
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ramon Andrade Bezerra De Mello | + 1562 word(s) | 1562 | 2020-11-18 10:30:49 | | | |
| 2 | Vicky Zhou | -86 word(s) | 1476 | 2020-12-01 09:04:14 | | |
Video Upload Options
Currently, lung cancer is a disease that acquired an impressive change in the clinical manangement due to the recent inovations regarding targeted therapies and immunne check point inhibitors.
1. Introduction
1.1. Epidemiology
Lung cancer (LC) is the most common neoplasm worldwide [1]. The World Health Organization (WHO) reported 2.1 million new cases of LC in 2018, with the highest incidence rates in Turkey, Japan, China, the United States, and the United Kingdom [2]. Specifically, in the United States, the American Cancer Society reports that LC was the malignancy with the second-highest number of estimated new cases in 2019, both in men and women. Lung and bronchus cancer had an estimated 228,150 new cases in the United States during 2019, divided between men (116,440 cases) and women (111,710 cases) [3]. The term “lung cancer” accounts for a heterogeneous set of tumors. They are divided into non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC). Among them there are more than 50 different histological subtypes [4] NSCLC, for instance, is subcategorized into adenocarcinoma, squamous cell carcinoma, and large cell carcinoma [5]. In this review of the literature, we will focus on NSCLC, which accounts for around 85% of all LC cases [6].
1.2. Mortality
LC is the main cause of death worldwide and accounts for approximately 1.6 million deaths per year [7]. In 2018, the WHO reported 1.8 million deaths due to this malignancy. The highest LC mortality rates were seen in countries such as Turkey, Canada, United States, United Kingdom, and Japan [2]. The American Cancer Society estimates that, in 2019, LC was the most important cause of cancer-related deaths in American men and women. During that year, lung and bronchus cancer had an estimated 76,650 male deaths and 66,020 female deaths, totaling 142,670 deaths in the United States. For all stages combined, LC has a 19% five-year relative survival rate [3]. The high mortality of this malignancy is heightened by the fact that many tumors are already advanced at the moment of diagnosis. Studies have found the presence of distant-organ metastasis in 47.3% of patients with NSCLC at their initial cancer diagnosis, which can greatly hinder treatment [8]. Therefore, mortality is still a very important problem related to this malignancy, and novel therapies aim to reduce its rates.
1.3. Risk Factors
The main risk factor for developing LC is tobacco smoking, which is accounted for in at least 80% of cases. Continuous smoking can increase LC risk by up to 50%, and in these cases, the association with squamous-cell LC is stronger than other tumor subtypes [7]. Other important environmental risk factors are passive smoking (regular passive smoking can increase LC risk up to 30%), pollution (a European cohort found that exposure to particulate matter is associated with a hazard ratio of 1.22 for LC), and occupational exposure to carcinogens (such as asbestos, chromium compounds, silica and diesel fumes) [9][10]. Furthermore, studies have shown that having a first-degree relative with a history of LC increases a person’s risk of developing this disease by 50% and that around 8% of all LC cases occur due to genetic predisposition [11].
1.4. Evolution in Treatment
Considerable advances have been made regarding LC treatment, which was initially restricted to cytotoxic chemotherapy [12]. Chemotherapy agents block the cell cycle and therefore attack rapidly dividing cells. This traditional treatment has low specificity, attacking normal cells as well as cancerous ones, leading to significant side effects. The recent advances in cancer treatment consist of the development of target therapies, which are drugs that block specific molecules that are found over-expressed or overactive in tumor cells only. These altered target molecules are a result of driver mutations in the cancer cells, which amplify proliferation and survival pathways, leading to tumor growth. There are roughly three types of target therapies: monoclonal antibodies, small molecule inhibitors, and immunotoxins [13][14]. The impact of this evolution of LC treatment is reflected in the increase of 5-year survival rates of patients, which jumped from 10.7% in the early 1970s to 19.8% in the 2010s [15]. However, some challenges remain, such as the identification of new driver gene alterations involved in carcinogenesis, knowledge of drug-resistance mechanisms, and recognition of response predictors to novel therapies [7].
