1000/1000
Hot
Most Recent

Saprochaete clavata and Saprochaete capitata are emerging fungal pathogens that are responsible for life-threatening infections in immunocompromised patients, particularly in the setting of profound neutropenia.
Invasive fungal infections (IFIs) constitute a major cause of mortality and morbidity especially in severely immunocompromised patients. These infections are being increasingly recognized worldwide, partly due to advancements in diagnostic testing, implementation of aggressive chemotherapeutic protocols and use of antifungal prophylaxis in immunocompromised patients. In one report, the incidence of IFIs increased from 23.2 cases / 100,000 patients in 2008 to 31.8 cases / 100,000 patients in 2015 [1]. To date, Aspergillus, Candida, Cryptococcus and Pneumocystis remain the most predominant fungal pathogens affecting immunocompromised patients [2]; however, over the past decade, we witnessed the emergence of less common fungal species as causative agents for life-threatening IFIs such as Saprochaetes and Trichosporon spp. [3]. These fungal organisms can cause blood stream infections as well as invasive and disseminated multiorgan disease.
Saprochaete clavata (formerly Geotrichum clavatum) and Saprochaete capitata (formerly Geotrichum capitatum, Blastoschizomyces capitatus, Magnusiomyces capitatus) are closely related organisms that are often misidentified due to their close phenotypical resemblances [3][4]. They are arthroconidial yeast-like filamentous fungi whose taxonomy has undergone multiple revisions over the years, largely due to changes in the rules of fungal nomenclature [5]. They are microbiologically and phylogenetically related to ascomycetous yeasts and are classified in the family Dipodascaceae, order Saccharomycetales [4][6].
The diagnosis of Saprochaete invasive infections remains challenging and relies primarily on clinical suspicion and isolation of these organisms from blood, tissue samples or sterile body fluids. Despite advances in diagnostic microbiology, S. clavata and S. capitata can often be misidentified even in laboratories that are equipped with advanced diagnostic tools such as automated identification systems, internal transcribed spacer (ITS) sequencing or matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) [4][5]. Both organisms appear to be predominantly resistant to echinocandins and fluconazole [7]. Reporting mortality from IFIs can be challenging as infected patients tend to be critically ill and death can often be attributed to numerous factors. Nevertheless, forty-two-day all-cause mortality for both Mucorales and Aspergillus spp. has been estimated at 28% in one study, compared to 20.5% for Candida [1]. Despite early isolation and initiation of adequate antifungal therapy, all-cause mortality rates as high as 40%-80% for invasive Saprochaete spp. infections have been reported in some patient groups [8][9][10].
S. capitata and S. clavata have emerged as causative agents responsible for multiple hospital outbreaks predominantly in Europe [3][9][11], with Italy having the highest number of reported cases to date. It is worth noting that outbreaks appear to be more peculiar to S. clavata compared to S. capitata, with the latter being associated with a high number of sporadic cases reported in several countries around the world such as Brazil [12], India [13][14], Iraq [15], Japan [16], Tunisia [17] and Turkey [18][19]. A recent cohort study in patients with cancer reported a rate of Saprochaete spp. infection approaching 3.4 per 100,000 patient admissions [20].
Saprochaete spp. can be found in nature, especially in soil [14][21]. They have also been isolated from dairy products [22], as well as dishwashers in household and healthcare facilities [23][24][25][26], making these contaminated sources potential suspects for some of the reported outbreaks. The majority of patients who develop invasive S. capitata or S. clavata infection are initially colonized with the organism [11]. The site of entry of these pathogens into the human host remains unclear; however, invasion through the respiratory or gastrointestinal tract is likely [3][15]. Damage to the gastrointestinal mucosal barrier and ulcerations induced by chemotherapy might allow fungal translocation, increasing the risk of fungemia [3]. The mode of transmission is not defined; however, the fact that isolates from the same clade were responsible for hospital outbreaks in France suggests that human–human infection in hospitalized patients is possible and could be related to environmental contamination or device colonization [5][9][26]. For instance, a recent French S. clavata outbreak was linked to a dishwasher with a deficient heating system [26]. Fly-to-human transmission is unlikely; however, S. clavata has been isolated from Drosophila fly body surfaces [2][12][14].
