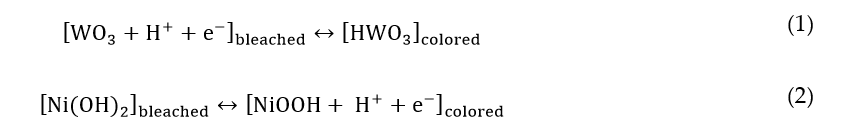| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Alessandro Cannavale | + 4660 word(s) | 4660 | 2020-12-14 11:43:51 | | | |
| 2 | Peter Tang | -96 word(s) | 4564 | 2020-12-19 11:41:42 | | |
Video Upload Options
Chromogenic materials and devices include a wide range of technologies that are capable of changing their spectral properties according to specific external stimuli. Several studies have shown that chromogenics can be conveniently used in building façades in order to reduce energy consumption, with other significant effects. First of all, chromogenics influence the annual energy balance of a building, achieving significant reductions in consumption for HVAC and artificial lighting. In addition, these technologies potentially improve the indoor level of visual comfort, reducing the risks of glare and excessive lighting.
1. Introduction
In recent years, awareness of global warming has grown a lot and this has pushed research groups all over the world to find new solutions to reduce the anthropogenic fingerprint on ecosystems [1]. A plethora of studies has highlighted the significant impact that the construction sector has on the global consumption of primary energy and, at the same time, on carbon dioxide and greenhouse gas emissions. It is possible to estimate that in the most developed countries, about 40% of primary energy is used for commercial and tertiary residential buildings [2]. In particular, in the last decades, the vast and multidisciplinary field of nanotechnologies has made available a large number of new materials with surprising properties that allow us to design systems, devices, and components that are capable of reducing impacts and manufacturing costs and of increasing performance as well. An extensive family of materials and devices of this kind is represented by so-called smart materials, i.e., materials with adaptive properties that change according to the application of an external stimulus [3]. Among smart materials, the so-called chromogenic materials are still under investigation: among them, electrochromic (EC), photochromic (PC), and thermochromic (TC) materials are of particular importance for use in buildings [4]. The vast class of chromogenics includes materials not considered in this work, such as gasochromics or liquid crystals [4][5][6][7]. In all cases, these materials undergo an alteration of their spectral properties in the visible and/or infrared wavelengths due to the application of a specific external stimulus. Consequently, chromogenic materials owe their name to the physical stimulus that triggers the activation of their mechanism. While in the case of EC materials and devices [8], the stimulus is represented by a voltage applied by means of an external circuit, PC [9] materials change color according to irradiation with precise wavelengths; on the other hand, TC materials [10] change their optical behavior due to the achievement of a precise critical temperature (TC). As mentioned above, EC materials change color depending on the application of an external bias to the device in which they are embedded. They have always been thought of as optimal for integration into buildings within the so-called "smart windows" [11]. In fact, windows are still considered a thermodynamically weak point of a building façade. Nowadays, increasing attention is paid to the design of materials capable of controlling and optimizing the energy flux, mainly through glazing. Windows allow the penetration of a wide range of thermal radiation, including visible and infrared wavelengths, with several thermal transfer mechanisms (conduction, convection, and irradiance) between the glass, surrounding gases, and sky. If, on the one hand, free heat gains are welcomed in winter (for example, reducing heating loads), in the summer season, they turn into undesired cooling loads [12]. For this reason, chromogenic glazing, with adaptive or adjustable spectral properties, can be a very useful tool to reduce energy consumption in several fields: automotive, transportation, and construction. EC materials, differently from PCs and TCs, work only if inserted within the architecture of a more complex device, embodying one or two transparent and conductive substrates, an electrolyte, and one or two EC materials with complementary behavior. EC materials can be organic [13] or inorganic; there is abundant scientific literature that investigates the properties of these materials under different profiles. Several companies, in recent years, have brought some types of EC devices to the market [13]. On the other hand, TC and PC materials do not allow, differently from EC ones, any type of control of their feature by users since the presence of the external light (or thermal stimulus, respectively) activates the chromatic modulation, at least until the stimulus itself is applied. It seems clear that these differences could be fundamental in finding the potential applications for these materials inside buildings or in other contexts.
2. Electrochromic Materials and Devices
The seminal studies by S. Deb [5][6] and C.G. Granqvist [7] have catalyzed the interest of numerous research groups worldwide towards the achievement of the dynamic behavior of glazing. An EC glass can be viewed as a battery embodying several materials in the form of thin films. The charge inserted can be related to the degree of transparency of an EC device. Typically, an EC device (Figure 1) includes two transparent conductive substrates; an EC film is deposited on top of each of them, with complementary behavior; the two substrates are separated by an electrolyte (liquid, gel, or solid). The electrolyte conducts ions, also acting as an insulator for electrons. It contains small cations (H+, Li+, or Na+, in general [14]) that can be shuttled through the electrolyte by applying an external bias.
Figure 1. Web-coated electrochromic (EC) devices in the form of large flexible sheets, as a foil for glass lamination. Reproduced from [15], Elsevier: 2018.
Coloration takes place as a consequence of redox reactions involving the EC films and the cations moving across the electrolyte. The most widely investigated EC materials are inorganic transition metal oxides, such as tungsten oxide (WO3), molybdenum oxide (MoO3), titanium dioxide (TiO2), and nickel oxide (NiO). EC material color, due to simultaneous cation and electron intercalation, is named "cathodic", whereas color due to cation extraction is called "anodic". The most investigated cathodic and anodic inorganic EC materials are WO3 and NiO, respectively [16][17]. Their complementary behavior allows for the simultaneous coloration/bleaching of both the materials being deposited on the two opposite substrates, oppositely charged during the processes. EC materials show "double conduction" of ions and electrons; for this reason, they have been defined as "mixed conductors". When cathodic EC materials are charged with electrons, due to the external bias (in general, very low voltages, 1–5 V dc) applied to the device, they tend to attract small cations inside the complex network of channels within their structure. Following this intercalation, EC materials undergo a reversible modification of absorption. Simplified forms of the complementary redox equations are as follows [18]:
In some cases, the anodic EC film can be replaced by a material providing ion storage to allow safe and full bleaching processes. The state of aggregation of an electrolyte influences the performance and durability of an EC device [19][20]. Liquid electrolytes containing volatile solvents may undergo evaporation and leakage, affecting the durability of devices [21]. They can be solid, as in the case of tantalum oxide (Ta2O5) [22][23], liquid [24][25], or gel [26][27]. Electrolytes based on polyethylene oxide (PEO) [28] or polymethyl methacrylate (PMMA) raise their ion conductivity by adding specific amounts of salts, such as lithium perchlorate LiClO4, LiTF, lithium iodide (LiI), or lithium sulfide (Li2SO4). Transparent conductive oxides [29][30][31] typically used are indium tin oxide (ITO) and fluorine-doped tin oxide (FTO). There are recent studies reporting the adoption of graphene and metal meshes, used effectively as conductive materials. Typically, the substrates used to make EC devices are made of glass. However, there are several studies using flexible substrates such as polyethylene terephthalate (PET). In this case, the weight of EC devices can be minimized. PET-based EC devices have also been used for lamination. EC devices may also be deposited on polyethylene terephthalate (polyester, PET), as reported in recent work by Granqvist et al. [15]. In this case, a gel polymer electrolyte was solidified by crosslinking; EC devices could be laminated in an insulated glass unit (IGU) using polyvinyl butyral (PVB) or ethylene vinyl acetate (EVA). Additionally, polyethylene naphthalate has been adopted for flexible EC devices [32]. The most widely studied EC materials are transition metal oxides and organic materials; more recently, ITO nanoparticles have shown relevant plasmon absorption within the near-infrared wavelength range, as demonstrated by Llordes et al. [33][34], who reported the independent tuning of infrared and visible light in a "dual-band" device. Organic EC molecules also undergo reversible coloration (and bleaching) upon specific redox processes. The most widespread organic EC materials are bipyrilidilium systems, conducting polymers, quinones, cyanobiphenyls, and phthalocyanines [4]. Jensen et al. [35] reported a relevant demonstration of flexible solid-state EC devices, fabricated using continuous roll-to-roll processing based on photo-crosslinking of an acrylate-based electrolyte. Dyer et al. [33] demonstrated a solar-powered EC device that includes PProDOT-(CH2OEtHx)2 and PProDOP-N-C18H37. The device includes PV cells that produce net positive energy, which is only partially used to modulate the thermal energy flux of windows.