2. Advanced Disease: From Cytotoxic Therapy to Target Agents
2.1. Cytotoxic Therapy
Chemotherapy was the backbone of lung cancer treatment for several years. Before the chemotherapy era, the median overall survival (OS) of metastatic lung cancer was 3.9 months with the best supportive care [16]. Platinum combinations have been playing the main role in treatment since the 80s, but the landmark study of these drugs was the meta-analysis by the Non-Small Cell Lung Cancer Collaborative Group in 1995, which demonstrated that platinum-based chemotherapy significantly improved OS over best supportive care (15% vs. 5% OS rate in 1 year) [17]. This method of chemotherapy is still prescribed to up to 80% of patients diagnosed with lung cancer, whether isolated or combined with radiotherapy. This modality has been used in several different scenarios: adjuvant for patients with compromised lymph nodes, neoadjuvant to reduce the tumor mass in advanced stages, as part of palliative systemic care in patients with metastatic disease, or in patients who can’t undergo surgery, regardless of tumor staging [1]. Platinum-based compounds (cisplatin, carboplatin) have been associated with third-generation cytotoxic agents. This combination is referred to as “platinum doublets” and can have double the response rate (RR) when compared to monotherapy regimens [18]. When comparing the effects of different doublets, studies found similar response rates for paclitaxel (RR = 0.89) and gemcitabine (RR = 0.92) in the doublets, while docetaxel had a smaller response rate (RR = 0.76) [19].
During the 2000s, another player entered the game. In phase 3 JMDB trial, pemetrexed-cisplatin doublets demonstrated clinical benefit (increase in OS of 1.7 months) on non-squamous NSCLC when compared to gemcitabine-cisplatin doublets, supporting the concept that histology does matter in the treatment of lung cancer [17]. Another phase 3 trial demonstrated the clinical benefit of pemetrexed maintenance treatment in non-squamous NSCLC. Pemetrexed was the only cytotoxic agent found to improve both progression-free survival and OS in maintenance therapy, and it was also the drug that caused the least toxic adverse effects in patients [20].
Figure 1 demonstrates the evolution of the clinical benefit of chemotherapy; as it becomes more precise, the higher is the overall survival [17].
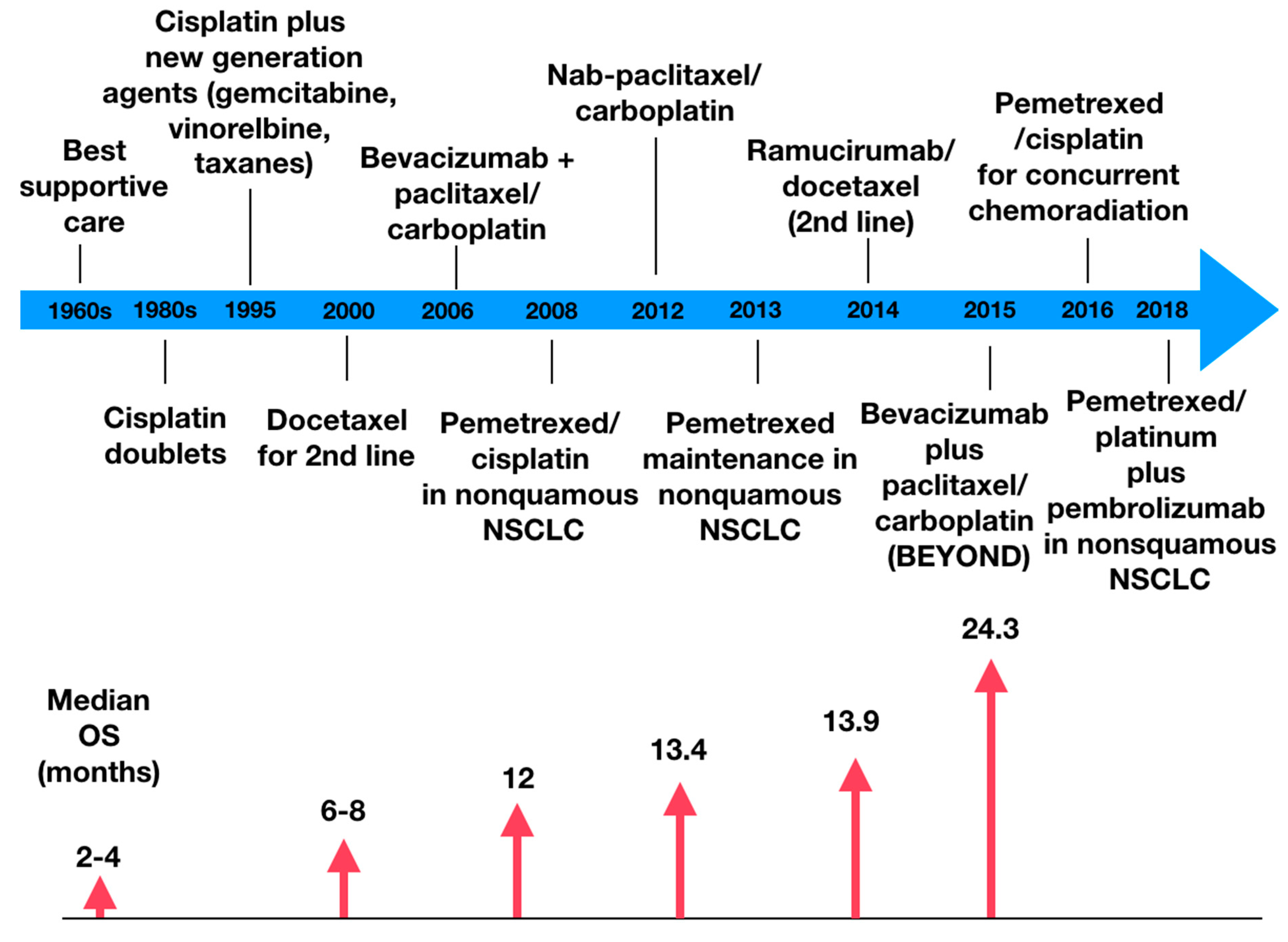
2.2. Chemotherapy with Other Agents