The majority of patients with invasive Saprochaete infections have, at the time of fungemia, an impaired immunity due to profound neutropenia secondary to chemotherapy administration in the setting of hematological malignancy. There appears to be a particular association with cytarabine use and acute leukemia [3][10][27][28][29][30][31][32]. Although exceedingly rare, invasive Saprochaete infections have been reported in solid organ transplant recipients receiving immunosuppressive therapy [33][34][35], and critically ill patients with prolonged intensive care unit stay without underlying malignancy [36][37]. Other underlying risk factors in immunocompromised patients include dysbiosis due to the prophylactic use of antibiotics and antifungal agents in patients prior to the infection [11][16][38]. A case of S. capitata infection has also been reported in a patient with CARD9 deficiency—a genetic immune disorder characterized by susceptibility to fungal infections [15]. Saprochaete pulmonary infections have been reported in immunocompetent patients with underlying lung disease such as asthma, chronic obstructive lung disease, history of tuberculosis infection or bacterial pneumonia [39][40][41][42][43].
Disseminated disease is common with Saprochaetes especially in severely immunosuppressed patients. Clinical presentation mimics that of invasive candidiasis, and fungemia is common, although Saprochaetes tend to affect the lungs and deep organs more frequently than Candida spp. [44][45]. Patients often present with an acute febrile illness, which could be the only initial manifestation, or could be accompanied by various other symptoms depending on the sites of disease involvement. Symptoms usually progress rapidly to multiorgan failure, shock and death in the majority of patients despite adequate antifungal therapy [11]. Mortality approaches 60–80%; however, rapid molecular identification and prompt initiation of appropriate antifungal therapy have been shown to decrease the numbers of disease-related deaths [3][9][46][47]. The clinical presentation in immunocompetent patients is less severe, and in this population, S. capitata has been exclusively isolated from sputum samples [42].
Table 1 (in the main article) lists common presenting symptoms and corresponding radiographic findings as reported in patients with invasive Saprochaete spp. infections depending on the site of disease involvement. Patients with pulmonary disease present with shortness of breath and cough which could be productive of purulent or bloody sputum. Most common radiographic findings include diffuse bilateral infiltrates, ground glass opacities, pleural effusion and parenchymal nodules. S. Clavata empyema has also been reported, highlighting the importance of obtaining prompt diagnostic thoracentesis and source control [48]. Clinical progression to respiratory failure is common, often requiring intubation and mechanical ventilation. Patients with intraabdominal disease can present with diarrhea, abdominal pain and jaundice in the setting of biliary duct obstruction. Dysuria and hematuria can be present in cases of renal and bladder involvement. Rarely, Saprochaetes can cause peritonitis and abdominal compartment syndrome [46][49]. The most common radiographic findings include hepatosplenomegaly and hypodense parenchymal lesions or nodules involving deep organs (Figure 1). Central nervous system (CNS) involvement can manifest as high-grade fevers, mental status changes and seizures that can progress to status epilepticus. Even if the patients recover from their illness and respond to antifungal therapy, they are at risk of developing long term neurological sequalae [10]. A brain mass or multiple lesions with or without surrounding edema and hemorrhages are usually seen on imaging. Skin lesions are seen in some of the patients with disseminated disease. Reported cutaneous manifestations include erythematous nodules and papules on the lower extremities and back mimicking lesions seen in disseminated candidiasis [50]. Black necrotic plaques involving the perianal area, and wound infection at the site of a laparotomy scar have also been reported [33].
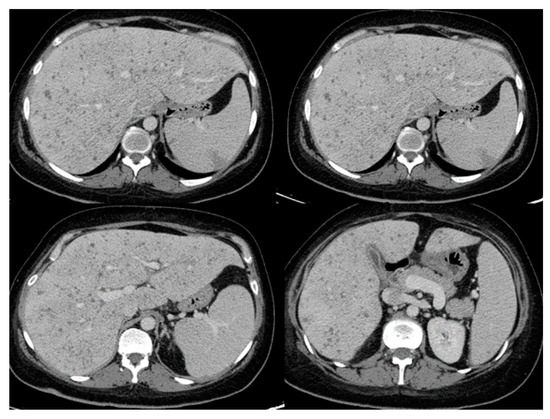
Figure 1. Abdominal computed tomography showing hepatosplenic abscesses in a patient with invasive Saprochaete clavata infection (reproduced from Del Principe, M.I et al. Mycoses 2016, 59, 594–601, doi:10.1111/myc.12508 with permission from John Wiley and Sons under license number 4915460075071).
