
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Emanuele Capra | + 2851 word(s) | 2851 | 2020-11-13 06:42:14 | | | |
| 2 | Dean Liu | -1291 word(s) | 1560 | 2020-11-20 06:41:44 | | |
Video Upload Options
Secretory extracellular vesicles (EVs) are membrane-enclosed microparticles that mediate cell to cell communication in proximity to, or distant from, the cell of origin. Extracellular vesicles mediate temporal and spatial interaction during many events in sexual reproduction and supporting embryo-maternal dialogue. Molecular characterization of EVs isolated in physiological and pathological conditions may increase our understanding of reproductive and obstetric diseases and assist the search for potential non-invasive biomarkers. Moreover, a more precise vision of the cocktail of biomolecules inside the EVs mediating communication between the embryo and mother could provide new insights to optimize the therapeutic action and safety of EV use.
1. Introduction
In mammals, the female reproductive trait, comprising ovaries, oviducts and uterus, plays a crucial role in the regulation of early and late reproductive events and provides the optimal environment for embryonic development. Secretory extracellular vesicles (EVs) are important for cell to cell communication, as they have a paracrine function being able to diffuse over a relatively short distance and produce a local action. It has been largely demonstrated that EV-associated activity is fundamental to reproductive success, mediating the fine-tuning of cellular cross-talk in the reproductive system, promoting embryo implantation and assisting successful pregnancy[1]. Especially in reproduction fields, many early studies used low-specific EV isolation methods that often co-isolated other soluble molecules such as growth factors, cytokines and metabolites, making difficult to attribute the activity specifically to EVs and not instead to low amount of other highly active soluble mediators. Extracellular vesicles (EVs) are membrane bound organelles which can convey information between cells through the transfer of functional protein and genetic information to alter phenotype and function of recipient cells[2][3]. Many recent reviews highlight the role of EVs in oogenesis, oocyte maturation and fertilization, embryo-maternal cross talk in the oviduct and embryo implantation[1][4][5][6][7][8][9][10][11][12][13].
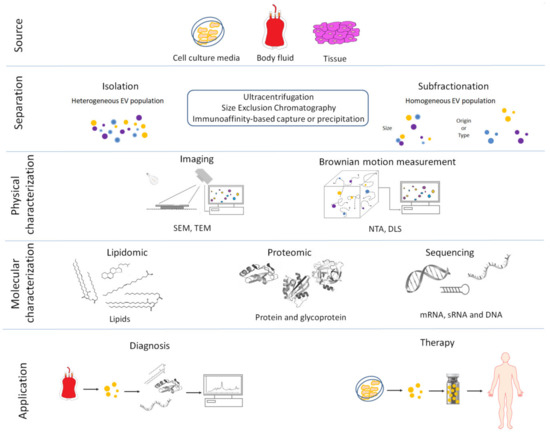
Figure 1. Overview of the main procedures used for the isolation, characterization and clinical use of extracellular vesicles (EVs). (1) Extracellular vesicles are released by cells into the culture media or from tissue into the extracellular environment. (2) Extracellular vesicles are separated or further purified to obtain a more homogeneous EV population using a variety of methods. (3) Isolated EVs are physically characterized by: Scanning Electron Microscopes (SEM), Transmission Electron Microscopes (TEM), Nanoparticle Tracking Analysis (NTA) and Dynamic Light Scattering (DLS). (4) Extracellular vesicles are molecularly characterized using several techniques. (5) Biomarkers can be obtained by molecular profiling of isolated EVs from in vitro cell cultures or body fluids in pathologic condition and used as diagnostic tools for several human and veterinary diseases. Isolated EVs can also be used as treatments in human and veterinary medicine.
2. Extracellular Vesicles and Biogenesis
Membrane-enclosed microparticles were first isolated from biological materials such as animal platelet-free plasma[14], human seminal plasma[15] and from a variety of mammalian cell lines[16]. The isolated vesicles were assumed to originate from outward budding of plasma membranes. It was not until years later that a novel pathway involving active vesicle secretion was described in reticulocytes and the term exosomes was used to define intracellular endosome vesicles released by exocytosis[17]. Although EVs have been isolated from all types of organisms including Archaea[18], Bacteria[19] and Eukarya[20], in animals they were classified into two main categories: exosomes and microvesicles depending on their size and biogenesis. The smallest exosomes (ranging in size between 50 and 150 nm in diameter) originate from the multivesicular endolytic compartment by the fusion of multivesicular endosome with the plasma membrane[21][22]. Microvesicles (ranging in size between 50 nm and 1,000 nm) are shed by an active outward budding mechanism mediated by reorganization of the actin-myosin cytoskeleton[23][24].
3. Methods for EV Isolation and Characterization
Cells produce and release a heterogeneous spectrum of EVs differing in size and chemical and physical characteristics. Many methods based on centrifugation, filtration, precipitation and affinity interaction have been used to isolate EVs[25]. The isolation of EVs from complex body fluids such as blood or follicular fluid often requires a combination of different techniques such as differential centrifugation, density gradient centrifugation, filtration, affinity-based method precipitation with polymers such as polyethylene glycol and size exclusion chromatography [25][26][27][28][29][30][31]. Recently, a new type of small (<50 nm), non-membranous nanoparticle named exomeres was separated from various secreted vesicles using asymmetric flow field-flow fractionation technology and ultracentrifugation[32][33]. Isolation efficiency is dependent on the type of sample and the research purposes and procedures must be carefully standardized[34].