Piccolo et al. adopted a lithium-conducting polymer made of PEO-PEGMA:Li to fabricate a large-scale EC window (12 by 12 cm) embodying two complementary inorganic EC materials: WO3 (cathodic) and NiOH:Li (anodic/ion storage). The device reported switching times of 5–6 min, with (visible transmittance) Tvis varying from 70% to 30%. Sibilio et al. [36] reviewed commercial EC glazing and made an examination of their average acceptable properties: minimum visible transmittance values in the clear state of 0.4–0.5; minimum solar heat gain coefficient from 0.29 to 0.32; Tvis and SHGC of the colored state ranging from 0.09 to 0.1 and from 0.1 to 0.13, respectively. Switching times may range from 7 to 20 min.
3. Thermochromic Materials
TC materials undergo reversible phase transition when a Tc is reached, with relevant changes in physical properties (transmittance and resistivity, for instance). TC behavior has been reported in organic compounds, ionic liquids, and composite materials [18][37]. Inorganic materials have reported higher stability even at temperatures higher than 200 °C. For example, Liu et al. have reported metal-doped Ca14Zn6Ga10O35-based TC materials, achieving reversible thermochromism in a wide temperature range (25–460 °C). The most widely investigated TC material is, by far, vanadium dioxide (VO2). If it reaches its Tc (68 °C), a strong modulation of near-infrared transmittance takes place. VO2 can be obtained by several methods: physical vapor deposition [37][38][39] has been the most studied method, though nanostructured VO2 has also been studied recently [40]. On the contrary, the modulation of visible transmittance (Tvis) is quite moderate (less than 10%). Cao et al. [41] have reviewed the open challenges and possible strategies for improving the properties of VO2. The open issues regarding VO2 were also summarized in work by Li et al. [42]: limited solar modulation (10%); luminous transmittance (Tlum) should not be higher than ~40%; ΔTsol should take place not so far from ambient temperature, but the Tc of VO2 is as high as 68 °C (Figure 2). TC materials indeed represent a chance to control solar radiation and thermal radiation passing through transparent façades, for various applications, according to the temperature variations.
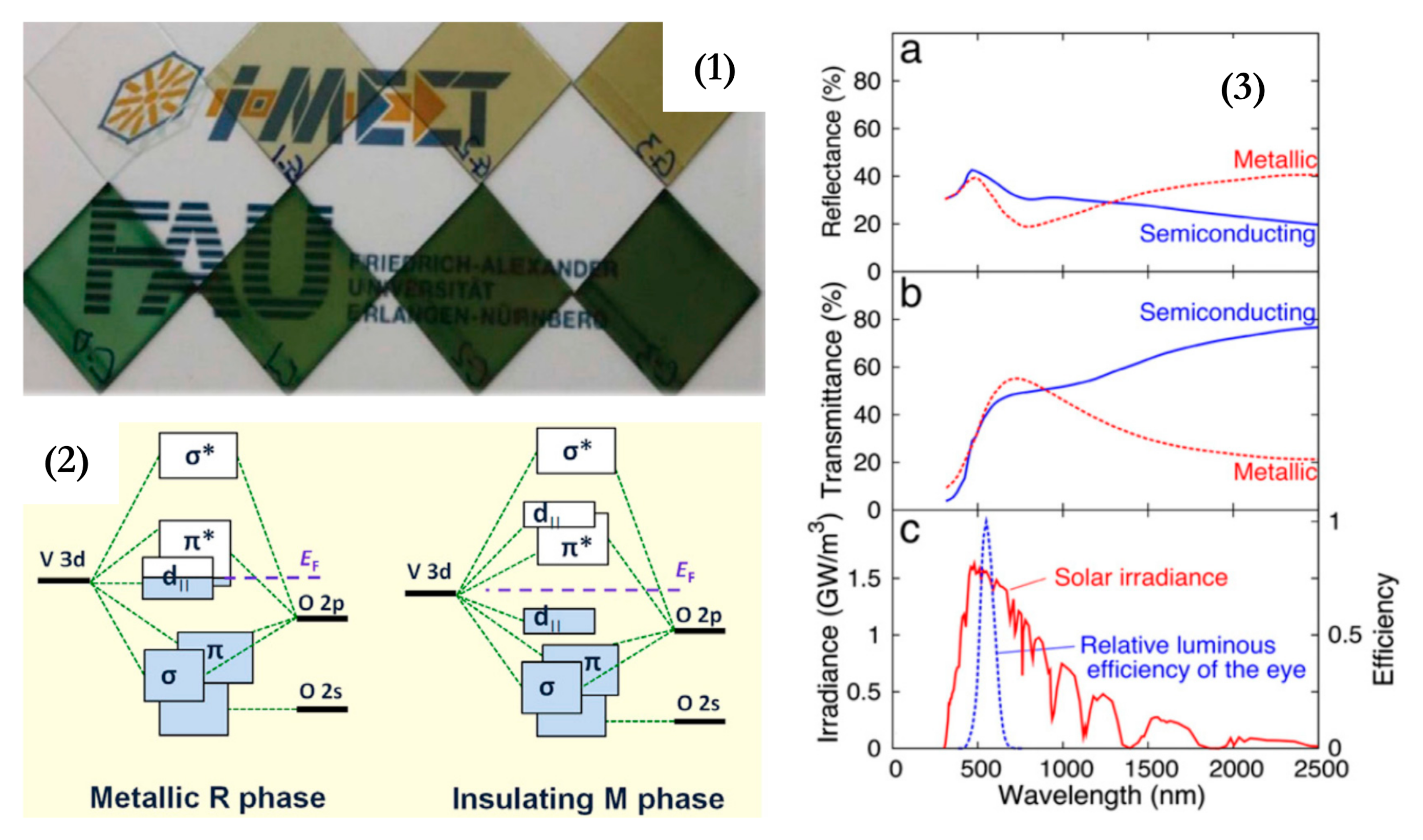
Figure 2. (1) Pictures of VO2-based thermochromic (TC) samples, (2) Band structures of the metallic rutile and insulating monoclinic phases of vanadium dioxide (VO2). Reproduced from [43], Elsevier: 2018. (3) Spectral reflectance (a) and transmittance (b) for a 50 nm VO2 film. Panel (c) illustrates spectra for the luminous efficiency of the human eye and solar irradiance. Reproduced from [42], Elsevier: 2012.
