated polysaccharides. Biochem. J. 1962, 84, 106–110.
- Dodgson, K.S. Determination of inorganic sulphate in studies on the enzymic and non-enzymic hydrolysis of carbohydrate and other sulphate esters. Biochem. J. 1961, 78, 312–319.
- Ruth Medeiros Oliveira; Rafael Barros Gomes Camara; Jéssyka Fernanda Santiago Monte; Rony Lucas Silva Viana; Karoline Rachel Teodosio Melo; Moacir Fernandes Queiroz; Luciana Guimaraes Alves Filgueira; Lila Missae Oyama; Hugo Alexandre Oliveira Rocha; Commercial Fucoidans from Fucus vesiculosus Can Be Grouped into Antiadipogenic and Adipogenic Agents. Marine Drugs 2018, 16, 193, 10.3390/md16060193.
- Rafael Barros Gomes Camara; Leandro Silva Costa; Gabriel Pereira Fidelis; Leonardo Thiago Duarte Barreto Nobre; Nednaldo Dantas-Santos; Sara Lima Cordeiro; Mariana Santana Santos Pereira Costa; Luciana Guimaraes Alves Filgueira; Hugo Alexandre Oliveira Rocha; Heterofucans from the Brown Seaweed Canistrocarpus cervicornis with Anticoagulant and Antioxidant Activities. Marine Drugs 2011, 9, 124-138, 10.3390/md9010124.
- Ho Duc Cuong; Thanh Thi Thu Thuy; Tran Thu Huong; Bui Minh Ly; Tran Thi Thanh Van; Structure and hypolipidaemic activity of fucoidan extracted from brown seaweedSargassum henslowianum. Natural Product Research 2014, 29, 411-415, 10.1080/14786419.2014.948436.
- Ace G. Galermo; Eshani Nandita; Mariana Barboza; Matthew J. Amicucci; Thai-Thanh Thi Vo; Carlito B. Lebrilla; Liquid Chromatography–Tandem Mass Spectrometry Approach for Determining Glycosidic Linkages. Analytical Chemistry 2018, 90, 13073-13080, 10.1021/acs.analchem.8b04124.
- Tullia M.C.C. Filisetti-Cozzi; Nicholas C. Carpita; Measurement of uronic acids without interference from neutral sugars. Analytical Biochemistry 1991, 197, 157-162, 10.1016/0003-2697(91)90372-z.
- Nelly Blumenkrantz; Gustav Asboe-Hansen; New method for quantitative determination of uronic acids. Analytical Biochemistry 1973, 54, 484-489, 10.1016/0003-2697(73)90377-1.
- T. Bitter; H.M. Muir; A modified uronic acid carbazole reaction. Analytical Biochemistry 1962, 4, 330-334, 10.1016/0003-2697(62)90095-7.
- Corradini, C.; Cavazza, A.; Bignardi, C. High-performance anion-exchange chromatography coupled with pulsed electrochemical detection as a powerful tool to evaluate carbohydrates of food interest: Principles and applications. Int. J. Carbohydr. Chem. 2012, 2012, 487564.
- Zhang, Z.; Khan, N.M.; Nunez, K.M.; Chess, E.K.; Szabo, C.M. Complete monosaccharide analysis by high-performance anion-exchange chromatography with pulsed amperometric detection. Anal. Chem. 2012, 84, 4104–4110.
- Balboa, E.M.; Rivas, S.; Moure, A.; Dominguez, H.; Parajo, J.C. Simultaneous extraction and depolymerization of fucoidan from Sargassum muticum in aqueous media. Mar. Drugs 2013, 11, 4612–4627.
- Flórez-Fernández, N.; Balboa, E.M.; Domínguez, H. Extraction and purification of fucoidan from marine sources. In Encyclopedia of Marine Biotechnology; Kim, S.K., Ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2020; pp. 1093–1125.
- Cristina De Castro Spadari; Luciana B. Lopes; Kelly Ishida; Potential Use of Alginate-Based Carriers As Antifungal Delivery System. Frontiers in Microbiology 2017, 8, 97, 10.3389/fmicb.2017.00097.
- Bouissil, S.; El Alaoui-Talibi, Z.; Pierre, G.; Michaud, P.; El Modafar, C.; Delattre, C. Use of alginate extracted from Moroccan brown algae to stimulate natural defense in date palm roots. Molecules 2020, 25, 720.
- Belattmania, Z.; Kaidi, S.; El Atouani, S.; Katif, C.; Bentiss, F.; Jama, C.; Reani, A.; Sabour, B.; Vasconcelos, V. Isolation and FTIR-ATR and 1H NMR characterization of alginates from the main alginophyte species of the Atlantic Coast of Morocco. Molecules 2020, 25, 4335.
- Estelle Deniaud-Bouët; Nelly Kervarec; Gurvan Michel; Thierry Tonon; Bernard Kloareg; Cécile Hervé; Chemical and enzymatic fractionation of cell walls from Fucales: insights into the structure of the extracellular matrix of brown algae. Annals of Botany 2014, 114, 1203-1216, 10.1093/aob/mcu096.
- Malyarenko, O.S.; Usoltseva, R.V.; Zvyagintseva, T.N.; Ermakova, S.P. Laminaran from brown alga Dictyota dichotoma and its sulfated derivative as radioprotectors and radiosensitizers in melanoma therapy. Carbohydr. Polym. 2019, 206, 539–547.
- Takei, M.N.; Kuda, T.; Taniguchi, M.; Nakamura, S.; Hajime, T.; Kimura, B. Detection and isolation of low molecular weight alginate- and laminaran-susceptible gut indigenous bacteria from ICR mice. Carbohydr. Polym. 2020, 238, 116205.