Each method present advantages and disadvantages and should be carefully chosen based on sample type, downstream application and scientific question. Some indications are reported in the guidelines presented by international EV body, ISEV[34][35] and summarized in Table 1.
Table 1. Summary of EV isolation techniques and main advantages (pros) and disadvantages (cons) for different methods.
| Method | Principle | Advantages | Disadvantages |
|---|---|---|---|
| Differential centrifugation | EVs isolation after different consequent centrifugation steps (from 300g to 100,000 g): depletion of cells, platelets and large apoptotic bodies by low-speed centrifugation steps. Larger EVs are pelleted at 10,000 20,000 g range. Smaller EVs are then pelleted at high speed (100,000 120,000 g). | Most used, intermediated recovery and specificity | Time consuming and extravesicular proteins complexes/aggregates, lipoprotein particles, and other contaminants may also sediment |
| Density gradient centrifugation | EVs isolation through density gradients of sucrose or iohexol or iodixanol | High purity (EVs float upward or move downwards into an overlaid density gradient) | Applicable to small volume (usually after differential centrifugation), sucrose or iohexol or iodixanol can influence downstream application |
| Filtration | EVs filtration with different molecular weight cutoff | Recovery and purity depend on the consequent centrifugation step and the cutoff of centrifugal filter employ | Low specificity, EV populations may adhere to the filter or filtering may cause deformation and breakup of large vesicles |
| Precipitation | EVs are precipitated with organic solvent or in presence of different chemical compound such as polyethylene glycol (PEG), sodium acetate or protamine | High recovery, fast and easy | Low specificity Coprecipitation of numerous non-EV contaminants such as lipoproteins. Rigorous assessment of contaminating particle is recommended |
| Size Exclusion Chromatography | EVs are separated by their ability to pass through a resin packed in a column | Well separated EVs from protein complexes biofluids | Not suitable for large volume |
| Affinity isolation | EVs bind specific antibodies against exosome-specific surface proteins or EV-binding molecules | High purity | Low recovery, requires specific antibody |
| Microfluidic devices | Microfluidics-based on-chip EVs isolation based on immunoaffinity, membrane filtration, nanowire-based traps, nano-sized deterministic lateral displacement, viscoelastic flow and acoustic isolation | high-throughput and low processing time | Not suitable for large volume |
| Nanoscale flow cytometric sorting | Fluorescent labelled EVs are sorted using flow cytometer | Very specific and high purity | Laborious and time consuming |
All procedures include methods for evaluation of vesicle morphology, size distribution and concentration so that the purified EVs can be correctly identified. A topographical EV view is obtained by direct imaging methods including scanning (SEM),transmission electronic microscopy (TEM) and cryo-electron microscopy (cryo-EM) that provide high resolution imaging and a more precise evaluation of vesicular size[36][37]. However, both techniques require laborious sample preparation procedures that limit sample throughput. Methods based on measurement of the Brownian motion of suspended particles include nanoparticle tracking analysis (NTA) and dynamic light scattering (DLS), or flow cytometry (FC) and tunable resistive pulse sensing (TRPS) methods can be used to measure EV concentration in solution or for determination of the EV size ranges when high-throughput information is desired. However, the detection of EVs becomes difficult when large vesicles are present in dynamic light scattering methods[38][39]. Noble et al.[40]reviewed a comparison between electron microscopy and optical methods for EV detection. Isolated vesicles are likely to be a combination of microvesicles and exosomes varying in size[40]. Thus, further biochemical characterization is needed to provide information on EV composition. Extracellular vesicle preparation identity and purity must be evaluated by detecting the presence of anchored protein localized at the external membrane such as transmembrane (CD63, CD81, CD82, CD47) or GPI-anchored protein (CD73) and the absence of major constituent proteins of non EV structures that co-isolate with EVs such as albumin (ALB) and apolipoproteins A1/2 and B (APO1/2, APOB). In addition, to evaluate EV integrity of lipid bilayers the presence of cytosolic protein (ALIX, heat shock proteins HSPs) should be taken into consideration[35]. Subtype characterization can be obtained by detecting the presence of proteins associated to different intracellular compartments. Extracellular vesicles subtypes separation from peripheral blood confirmed the specific isolation of microvescicles using specific antibody targeting proteins, reflecting their biogenesis such as tubulin, actinin-4 or mitofilin for microvesicles and tetraspanin antibodies (e.g., CD9, CD81) for exosomes[41].Protein markers for EV characterization are detected using different technologies. Western blotting and enzyme-linked immunosorbent assay (ELISA) are the most commonly used method to evaluate the presence of EV associated markers. In general, both techniques require a large sample volume and long processing. The limit of detection is similar for both methods, but ELISA can be easily scaled up for higher-throughput measurements. In alternative, EV protein composition can be assessed by mass spectrometry, that provides quantitative and comparative EV proteomic characterization, although requires significant preparatory and processing time. Surface protein marker can be detected using other methods such as small particle flow cytometry, surface plasma resonance. Methods for EV protein characterization were extensively reviewed by Shao et al.[42].