VO2 shows a monoclinic structure (insulator) in its transparent state, which turns rutile (metal) upon reaching its Tc (Figure 2, left side). In its basic form, the Tc is too high for building fenestrations. For this reason, several methods have been adopted to decrease it (40 °C is considered an acceptable value). Other issues in the development of TC coatings for industrial applications include the stability of performance in time and increasing the modulation of visible and infrared transmittance. To reduce the Tc, doping with cations larger than vanadium (such as tungsten, molybdenum, and niobium) and anions smaller than O2- (like fluorine) is the most adopted strategy [44][45][46]. In fact, it was observed that the direction of the change in Tc could be related to the relative size of the doping ion compared with vanadium [47] or to the charge-transfer mechanism associated with the introduction of extra electrons into the vanadium d bands [48]. On the other hand, to increase the luminous transmittance of VO2 films, antireflection coatings or structures are adopted. For instance, Kolenaty et al. reported a three-layer structure, with ZrO2 antireflective layers acting as a protection layer and structure template [49]. Several works have used SiO2, TiO2, and ZrO2 antireflective layers [37]. A more sophisticated approach was proposed by Liu et al. [50], who reported the design of nanocone antireflective layers made of TiO2, biomimicking moth-eye morphology and reaching a Tlum of 55.4%, with ΔTsol of 11.3%. Moreover, the stability of VO2 coatings is limited by chemical and physical deterioration issues. Durability is indeed a special figure of merit for TC coatings: at least 10 years of service life is required. Several forms of VO2 exist, but, unfortunately, the most stable is V2O5. Moisture and acidic environment tend to affect the original TC properties of VO2. From the physical point of view, a small volume change (roughly 0.3%) takes place upon each phase-transition process, and this point may result in cracks and deterioration of TC performance [41]. This fact may influence the durability of core-shell structures embodying VO2.
Figure 3 shows spectral transmittance for VO2 films at different temperatures, lying below and above the Tc. Mlyuka et al. [51] used computations to optimize multilayer films and demonstrated that TiO2/VO2/TiO2 structures may report higher transmittance than bare VO2 films. VO2 transmittance drops at λ < 600 nm: such a feature is due to inherent band-to-band absorption. This issue can be partially solved by Mg doping, leading to a band-gap widening. Doping strategies of VO2 have led to much lower Tc ≈ 25 °C [42]. Zhan et al. [39] reported the TC performance of VOx-based multilayer films that are effectively controlled by annealing pressure: the Tc was reduced to 54 °C by changing the annealing pressure, without doping. Xu et al. reviewed a recent advance in TC composites for smart windows [52], with special reference to multilayer composite structures that improve the VO2 properties. A synthesis of the three main challenges of TC materials’ performance, which limit their building integration, was offered by Li et al. [42]: VO2 should accomplish high solar transmittance modulation (ΔTsol ≫ 10%), high luminous transmittance modulation (Tlum ≫ 40%), and low critical temperature (Tc ≈ 25 °C).
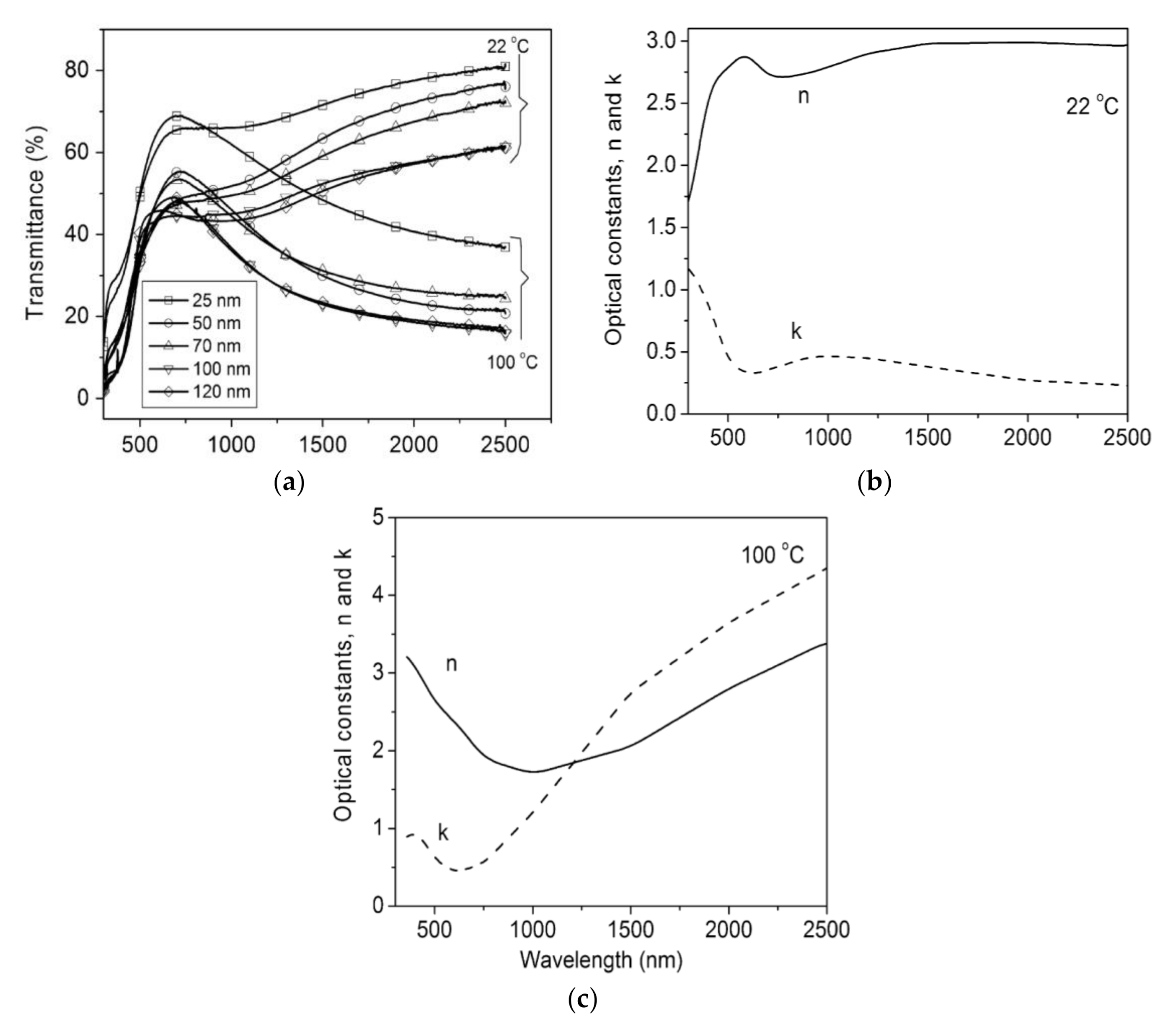
Figure 3. (a) Spectral transmittance for VO2, measured above and below its precise critical temperature (TC); (b) spectral optical constants, n and k, for VO2 films, measured above and below its Tc; (c) Spectral opticalconstants, n and k, for VO2 films, above the Tc. Reproduced from [51], Elsevier: 2009.