Bevacizumab is a monoclonal antibody that binds to vascular endothelial growth factor receptor (VEGFR) in cancer cells, blocking angiogenic pathways and therefore decreasing a tumor’s vascular permeability, which can decrease tumor growth from 25 to 95% [21]. There are several trials in which the efficacy of this drug has been proved. A meta-analysis demonstrated that the use of a platinum doublet plus bevacizumab showed significant OS benefit over platinum doublet alone, reducing mortality by 11% [22]. In the phase III ECOG 4599 trial, bevacizumab was combined with paclitaxel/carboplatin, leading to higher response rates and OS (12.3 months vs. 10.3 months, p = 0.003) when compared to paclitaxel/carboplatin alone [23]. Several other trials demonstrated the clinical benefit of bevacizumab, as the AVAil trial and the BEYOND trial [24]. Despite the benefits, this agent is associated with life-threatening bleeding and is only recommended for tumors or non-squamous histology [25]. Combinations with platinum doublets have been evaluated in the adjuvant setting but failed to prove OS benefit [26].
Ramucirumab is a human monoclonal antibody with a high affinity to the VEGFR2 extracellular domain. It can be used to treat patients with locally advanced or metastatic NSCLC [27]. This indication is supported by several studies, for instance, the REVEAL trial, a multicenter, double-blind, randomized phase III study that compared Ramucirumab plus Docetaxel versus placebo plus Docetaxel as a second-line treatment of NSCLC after disease progression on platinum-based chemotherapy. The results showed a higher median OS in the first group (10.5 months) compared to the second group (9.1 months). Median progression-free survival (PFS) has also been proven higher in the Ramucirumab group. However, almost all patients in both arms presented treatment-emergent adverse effects, and the common grade 3 adverse effects in the Ramucirumab group were neutropenia, leucopenia, fatigue, and hypertension [28].
Albumin-bound paclitaxel is a microtubule inhibitor best efficient when used to treat NSCLC, either as a single therapy or as part of some combination [29] Albumin-bound paclitaxel, also known as nab-Paclitaxel, is a solvent-free formulation developed over a decade ago that delivers a higher dose of Paclitaxel to solid tumors while reducing the incidence of toxicities [30]. Regarding NSCLC, a phase III trial shows that, based on independent assessment, weekly nab-Paclitaxel plus Carboplatin demonstrated a higher overall response rate (ORR, 33%) than solvent based-Paclitaxel plus Carboplatin (25%). The nab-Paclitaxel arm also presented a higher median OS (12.1 vs. 11.2 months) and median PFS (6.3 vs. 5.8 months) compared to the other group (p values = 0.271 and 0.214, respectively). Nab-paclitaxel has also shown benefit as the patients who received it presented significantly less grade 3 or higher adverse effects, such as neuropathy, neutropenia, arthralgia, and myalgia. However, the drug demonstrated a higher incidence of thrombocytopenia and anemia [31]. Current guidelines recommend nab-paclitaxel as a first-line replacement of docetaxel or paclitaxel for those who experience hypersensitivity reactions despite pre-medications or where pre-medications are contraindicated [32].