The diagnosis of invasive Saprochaete spp. infection is proven by histopathologic or direct microscopic visualization of the fungi from a normally sterile body site [51]. The clinical significance of isolating these organisms from respiratory, urinary or stool cultures of immunosuppressed patients remains unclear; however, in the absence of other identifiable organisms and in the setting of a clinically documented infection, this represents probable invasive fungal disease [9][44]. In the absence of host factors such as immunosuppression or prolonged neutropenia, the isolation of Saprochaetes from non-sterile sites likely represents a contamination and care must be exerted when interpreting such microbiological results [14][42].
The colonies of Saprochaete spp. grow on a multitude of fungal media within 24–48 hours; however, cultures may require incubation for up to 5 days. S. clavata and S. capitata are often misidentified as they are identical in macroscopic and microscopic analysis [4][28]. This misidentification may have contributed to the significantly lower numbers of reported S. clavata infections prior to the availability of more advanced diagnostic modalities [3][11]. Distinguishing between these 2 organisms to the species level is important for epidemiological and outbreak investigation purposes, as well as for clinical reasons, as S. clavata and S. capitata can have different antifungal susceptibility profiles [7]. Macroscopically, Saprochaete spp. form yeast-like, farinose, dry cottony colonies with frosted glass appearance on the plate (Figure 2) [3][30]. Microscopically, they form true fragmented hyphae, pseudo-hyphae, arthroconidia, annelloconidia and blastoconidia [3][42][52] (Figure 3).
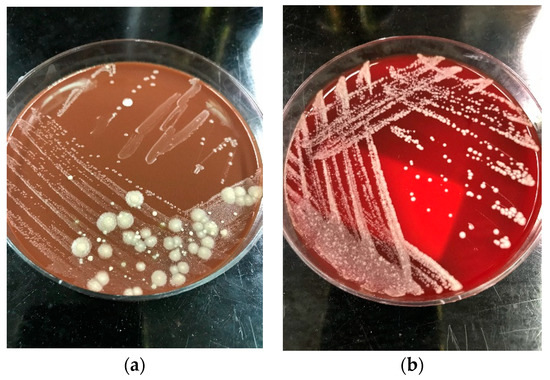
Figure 2. Dry cottony colonies with frosted glass appearance of Saprochaete spp. on (a) blood agar and (b) chocolate agar, respectively. The isolates were incubated at 26 °C for 72 h and later identified as S. capitata by MALDI-TOF.
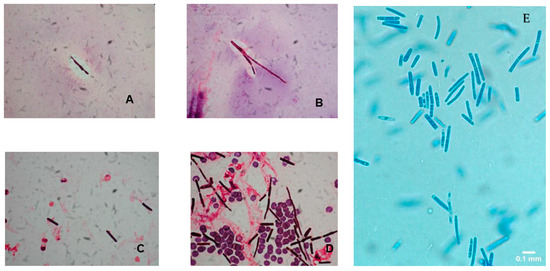
Figure 3. (A–D) Gram stain preparation of blood cultures showing arthroconidia and hyphal elements of Saprochaete clavata. Magnification ×1000 (reproduced from Del Principe, M.I et al. Mycoses 2016, 59, 594–601, doi:10.1111/myc.12508 with permission from John Wiley and Sons under license number 4915460075071). (E) Lactophenol cotton blue preparation of peritoneal fluid cultures showing arthroconidia suggestive of Saprochaete spp. infection. The organism was identified as S. capitata by MALDI-TOF. Magnification ×40.
Saprochaete spp. are urease negative, a characteristic that helps distinguish them from Trichosporon spp. [28]. S. clavata, unlike S. capitata, has the ability to assimilate carbon sources such as cellobiose, salicin and arbutin [5][12]; however, many S. capitata strains could grow on all carbon sources [5], and as many as 15% of S. clavata strains do not assimilate cellobiose, making biochemical testing alone unreliable for accurate species identification [11]. Moreover, commercial test kits often lack salicin and arbutin [4][53]. Both species are thermotolerant with optimal growth observed at temperatures between 30 °C and 40 °C [4][5]. In one study, S. clavata, unlike S. capitata isolates, did not grow at temperatures above 45 °C, and isolates preincubated at that temperature became nonviable and demonstrated no regrowth [5]. Differentiation between both species based on incubation temperatures, however, is unreliable as other studies demonstrated that both species exhibited similar growth patterns at different temperatures [23][26].