4. Photochromic Materials
Photochromic (PC) materials undergo reversible photoinduced switching (Figure 4) between different states (or isomers) that have remarkably different absorption spectra [53]. The word PC derives from the transliteration of the ancient Greek words "φώς" (light) and "χρώμα" (color). To activate the coloring process, PC materials must be exposed to certain wavelengths of light. Just like TC materials, PC devices can be fabricated without any extra energy input for use in and maintenance of windows. In these materials, light acts as a free, energy-efficient, and fast power source, activating the photoswitching. PCs can be exploited not only for optical lenses and smart windows, photonics, and optoelectronics, but also in molecular switches, optical data storage, molecular electronics, and sensors [53][54]. The interconversion between different states is also witnessed by parallel changes in chemical and physical properties upon light irradiation of PC materials (refractive index, polarizability, as well as electric, magnetic, and mechanical properties). As reported by Barachevsky, a current goal in the design of PC nanoparticles is represented by the development of PC nanoparticles or hybrid core-shells of PC compounds based on noble metals, silica, and PC organic compounds [55]. The relevant potential of organic synthesis allows for an unlimited fine-tuning of molecules, according to specific design choices. In organic PC materials, coloration is the result of a chemical bond rearrangement, effecting changes in structural and electronic properties. They can be fabricated by embedding active PC materials into a transparent matrix [56]. Several classes of PC organic molecules exhibit the phenomenon named as photochromism: fulgides, diarylethenes [57], spiropyrans [58], spirooxazines [59], naphthopyrans [60], and azobenzenes [61]. The main figures of merit for these materials, as reported by Ke et al. [62], are fast kinetics, high contrast, and, above all, photochemical stability. Azobenzenes can undergo trans-to-cis isomerization when UV-irradiated and the cis isomer may be bleached back to the trans form by visible light as well as thermal relaxation. They photoisomerize upon UV exposure and show opposite properties compared with other PCs. In fact, azobenzenes are originally opaque and turn transparent when UV-irradiated. When UV-exposed, spiropyrans will isomerize, showing a transition to dark blue—such photo-induced process of coloration is thermally unstable; for this reason, it gives rise to spontaneous bleaching after UV exposure ceases. Spiropyrans are generally used for transitional PC sunglasses.
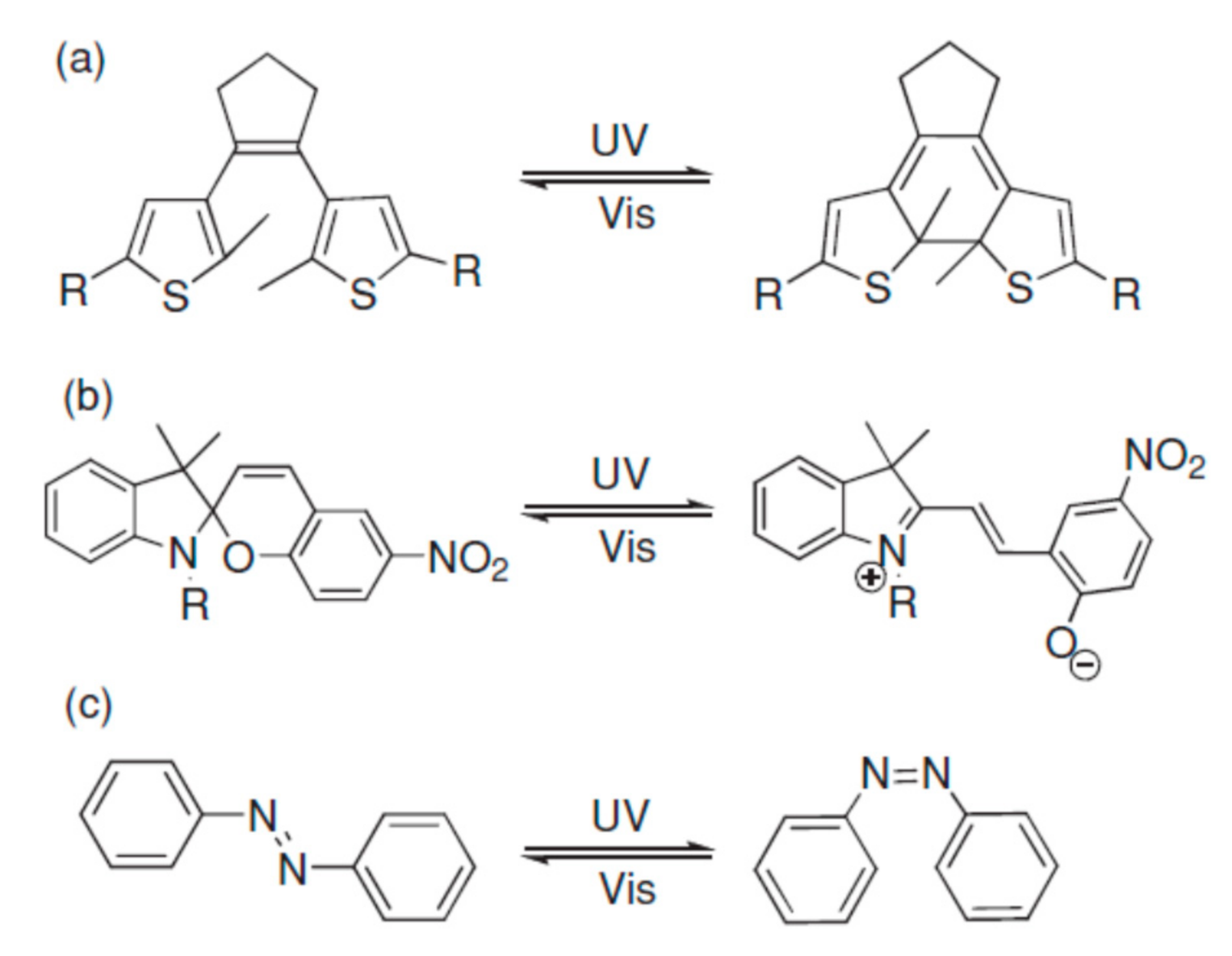
Figure 4. Photochromism of dithienylethene (a), spiropyran (b), and azobenzene (c). Reproduced from [53], WILEY-VCH: 2013.
On the other hand, the coloration of diarylethenes [63] is due to reversible photoinduced valence isomerization between the colorless ring-open state and the colored cyclic form. Four kinds of diarylethenes are reported in the literature, depending on the benzofuran and benzene moieties contained by the molecules. They can turn from colorless to red, purple, or orange. The colored form is thermally stable and cannot be bleached in the dark (simply leave the material at room temperature). On the contrary, the bleaching process—also observed in fulgides [64]—requires exposure under visible light (or elevated temperatures) to recover its original color. As reported by Nakamura et al. [65], the colored ring-closed forms of diarylethenes tend to remain stable for more than 12 h at 80 °C and can be reversed to their original colorless form only by exposing them to visible light.