References
- Visconti, R.; Morra, F.; Guggino, G.; Celetti, A. The between Now and Then of Lung Cancer Chemotherapy and Immunotherapy. Int. J. Mol. Sci. 2017, 18, 1374.
- World Health Organization: Regional Office for Europe. World Cancer Report: Cancer Research for Cancer Development; IARC: Lyon, France, 2020; ISBN 978-92-832-0447-3.
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34.
- Osmani, L.; Askin, F.; Gabrielson, E.; Li, Q.K. Current WHO guidelines and the critical role of immunohistochemical markers in the subclassification of non-small cell lung carcinoma (NSCLC): Moving from targeted therapy to immunotherapy. Semin. Cancer Biol. 2018, 52, 103–109.
- Inamura, K. Lung Cancer: Understanding Its Molecular Pathology and the 2015 WHO Classification. Front. Oncol. 2017, 7, 193.
- Duma, N.; Santana-Davila, R.; Molina, J.R. Non-Small Cell Lung Cancer: Epidemiology, Screening, Diagnosis, and Treatment. Mayo Clin. Proc. 2019, 94, 1623–1640.
- Herbst, R.S.; Morgensztern, D.; Boshoff, C. The biology and management of non-small cell lung cancer. Nature 2018, 553, 446–454.
- Tamura, T.; Kurishima, K.; Nakazawa, K.; Kagohashi, K.; Ishikawa, H.; Satoh, H.; Hizawa, N. Specific organ metastases and survival in metastatic non-small-cell lung cancer. Mol. Clin. Oncol. 2015, 3, 217–221.
- Malhotra, J.; Malvezzi, M.; Negri, E.; La Vecchia, C.; Boffetta, P. Risk factors for lung cancer worldwide. Eur. Respir. J. 2016, 48, 889–902.
- Raaschou-Nielsen, O.; Andersen, Z.J.; Beelen, R.; Samoli, E.; Stafoggia, M.; Weinmayr, G.; Hoffmann, B.; Fischer, P.; Nieuwenhuijsen, M.J.; Brunekreef, B.; et al. Air pollution and lung cancer incidence in 17 European cohorts: Prospective analyses from the European Study of Cohorts for Air Pollution Effects (ESCAPE). Lancet Oncol. 2013, 14, 813–822.
- Kanwal, M.; Ding, X.-J.; Cao, Y. Familial risk for lung cancer. Oncol. Lett. 2017, 13, 535–542.
- Naylor, E.C.; Desani, J.K.; Chung, P.K. Targeted Therapy and Immunotherapy for Lung Cancer. Surg. Oncol. Clin. N. Am. 2016, 25, 601–609.
- Baudino, T.A. Targeted Cancer Therapy: The Next Generation of Cancer Treatment. Curr. Drug. Discov. Technol. 2015, 12, 3–20.
- Gridelli, C.; Rossi, A.; Carbone, D.P.; Guarize, J.; Karachaliou, N.; Mok, T.; Petrella, F.; Spaggiari, L.; Rosell, R. Non-small-cell lung cancer. Nat. Rev. Dis. Primers 2015, 1, 1–16.
- Lu, T.; Yang, X.; Huang, Y.; Zhao, M.; Li, M.; Ma, K.; Yin, J.; Zhan, C.; Wang, Q. Trends in the incidence, treatment, and survival of patients with lung cancer in the last four decades. Cancer Manag. Res. 2019, 11, 943–953.
- Dietrich, M.F.; Gerber, D.E. Chemotherapy for Advanced Non-small Cell Lung Cancer. Cancer Treat. Res. 2016, 170, 119–149.
- Lee, S.H. Chemotherapy for Lung Cancer in the Era of Personalized Medicine. Tuberc. Respir. Dis. 2019, 82, 179–189.
- Baxevanos, P.; Mountzios, G. Novel chemotherapy regimens for advanced lung cancer: Have we reached a plateau? Ann. Transl. Med. 2018, 6, 139.