Commercial systems for yeast identification such as VITEK-2 (ID-YEST card; BioMérieux) and API ID32C (BioMérieux) can successfully identify some of the S. capitata isolates; however, none of the systems cover S. clavata [3][11][30][35][54]. Nucleotide sequencing of the ITS and partial large subunit (LSU) loci can discriminate between Saprochaetes at the species level; however, misidentification is common as the diagnostic ability of these tests is dependent on the accuracy and public availability of the nucleotide sequences (e.g., GenBank) [4][5]. Multilocus sequencing of protein binding loci such as Rbp2, Act or Tef1α might allow for more accurate identification [5][11]. MALDI-TOF mass spectrometry is a useful and reliable modality for the identification and discrimination between Saprochaete spp. and other arthroconidial fungi; however, as with nucleotide sequencing, the accuracy of this test is highly limited by the quality and number of mass spectra available in commercial and in-house fungal libraries [11][55]. S. capitata and S. clavata are better distinguished by MALDI-TOF when newer databases such as Bruker BioTyper (3.0 or 3.1) (Bruker Daltonics, Bremen, Germany) or expanded in-house databases are used [8]. The development of polymerase chain reaction (PCR) assays that are highly species specific may be useful in early identification of the organisms from blood or tissue samples, allowing prompt initiation of adequate antifungal therapy; however, such assays are not yet available for commercial use [56]. In the setting of a suspected outbreak, whole-genome sequence (WGS) typing is the best method to determine clonality and to infer strain relatedness [3]. A single clone was identified by WGS typing as responsible for most cases of a French cluster of infections [9][57]. Recently, the investigation of an S. clavata outbreak in France demonstrated that clinical and environmental isolates were clustered within the same clade and the outbreak ended after discarding the contaminated dishwasher and kitchen utensils [26].
Arthroconidial fungi can cross react with Aspergillus galactomannan (GM) and serum 1,3-beta-D-glucan (BDG); however, these tests are not sensitive, nor specific for Saprochaetes [3][17][58] and therefore have little clinical utility for the diagnosis of invasive infections with these organisms. It is worth noting, however, that a positive GM test in a profoundly neutropenic patient with clinical findings suggestive of invasive Candida infection should prompt physicians to suspect invasive Saprochaete disease, especially that these organisms are resistant to echinocandins and fluconazole, which are the drugs of choice used for the treatment of invasive candidiasis [58].
To this date, there is no established therapeutic regimen for the treatment of invasive Saprochaete spp. infections, largely due to the rarity and challenging diagnosis of these organisms and lack of standardized antifungal breakpoints [3]. All treatment recommendations are based on expert opinion and extrapolated data from case reports and small case series. In general, echinocandins and fluconazole should be avoided given the high in vitro MIC values and the frequent numbers of breakthrough Saprochaete infections reported while patients are receiving these drugs [7]. High dose fluconazole might be an appropriate treatment when isolates are susceptible [17][28], however, avoidance of this drug for empiric therapy might be prudent given the high probability of resistance. The European Society of Clinical Microbiology and Infectious Diseases (ESCMID) guidelines published in 2014 recommend any formulation of amphotericin B with or without flucytosine as initial therapy [59].
The presence of a CVC may be a risk factor for the development of catheter- related Saprochaete spp. infections. Removal of the CVC within 5 days after onset of infection appeared to positively influence outcomes in patients with S. capitata catheter- related infection in one study [44], however, no firm evidence in the form of randomized controlled trials (RCTs), quasi-RCTs or large observational studies exists in the literature to support this practice. Timely administration of antifungal agents and source control (e.g. drainage of an empyema or retroperitoneal fluid collection) are a mainstay for the treatment of invasive Saprochaete spp. infections [3][60][61]. Recovery from immunosuppression likely plays a crucial role in successful treatment [11][31].The use of granulocyte stimulating factor (G-CSF) or Interferon-gamma in combination with antifungal therapy has been successful in eliminating the infection in some patients [29], however, the impact of these regimens on clinical outcomes remains unclear.