This is typical behavior reported of the so-called "thermally irreversible" PCs. On the other hand, spiropyrans, spirooxazines, azobenzenes, naphthopyrans, and dipyrrolylethenes show "thermally reversible" PC behavior [66][67], i.e., their bleaching may be activated by visible light or heating. They are used for eyeglasses due to their behavior. Naphthopyrans embedded in a solgel matrix [68] demonstrate a relevant reduction (50%) of the visible transmittance upon coloration. Bleaching back occurs after 30 min from ceasing UV irradiation [68]. Fulgides doped in PMMA showed stable and multilevel photoisomerization due to UV irradiation and consequent absorption at 382 nm [69]. As reported by Wang et al. [70], a recent trend of research in PC materials is represented by the challenge to obtain more color states by designing specific functional groups in order to tune the HOMO–LUMO gap of PC molecules. However, organic PCs require complex syntheses and, in some cases, do not show thermal stability [71]. Some authors have also highlighted the persisting drawbacks: cost of synthesis, limited thermal stability, and toxicity issues [72]. Ortica [73] reviewed a series of PC systems (spirooxazines, chromenes, and diarylethenes), investigating the effect of temperature on their behavior. Temperature may increase the bleaching rate in thermo-reversible molecules and also decrease the coloration due to the light stimulus; on the other hand, for spirooxazines and chromenes, a description was made by considering a combination of the TC and PC data obtained. Another phenomenon under wide investigation in PC materials is represented by the upconversion process: two or more near-infrared photons can convert into visible or ultraviolet light, generating high energy luminescence, absorbing two or more photons of low frequency [72][74]. Kang et al. [75] observed that the range of the UV is quite limited in the solar spectrum (about 5%), and this fact limits the maximum theoretical efficiency of PC windows, which are typically triggered by UV absorption. For this reason, they suggested the use of phosphor materials (i.e., lanthanide metal ions like Pr3+, Tm3+, Eu3+, and Er3+) to upconvert visible or IR wavelengths. They suggested praseodymium-doped yttrium silicate (Y2SiO5:Pr3+, YSO:Pr3+) as a suitable phosphor material that is capable of absorbing nm light with a wavelength of ∼505 and emitting UV light (between 270 and 380 nm). In their work, they proposed a hybrid double-layer film embodying a phosphor devoted to upconversion and a PC layer (Y2SiO5:Pr3+/H3PW12O40). The PC effect and kinetics that resulted increased several times compared to a single layer due to the upconversion effect. Reversible PC modulation has also been reported in inorganic materials and hybrid organic–inorganic films, showing, on the other hand, chemical stability, mechanical strength, and oxidation resistance. Inorganic materials that have also been reported to have PC properties, such as transition metal oxides (WO3, TiO2, MoO3, V2O5, Nb2O5), are considered cost-efficient for large-scale applications; other materials like metal halides and rare-earth complexes are typically used for lenses. Fair photochromism in WO3 takes place due to the electron transfer between tungsten atoms, with different valence states, if the material is in contact with water and irradiated with light. Poor revisability of its PC behavior has compelled researchers to improve its properties, adopting different strategies. TiO2 has also been reported to have PC behavior in the form of gels and nanoparticles. Similar to WO3, an electron–hole pair is created by light irradiation of the material. Titanium ions undergo reduction due to electron insertion, producing colored Ti3+. Zuo [76] reported the PC postannealing properties of RF magnetron-sputtered TiO2 thin films with embedded silver nanoparticles. These films can also be deposited using other techniques (solgel, spin-coating, photoreduction by UV irradiation). As well known, WO3 exhibits PC properties due to W6+ to W5+ reduction upon UV irradiation, activating a charge transfer from oxygen to metal. WO3—with a bandgap ranging from 2.7 to 3.1 eV—can suitably work as a PC component in a cellulose matrix, being deposited by the solvent casting method, reporting full cycles of coloration/bleaching, from pale yellow into dark blue in about 1 min, and gradually recovering its bleached form in the dark within 20 min, lasting about 20 min [77].
Several studies have recently demonstrated the benefits associated with the building integration of chromogenic devices, mainly ECs [16][78][79][80][81]. Sibilio et al. [36] observed that EC devices may allow high energy savings (up to 39–59%, in some cases), but such benefits are indeed influenced by orientation, the control strategy adopted, climatic conditions, and location. As to the building integration of TC materials, it is claimed that they have the ability to reduce both heating and cooling energy demand from 5.0% to 84.7% when compared to clear glass as a reference. Nevertheless, such performance was strongly dependent on film type and location [82]. On the other hand, Giovannini et al. [83] tested large-scale samples of TC layers laminated in glazing and compared them to standard selective glass. They found a strict dependence of the results on latitude and location; in the case investigated, TC glazing required lower energy use than selective glazing, although cooling demand was higher because of secondary heating flows towards the interior of the building, associated with increased TC glazing absorption in its dark state. The number of studies concerning the integration of PC glass in buildings is significantly lower. A recent work by Tällberg et al. [84] reported a comparison among the chromogenic technologies studied in this work. They observed that the performance of PC glazing was affected by the narrower interval between the clearest and darkest state (i.e., 0.40–0.36 in the case investigated in their work). Moreover, TC and PC glass showed low values for both the bleached/colored states compared to EC glass, which had higher modulation of SHGC in the bleached and colored states, respectively (0.46–0.09). Ke et al. [62] also observed that just like TC glass, PCs allow simple fabrication without extra energy input. Moreover, according to their outcomes, they suggested that PC windows should have high contrasts and fast reversible kinetics to be suitable for building integration; moreover, they stated that PC windows would not be desirable in "four-seasoned countries" since they could impede energy flow through the glazing even in winter in the colored state.
Piccolo et al. observed that EC layers have intrinsic low-e properties due to the ITO conductive layer. Moreover, they suggested placing EC films in Surface 2, i.e., the inner side of the outer glass pane of a standard argon/air-filled IGU. Such placement allows the reduction of high rates of secondary heat gain, as well as glass overheating.
Many studies have dealt with the potential benefits deriving from chromogenic systems used in glazing [18]. Some of them were carried out using numerical simulation software; others were based on in-situ measurements of prototypes made on a large scale. In any case, the significant modulation of Tsol and Tvis of glass entails a dramatic opportunity to control the energy flow that passes through the windowed component, according to seasonal requirements, to achieve indoor comfort. This can translate into the possibility of reducing energy consumption in the construction sector and, at the same time, an improvement in the conditions of comfort in offices and homes. Moreover, they may allow for a relevant downsizing of HVAC systems, with further economic and environmental advantages. In this brief review, the most common chromogenic systems (thermochromic, electrochromic, photochromic) have been described, as well as the fabrication techniques typically used to manufacture them, although in a nonexhaustive way. The current research trends have been reported for each class of materials. The interest in chromogenics is very high in the scientific field as they offer significant economic, comfort, and environmental benefits for users. What unites TC and PC glass is the fact that the modulation in these cases is not controllable or customizable by the user. On the contrary, the user can overrun the modulation of EC glass or activate it at different degrees, according to specific needs. On the other hand, the fact that EC glass requires activation by users strongly relates their advantages to the management strategy adopted for such devices. For example, it has been observed that the degree of coloration of EC glazing should ideally depend on the degree of illuminance required on a work surface: such a strategy offers the maximum energy benefit in the face of an optimal condition of visual comfort. Other strategies, such as the one based on glare control, allows the maximization of visual comfort at the expense of energy consumption [5][12][78].