- Vasconcellos, V.F.; Marta, G.N.; da Silva, E.M.; Gois, A.F.; de Castria, T.B.; Riera, R. Cisplatin versus carboplatin in combination with third-generation drugs for advanced non-small cell lung cancer. Cochrane Database Syst. Rev. 2020, 1, CD009256.
- Minami, S.; Kijima, T. Pemetrexed in maintenance treatment of advanced non-squamous non-small-cell lung cancer. Lung Cancer 2015, 6, 13–25.
- Assoun, S.; Brosseau, S.; Steinmetz, C.; Gounant, V.; Zalcman, G. Bevacizumab in advanced lung cancer: State of the art. Future Oncol. 2017, 13, 2515–2535.
- Lima, A.B.C.; Macedo, L.T.; Sasse, A.D. Addition of bevacizumab to chemotherapy in advanced non-small cell lung cancer: A systematic review and meta-analysis. PLoS ONE 2011, 6, e22681.
- Qiang, H.; Chang, Q.; Xu, J.; Qian, J.; Zhang, Y.; Lei, Y.; Han, B.; Chu, T. New advances in antiangiogenic combination therapeutic strategies for advanced non-small cell lung cancer. J. Cancer Res. Clin. Oncol. 2020, 146, 631–645.
- Zhou, C.; Wu, Y.-L.; Chen, G.; Liu, X.; Zhu, Y.; Lu, S.; Feng, J.; He, J.; Han, B.; Wang, J.; et al. BEYOND: A Randomized, Double-Blind, Placebo-Controlled, Multicenter, Phase III Study of First-Line Carboplatin/Paclitaxel Plus Bevacizumab or Placebo in Chinese Patients With Advanced or Recurrent Nonsquamous Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2015, 33, 2197–2204.
- Hang, X.F.; Xu, W.S.; Wang, J.X.; Wang, L.; Xin, H.G.; Zhang, R.Q.; Ni, W. Risk of high-grade bleeding in patients with cancer treated with bevacizumab: A meta-analysis of randomized controlled trials. Eur. J. Clin. Pharmacol. 2011, 67, 613–623.
- Weiss, J. Bevacizumab in adjuvant treatment of non-small-cell lung cancer. Lancet Oncol. 2017, 18, 1558–1560.
- De Mello, R.A.; Aguiar, P.N.; Tadokoro, H.; Farias-Vieira, T.M.; Castelo-Branco, P.; de Lima Lopes, G.; Pozza, D.H. MetaLanc9 as a novel biomarker for non-small cell lung cancer: Promising treatments via a PGK1-activated AKT/mTOR pathway. J. Thorac Dis. 2018, 10 (Suppl. 17), S2076–S2078.
- Garon, E.B.; Ciuleanu, T.-E.; Arrieta, O.; Prabhash, K.; Syrigos, K.N.; Goksel, T.; Park, K.; Gorbunova, V.; Kowalyszyn, R.D.; Pikiel, J.; et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): A multicentre, double-blind, randomised phase 3 trial. Lancet 2014, 384, 665–673.
- Adrianzen Herrera, D.; Ashai, N.; Perez-Soler, R.; Cheng, H. Nanoparticle albumin bound-paclitaxel for treatment of advanced non-small cell lung cancer: An evaluation of the clinical evidence. Expert Opin. Pharmacother. 2019, 20, 95–102.
- Kundranda, M.N.; Niu, J. Albumin-bound paclitaxel in solid tumors: Clinical development and future directions. Drug Des. Dev. Ther. 2015, 9, 3767–3777.
- Socinski, M.A.; Bondarenko, I.; Karaseva, N.A.; Makhson, A.M.; Vynnychenko, I.; Okamoto, I.; Hon, J.K.; Hirsh, V.; Bhar, P.; Zhang, H.; et al. Weekly nab-paclitaxel in combination with carboplatin versus solvent-based paclitaxel plus carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer: Final results of a phase III trial. J. Clin. Oncol. 2012, 30, 2055–2062.
- Ettinger, D.S.; Aisner, D.L.; Wood, D.E.; Akerley, W.; Bauman, J.; Chang, J.Y.; Chirieac, L.R.; D’Amico, T.A.; Dilling, T.J.; Dobelbower, M.; et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 5.2018. J. Natl. Compr. Cancer Netw. 2018, 16, 807–821.