- Ahmed Zayed; Roland Ulber; Fucoidans: Downstream Processes and Recent Applications. Marine Drugs 2020, 18, 170, 10.3390/md18030170.
- O H Lowry; N J Rosebrough; A L Farr; R J Randall; Protein measurement with the Folin phenol reagent.. Journal of Biological Chemistry 1951, 193, 265–275.
- Wu Qianqian; Ma Shuang; Xiao Hourong; Zhang Min; Jingmin Cai; Purification and the Secondary Structure of Fucoidanase fromFusariumsp. LD8. Evidence-Based Complementary and Alternative Medicine 2011, 2011, 1-8, 10.1155/2011/196190.
- Tzong-Shi Lu; Szu-Yu Yiao; Kenneth Lim; Roderick V. Jensen; Li-Li Hsiao; Interpretation of biological and mechanical variations between the Lowry versus Bradford method for protein quantification. North American Journal of Medical Sciences 2010, 2, 325-328, 10.4297/najms.2010.2325.
- Brady, P.N.; Macnaughtan, M.A. Evaluation of colorimetric assays for analyzing reductively methylated proteins: Biases and mechanistic insights. Anal. Biochem. 2015, 491, 43–51.
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254.
- Setsuro Matsushita; Nobuko Iwami; Yuki Nitta; Colorimetric estimation of amino acids and peptides with the Folin phenol reagent. Analytical Biochemistry 1966, 16, 365-371, 10.1016/0003-2697(66)90168-0.
- Gary L. Peterson; Review of the folin phenol protein quantitation method of lowry, rosebrough, farr and randall. Analytical Biochemistry 1979, 100, 201-220, 10.1016/0003-2697(79)90222-7.
- Sónia A. O. Santos; Rafael Félix; Adriana C.S. Pais; Sílvia M. Rocha; Armando J. D. Silvestre; The Quest for Phenolic Compounds from Macroalgae: A Review of Extraction and Identification Methodologies. Biomolecules 2019, 9, 847, 10.3390/biom9120847.
- Rohwer, K.; Neupane, S.; Bittkau, K.S.; Galarza Pérez, M.; Dörschmann, P.; Roider, J.; Alban, S.; Klettner, A. Effects of crude Fucus distichus subspecies evanescens fucoidan extract on retinal pigment epithelium cells-implications for use in age-related macular degeneration. Mar. Drugs 2019, 17, 538.
- Chale-Dzul, J.; Moo-Puc, R.; Robledo, D.; Freile-Pelegrín, Y. Hepatoprotective effect of the fucoidan from the brown seaweed Turbinaria tricostata. J. Appl. Phycol. 2015, 27, 2123–2135.
- Ricardo M. Ferreira; Ana Ramalho Ribeiro; C. Patinha; Artur M.S. Silva; Susana M. Cardoso; Rui Costa; Water Extraction Kinetics of Bioactive Compounds of Fucus vesiculosus.. Molecules 2019, 24, 3408, 10.3390/molecules24183408.
- Jueun Lee; Youngae Jung; Jeoung-Hwa Shin; Ho Kyoung Kim; Byeong Cheol Moon; Do Hyun Ryu; Geum-Sook Hwang; Secondary Metabolite Profiling of Curcuma Species Grown at Different Locations Using GC/TOF and UPLC/Q-TOF MS. Molecules 2014, 19, 9535-9551, 10.3390/molecules19079535.
- Thomas Hahn; Ahmed Zayed; Mariya Kovacheva; Ralf Stadtmüller; Siegmund Lang; Kai Muffler; Roland Ulber; Dye affinity chromatography for fast and simple purification of fucoidan from marine brown algae. Engineering in Life Sciences 2015, 16, 78-87, 10.1002/elsc.201500044.
- Marta Szekalska; Katarzyna Sosnowska; Anna Czajkowska-Kośnik; Katarzyna Winnicka; Calcium Chloride Modified Alginate Microparticles Formulated by the Spray Drying Process: A Strategy to Prolong the Release of Freely Soluble Drugs. Materials 2018, 11, 1522, 10.3390/ma11091522.
- Ahmed Zayed; Kai Muffler; Thomas Hahn; Steffen Rupp; Doris Finkelmeier; Anke Burger-Kentischer; Roland Ulber; Physicochemical and Biological Characterization of Fucoidan from Fucus vesiculosus Purified by Dye Affinity Chromatography. Marine Drugs 2016, 14, 79, 10.3390/md14040079.
- Nguyen, T.T.; Mikkelsen, M.D.; Tran, V.H.N.; Trang, V.T.D.; Rhein-Knudsen, N.; Holck, J.; Rasin, A.B.; Cao, H.T.T.; Van, T.T.T.; Meyer, A.S. Enzyme-assisted fucoidan extraction from brown macroalgae Fucus distichus subsp. evanescens and Saccharina latissima. Mar. Drugs 2020, 18, 296.
- Dörschmann, P.; Mikkelsen, M.D.; Thi, T.N.; Roider, J.; Meyer, A.S.; Klettner, A. Effects of a newly developed enzyme-assisted extraction method on the biological activities of fucoidans in ocular cells. Mar. Drugs 2020, 18, 282.
- Marcelo D. Catarino; Artur M.S. Silva; Susana M. Cardoso; Phycochemical Constituents and Biological Activities of Fucus spp.. Marine Drugs 2018, 16, 249, 10.3390/md16080249.
- Jean-Pierre Hallé; Danielle Landry; Alain Fournier; Michèle Beaudry; François A. Leblond; Method for the Quantification of Alginate in Microcapsules. Cell Transplantation 1993, 2, 429-436, 10.1177/096368979300200511.