In recent years, beyond the strategies aiming at improving device performance and new optimization processes for materials on the nanoscale, there has been a clear trend to design devices with extended functionalities, coupling several functions; in the case of photoelectrochromic devices [85][86] and photovoltachromic ones [24], this would allow the separation of the control of optical modulation from photovoltaic conversion. A further approach, aiming at a separation of visible and near-infrared modulation, has been adopted in the design of so-called dual-band devices, produced by the research group of the LBNL [33][34]. It allows a wide customization of device features, according to the varying external conditions. DeForest et al. analyzed the energy savings associated with the building integration of such devices in the US, reporting fair output that was strictly related to the climatic zone [87][88][89]. One more suitable strategy would be represented by the combination of more chromogenic technologies in a single device. For instance, Detsi et al. [90] have recently proposed a combination of EC and TC materials, demonstrating an 18.5% and 8.1% reduction in annual primary energy use for Athens and Stockholm, respectively. Multistage coloring devices could also be of particular interest for precise practical applications [42]. The application of the devices, in each case, is finally one of the main points to be carefully evaluated for each technology. For example, a system that does not allow users to control the optical properties (such as TC and PC glazing) could be applied in contexts where the illuminance on a worktop may not be relevant, while the control of incoming radiation and visual comfort are, thus preferring the energetic aspect. For these reasons, the choice of the most suitable chromogenic technology to be used will be made on the basis of specific design needs and constraints, case by case.
References
- IEA and UNEP International Energy Agency and the United Nations Environment Programme. Global Status Report 2018: Towards a Zero-Emission, Efficient and Resilient Buildings and Construction Sector; IEA and UNEP: Paris, France, 2018; p. 325
- Nations Unies. Convention – cadre sur les changements climatiques. In Proceedings of the Cop 21, Paris, France, 30 November–11 December 2015; Volume 21930, p. 39.
- Addington, D.M.; Schodek, D.L. Smart Materials and Technologies in Architecture: For the Architecture and Design Professions; Harvard University Press: Cambridge, MA, USA, 2012.
- Lampert, C.M. Chromogenic smart materials. Mater. Today 2004, 7, 28–35.
- Casini, M. Active dynamic windows for buildings: A review. Renew. Energy 2018, 119, 923–934.
- Lampert, C.M. Optical switching technology for glazings. Thin Solid Films 1993, 236, 6–13.
- Cupelli, D.; Nicoletta, F.P.; Manfredi, S.; Vivacqua, M.; Formoso, P.; De Filpo, G.; Chidichimo, G. Self-adjusting smart windows based on polymer-dispersed liquid crystals. Sol. Energy Mater. Sol. Cells 2009, 93, 2008–2012.
- Granqvist, C.G. Handbook of Inorganic Electrochromic Materials; Elsevier : Amsterdam, The Netherlands, 1995.
- Nie, H.; Self, J.L.; Kuenstler, A.S.; Hayward, R.C.; Read de Alaniz, J. Multiaddressable Photochromic Architectures: From Molecules to Materials. Adv. Opt. Mater. 2019, 7, 1900224.
- Garshasbi, S.; Santamouris, M. Using advanced thermochromic technologies in the built environment: Recent development and potential to decrease the energy consumption and fight urban overheating. Sol. Energy Mater. Sol. Cells 2019, 191, 21–32.
- Piccolo, A. Thermal performance of an electrochromic smart window tested in an environmental test cell. Energy Build. 2010, 42, 1409–1417.
- Cannavale, A.; Martellotta, F.; Ayr, U. Energy performance of building-integrated electrochromic and photovoltaic systems. IOP Conf. Ser. Mater. Sci. Eng. 2019, 609, 062004.
- Baetens, R.; Jelle, B.P.; Gustavsen, A. Properties, requirements and possibilities of smart windows for dynamic daylight and solar energy control in buildings: A state-of-the-art review. Sol. Energy Mater. Sol. Cells 2010, 94, 87–105.
- De Matteis, V.; Cannavale, A.; Blasi, L.; Quarta, A.; Gigli, G. Chromogenic device for cystic fibrosis precocious diagnosis: A “point of care” tool for sweat test. Sens. Actuators B Chem. 2016, 225, 474–480.
- Granqvist, C.G.; Bayrak Pehlivan, İ.; Niklasson, G.A. Electrochromics on a roll: Web-coating and lamination for smart windows. Surf. Coatings Technol. 2018, 336, 133–138.
- Azens, A.; Granqvist, C.G. Electrochromic smart windows: Energy efficiency and device aspects. J. Solid State Electrochem. 2003, 7, 64–68.
- Granqvist, C.G. Chapter 3—Tungsten Oxide Films: Preparation, Structure, and Composition of Evaporated Films. In Granqvist, CGBT-H of IEM; Elsevier Science BV: Amsterdam, The Netherlands, 1995; pp. 29–53.
- Granqvist, C.G.; Lansåker, P.C.; Mlyuka, N.R.; Niklasson, G.A.; Avendaño, E. Progress in chromogenics: New results for electrochromic and thermochromic materials and devices. Sol. Energy Mater. Sol. Cells 2009, 93, 2032–2039.
- Lee, S.J.; Lee, T.G.; Nahm, S.; Kim, D.H.; Yang, D.J.; Han, S.H. Investigation of all-solid-state electrochromic devices with durability enhanced tungsten-doped nickel oxide as a counter electrode. J. Alloys Compd. 2020, 815, 152399.
- Lee, E. Application issues for large-area electrochromic windows in commercial buildings. Sol. Energy Mater. Sol. Cells 2002, 71, 465–491.
- Piccolo, A.; Simone, F. Performance requirements for electrochromic smart window. J. Build. Eng. 2015, 3, 94–103.
- Niwa, T.; Takai, O. All-solid-state reflectance-type electrochromic devices using iridium tin oxide film as counter electrode. Thin Solid Films 2010, 518, 5340–5344.
- Niwa, T.; Takai, O. Optical and electrochemical properties of all-solid-state transmittance-type electrochromic devices. Thin Solid Films 2010, 518, 1722–1727.
- Cannavale, A.; Martellotta, F.; Fiorito, F.; Ayr, U. The Challenge for Building Integration of Highly Transparent Photovoltaics and Photoelectrochromic Devices. Energies 2020, 13, 1929.
- Cannavale, A.; Cossari, P.; Eperon, G.E.; Colella, S.; Fiorito, F.; Gigli, G.; Snaith, H.J.; Listorti, A. Forthcoming perspectives of photoelectrochromic devices: a critical review. Energy Environ. Sci. 2016, 9, 2682–2719.
- Theodosiou, Κ.; Dokouzis, A.; Antoniou, I.; Leftheriotis, G. Gel electrolytes for partly covered photoelectrochromic devices. Sol. Energy Mater. Sol. Cells 2019, 202, 110124.
- Wu, C.H.; Hsu, C.Y.; Huang, K.C.; Nien, P.C.; Lin, J.T.; Ho, K.C. A photoelectrochromic device based on gel electrolyte with a fast switching rate. Sol. Energy Mater. Sol. Cells 2012, 99, 148–153.
- Cannavale, A.; Eperon, G.E.; Cossari, P.; Abate, A.; Snaith, H.J.; Gigli, G. Perovskite photovoltachromic cells for building integration. Energy Environ. Sci. 2015, 8, 1578–1584.
- Granqvist, C.G.; Azens, A.; Heszler, P.; Kish, L.B.; Österlund, L. Nanomaterials for benign indoor environments: Electrochromics for “smart windows”, sensors for air quality, and photo-catalysts for air cleaning. Sol. Energy Mater. Sol. Cells 2007, 91, 355–365.
- Alam, M.J.; Cameron, D.C. Optical and electrical properties of transparent conductive ITO thin films deposited by sol-gel process. Thin Solid Films 2000, 377, 455–459.
- Granqvist, C.G. Transparent conductors as solar energy materials: A panoramic review. Sol. Energy Mater. Sol. Cells 2007, 91, 1529–1598.
- Cossari, P.; Cannavale, A.; Gambino, S.; Gigli, G. Room temperature processing for solid-state electrochromic devices on single substrate: From glass to flexible plastic. Sol. Energy Mater. Sol. Cells 2016, 155, 411–420.
- Llordés, A.; Garcia, G.; Gazquez, J.; Milliron, D.J. Tunable near-infrared and visible-light transmittance in nanocrystal-in-glass composites. Nature 2013, 500, 323–326.
- Garcia, G.; Buonsanti, R.; Llordes, A.; Runnerstrom, E.L.; Bergerud, A.; Milliron, D.J. Near-Infrared Spectrally Selective Plasmonic Electrochromic Thin Films. Adv. Opt. Mater. 2013, 1, 215–220.
- Jensen, J.; Krebs, F.C. From the bottom up—Flexible solid state electrochromic devices. Adv. Mater. 2014, 26, 7231–7234.36. Sibilio, S.; Rosato, A.; Scorpio, M.; Iuliano, G.; Ciampi, G.; Vanoli, G.; Rossi, F. A Review of Electrochromic Windows for Residential Applications. Int. J. Heat Technol. 2016, 34, S481–S488.
- Chang, T.C.; Cao, X.; Bao, S.H.; Ji, S.D.; Luo, H.J.; Jin, P. Review on thermochromic vanadium dioxide based smart coatings: from lab to commercial application. Adv. Manuf. 2018, 6, 1–19.
- Marvel, R.E.; Appavoo, K.; Choi, B.K.; Nag, J.; Haglund, R.F. Electron-beam deposition of vanadium dioxide thin films. Appl. Phys. A 2013, 111, 975–981.
- Kumar, M.; Singh, J.P.; Chae, K.H.; Park, J.; Lee, H.H. Annealing effect on phase transition and thermochromic properties of VO2 thin films. Superlattices Microstruct. 2020, 137, 106335.
- Zhan, Y.; Lu, Y.; Xiao, X.; Wang, J.; Liu, Y.; Zhang, S.; Shen, C.; Xu, X.; Xu, G. Tuning thermochromic performance of VOx-based multilayer films by controlling annealing pressure. Ceram. Int. 2020, 46, 2079–2085.
- Wang, S.; Li, C.; Tian, S.; Liu, B.; Zhao, X. Facile synthesis of VO2 (D) and its transformation to VO2(M) with enhanced thermochromic properties for smart windows. Ceram. Int. 2020, 46, 14739–14746.
- Cao, X.; Chang, T.; Shao, Z.; Xu, F.; Luo, H.; Jin, P. Challenges and Opportunities toward Real Application of VO2-Based Smart Glazing. Matter 2020, 2, 862–881.
- Li, S.Y.; Niklasson, G.A.; Granqvist, C.G. Thermochromic fenestration with VO 2-based materials: Three challenges and how they can be met. Thin Solid Films 2012, 520, 3823–3828.
- Cui, Y.; Ke, Y.; Liu, C.; Chen, Z.; Wang, N.; Zhang, L.; Zhou, Y.; Wang, S.; Gao, Y.; Long, Y. Thermochromic VO2 for Energy-Efficient Smart Windows. Joule 2018, 2, 1707,1746.
- Zhao, Z.; Liu, Y.; Wang, D.; Ling, C.; Chang, Q.; Li, J.; Zhao, Y.; Jin, H. Sn dopants improve the visible transmittance of VO2 films achieving excellent thermochromic performance for smart window. Sol. Energy Mater. Sol. Cells 2020, 209, 110443.
- Fan, L.; Zhu, Y.; Zhao, S.; Wang, Z.; Liu, Z.; Zhu, L.; Wang, B.; Zhang, Q. Modulation of VO2 metal-insulator transition by co-doping of hydrogen and oxygen vacancy. Sol. Energy Mater. Sol. Cells 2020, 212, 110562.
- Ji, H.; Liu, D.; Cheng, H. Infrared optical modulation characteristics of W-doped VO2(M) nanoparticles in the MWIR and LWIR regions. Mater. Sci. Semicond. Process. 2020, 119, 105141.
- Moon-Hee Lee, Myoung-Geun Kim, H.-K.S. Thermochromism of rapid thermal annealed VO2 and Sn-doped VO2 thin films. Thin Solid Films 1996, 290, 30–33.
- Jin, P.; Nakao, S.; Tanemura, S. Tungsten doping into vanadium dioxide thermochromic films by high-energy ion implantation and thermal annealing. Thin Solid Films 1998, 324, 151–158.
- Kolenatý, D.; Vlček, J.; Bárta, T.; Rezek, J.; Houška, J.; Haviar, S. High-performance thermochromic VO2-based coatings with a low transition temperature deposited on glass by a scalable technique. Sci. Rep. 2020, 10, 1–12.
- Liu, S.; Tso, C.Y.; Lee, H.H.; Zhang, Y.; Yu, K.M.; Chao, C.Y.H. Bio-inspired TiO2 nano-cone antireflection layer for the optical performance improvement of VO2 thermochromic smart windows. Sci. Rep. 2020, 10, 1–14.
- Mlyuka, N.R.; Niklasson, G.A.; Granqvist, C.G. Thermochromic multilayer films of VO2 and TiO2 with enhanced transmittance. Sol. Energy Mater. Sol. Cells 2009, 93, 1685–1687.
- Xu, F.; Cao, X.; Luo, H.; Jin, P. Recent advances in VO2-based thermochromic composites for smart windows. J. Mater. Chem. C 2018, 6, 1903–1919.
- Zhang, J.; Zou, Q.; Tian, H. Photochromic materials: More than meets the eye. Adv. Mater. 2013, 25, 378–399.
- Wang, L.; Li, Q. Photochromism into nanosystems: Towards lighting up the future nanoworld. Chem. Soc. Rev. 2018, 47, 1044–1097.
- Barachevsky, V.A. Photochromic Nanoparticles and Their Properties. Crystallogr. Reports 2018, 63, 271–275.
- Wu, L.; Zhang, S.; Gao, J.; Qiang, P.; Lei, J. Preparation of a spirooxazine grafted PMMA and its photochromic properties. Synth. Commun. 2016, 46, 818–830.
- Lvov, A.G.; Kavun, A.M.; Kachala, V. V.; Nelyubina, Y. V.; Metelitsa, A. V.; Shirinian, V.Z. Structural and Spectral Properties of Photochromic Diarylethenes: Size Effect of the Ethene Bridge. J. Org. Chem. 2017, 82, 1477–1486.
- Barachevsky, V.A.; Butenko, V.G. Photoelectrochromic Organic Systems. Russ. J. Gen. Chem. 2018, 88, 2747–2772.
- Li, X.; Li, C.; Wang, S.; Dong, H.; Ma, X.; Cao, D. Synthesis and properties of photochromic spirooxazine with aggregation-induced emission fluorophores polymeric nanoparticles. Dye. Pigment. 2017, 142, 481–490.
- Song, L.; Yang, Y.; Zhang, Q.; Tian, H.; Zhu, W. Synthesis and photochromism of naphthopyrans bearing naphthalimide chromophore: Predominant thermal reversibility in color-fading and fluorescence switch. J. Phys. Chem. B 2011, 115, 14648–14658.
- Tsuda, K.; Dol, G.C.; Gensch, T.; Hofkens, J.; Latterini, L.; Weener, J.W.; Meijer, E.W.; De Schryver, F.C. Fluorescence from azobenzene functionalized poly(propylene imine) dendrimers in self-assembled supramolecular structures. J. Am. Chem. Soc. 2000, 122, 3445–3452.
- Ke, Y.; Chen, J.; Lin, G.; Wang, S.; Zhou, Y.; Yin, J.; Lee, P.S.; Long, Y. Smart Windows: Electro-, Thermo-, Mechano-, Photochromics, and Beyond. Adv. Energy Mater. 2019, 9, 1–38.
- Cipolloni, M.; Heynderickx, A.; Maurel, F.; Perrier, A.; Jacquemin, D.; Siri, O.; Ortica, F.; Favaro, G. Multiswitchable acidichromic and photochromic bisdiarylethene. An experimental and theoretical study. J. Phys. Chem. C 2011, 115, 23096–23106.
- Seibold, M.; Handschuh, M.; Port, H.; Wolf, H.C. Photochromic fulgides: Towards their application in molecular electronics. J. Lumin. 1997, 72–74, 454–456.
- Nakamura, S.; Irie, M. Thermally Irreversible Photochromic Systems. A Theoretical Study. J. Org. Chem. 1988, 53, 6136–6138.
- Inaba, K.; Iwai, R.; Morimoto, M.; Irie, M. Thermally reversible photochromism of dipyrrolylethenes. Photochem. Photobiol. Sci. 2019, 18, 2136–2141.
- Uchida, K.; Matsuoka, T.; Sayo, K.; Iwamoto, M.; Hayashi, S.; Irie, M. Thermally reversible photochromic systems. Photochromism of a dipyrrolylperfluorocyclopentene. Chem. Lett. 1999, 835–836.
- Wu, L.; Zhao, Q.; Huang, H.; Lim, R.J. Sol-gel based photochromic coating for solar responsive smart window. Surf. Coatings Technol. 2017, 320, 601–607.
- Chen, Y.; Li, T.; Fan, M.; Mai, X.; Zhao, H.; Xu, D. Photochromic fulgide for multi-level recording. Mater. Sci. Eng. B 2005, 123, 53–56.
- Wang, Y.; Runnerstom, E.L.; Milliron, D.J. Switchable Materials for Smart Windows. Annu. Rev. Chem. Biomol. Eng. 2016, 7, 283–304.
- Mukhopadhyay, A.; Moorthy, J.N. Phenomenon to functions: Photochromism of diarylpyrans, spectrokinetic properties and functional materials. J. Photochem. Photobiol. C Photochem. Rev. 2016, 29, 73–106.
- Wei, T.; Jia, B.; Shen, L.; Zhao, C.; Wu, L.; Zhang, B.; Tao, X.; Wu, S.; Liang, Y. Reversible upconversion modulation in new photochromic SrBi2Nb2O9 based ceramics for optical storage and anti-counterfeiting applications. J. Eur. Ceram. Soc. 2020, 40, 4153–4163.
- Ortica, F. The role of temperature in the photochromic behaviour. Dye. Pigment. 2012, 92, 807–816.
- Massaro, G.; Hernando, J.; Ruiz-Molina, D.; Roscini, C.; Latterini, L. Thermally Switchable Molecular Upconversion Emission. Chem. Mater. 2016, 28, 738–745.
- Kang, M.J.; Santoro, E.G.; Kang, Y.S. Enhanced Efficiency of Functional Smart Window with Solar Wavelength Conversion Phosphor-Photochromic Hybrid Film. ACS Omega 2018, 3, 9505–9512.
- Zuo, J. Annealing effect on reversible photochromic properties of Ag@TiO 2 nanocomposite film. Key Eng. Mater. 2013, 537, 201–204.
- Evdokimova, O.L.; Kusova, T. V.; Ivanova, O.S.; Shcherbakov, A.B.; Yorov, K.E.; Baranchikov, A.E.; Agafonov, A. V.; Ivanov, V.K. Highly reversible photochromism in composite WO3/nanocellulose films. Cellulose 2019, 26, 9095–9105.
- Piccolo, A.; Simone, F. Effect of switchable glazing on discomfort glare from windows. Build. Environ. 2009, 44, 1171–1180.
- Cannavale, A.; Martellotta, F.; Cossari, P.; Gigli, G. Energy savings due to building integration of innovative solid-state electrochromic devices. Appl. Energy 2018, 225, 975–985.
- Tavares, P.; Bernardo, H.; Gaspar, A.; Martins, A. Control criteria of electrochromic glasses for energy savings in mediterranean buildings refurbishment. Sol. Energy 2016, 134, 236–250.
- Wen, R.T.; Arvizu, M.A.; Niklasson, G.A.; Granqvist, C.G. Electrochromics for energy efficient buildings: Towards long-term durability and materials rejuvenation. Surf. Coatings Technol. 2015, 278, 121–125.
- Cannavale, A.; Ayr, U.; Fiorito, F.; Martellotta, F. Smart electrochromic windows to enhance building energy efficiency and visual comfort. Energies 2020, 13, 1–17.
- Aburas, M.; Soebarto, V.; Williamson, T.; Liang, R.; Ebendorff-Heidepriem, H.; Wu, Y. Thermochromic smart window technologies for building application: A review. Appl. Energy 2019, 255, 113522.
- Giovannini, L.; Favoino, F.; Serra, V.; Zinzi, M. Thermo-chromic glazing in buildings: A novel methodological framework for a multi-objective performance evaluation. Energy Procedia 2019, 158, 4115–4122.
- Tällberg, R.; Jelle, B.P.; Loonen, R.; Gao, T.; Hamdy, M. Comparison of the energy saving potential of adaptive and controllable smart windows: A state-of-the-art review and simulation studies of thermochromic, photochromic and electrochromic technologies. Sol. Energy Mater. Sol. Cells 2019, 200, 109828.
- Dokouzis, A.; Bella, F.; Theodosiou, K.; Gerbaldi, C.; Leftheriotis, G. Photoelectrochromic devices with cobalt redox electrolytes. Mater. Today Energy 2020, 15, 100365.
- Leftheriotis, G.; Syrrokostas, G.; Yianoulis, P. Development of photoelectrochromic devices for dynamic solar control in buildings. Sol. Energy Mater. Sol. Cells 2010, 94, 2304–2313.
- DeForest, N.; Shehabi, A.; Selkowitz, S.; Milliron, D.J. A comparative energy analysis of three electrochromic glazing technologies in commercial and residential buildings. Appl. Energy 2017, 192, 95–109.
- DeForest, N.; Shehabi, A.; Garcia, G.; Greenblatt, J.; Masanet, E.; Lee, E.S.; Selkowitz, S.; Milliron, D.J. Regional performance targets for transparent near-infrared switching electrochromic window glazings. Build. Environ. 2013, 61, 160–168.
- DeForest, N.; Shehabi, A.; O’Donnell, J.; Garcia, G.; Greenblatt, J.; Lee, E.S.; Selkowitz, S.; Milliron, D.J. United States energy and CO2 savings potential from deployment of near-infrared electrochromic window glazings. Build. Environ. 2015, 89, 107–117.