Extracellular vesicles represent an important mode of intercellular communication by facilitating the horizontal cell to cell transfer of lipids, proteins, RNA species and DNA fragments. However, the functionality and characterization of EV molecular cargo is not always easy to interpret due to the presence of different isolate subtypes and the inability to further separate and to stratify these into distinct sub-populations[43][44].
References
- Ng, Y.H.; Rome, S.; Jalabert, A.; Forterre, A.; Singh, H.; Hincks, C.L.; Salamonsen, L.A. Endometrial exosomes/microvesicles in the uterine microenvironment: A new paradigm for embryo-endometrial cross talk at implantation. PLoS ONE 2013, 8, e58502.
- Xu, R.; Greening, D.W.; Zhu, H.J.; Takahashi, N.; Simpson, R.J. Extracellular vesicle isolation and characterization: Toward clinical application. J. Clin. Invest. 2016, 126, 1152–1162.
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289.
- Andronico, F.; Battaglia, R.; Ragusa, M.; Barbagallo, D.; Purrello, M.; Di Pietro, C. Extracellular vesicles in human oogenesis and implantation. Int. J. Mol. Sci. 2019, 20, 2162.
- Almiñana, C.; Bauersachs, S. Extracellular vesicles: Multi-signal messengers in the gametes/embryo-oviduct cross-talk. Theriogenology 2020, 150, 59–69.
- Bridi, A.; Perecin, F.; Silveira, J.C.D. Extracellular vesicles mediated early embryo-maternal interactions. Int. J. Mol. Sci. 2020, 21, 1163.
- Gurung, S.; Greening, D.W.; Catt, S.; Salamonsen, L.; Evans, J. Exosomes and soluble secretome from hormone-treated endometrial epithelial cells direct embryo implantation. Mol. Hum. Reprod. 2020, 26, 510–520.
- Vilella, F.; Moreno-Moya, J.M.; Balaguer, N.; Grasso, A.; Herrero, M.; Martinez, S.; Marcilla, A.; Simon, C. Hsa-miR-30d, secreted by the human endometrium, is taken up by the pre-implantation embryo and might modify its transcriptome. Development 2015, 142, 3210–3221.
- Balaguer, N.; Moreno, I.; Herrero, M.; González, M.; Simón, C.; Vilella, F. Heterogeneous nuclear ribonucleoprotein C1 may control miR-30d levels in endometrial exosomes affecting early embryo implantation. Mol. Hum. Reprod. 2018, 24, 411–425.
- Greening, D.W.; Nguyen, H.P.; Elgass, K.; Simpson, R.J.; Salamonsen, L.A. Human endometrial exosomes contain hormone-specific cargo modulating trophoblast adhesive capacity: Insights into endometrial-embryo interactions. Biol. Reprod. 2016, 94, 38.
- Evans, J.; Rai, A.; Nguyen, H.P.T.; Poh, Q.H.; Elglass, K.; Simpson, R.J.; Salamonsen, L.A.; Greening, D.W. Human endometrial extracellular vesicles functionally prepare human trophectoderm model for implantation: Understanding bidirectional maternal-embryo communication. Proteomics 2019, 19, e1800423.
- Simon, C.; Greening, D.W.; Bolumar, D.; Balaguer, N.; Salamonsen, L.A.; Vilella, F. Extracellular vesicles in human reproduction in health and disease. Endocr. Rev. 2018, 39, 292–332.
- Burnett, L.A.; Nowak, R.A. Exosomes mediate embryo and maternal interactions at implantation and during pregnancy. Front. Biosci. (Schol Ed.) 2016, 8, 79–96.
- Crawford, N. The presence of contractile proteins in platelet microparticles isolated from human and animal platelet-free plasma. Br. J. Haematol. 1971, 21, 53–69.
- Stegmayr, B.; Ronquist, G. Promotive effect on human sperm progressive motility by prostasomes. Urol. Res. 1982, 10, 253–257.
- Trams, E.G.; Lauter, C.J.; Salem, N., Jr.; Heine, U. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim. Biophys. Acta 1981, 645, 63–70.
- Johnstone, R.M.; Hammond, J.R.; Turbide, C. Vesicle formation during reticulocyte maturation. association of plasma membrane activities with released vesicles (exosomes). J. Biol. Chem. 1987, 262, 9412–9420.
- Makarova, K.S.; Yutin, N.; Bell, S.D.; Koonin, E.V. Evolution of diverse cell division and vesicle formation systems in Archaea. Nat. Rev. Microbiol. 2010, 8, 731–741.
- Kim, J.H.; Lee, J.; Park, J.; Gho, Y.S. Gram-negative and Gram-positive bacterial extracellular vesicles. Semin. Cell Dev. Biol. 2015, 40, 97–104.
- Oliveira, D.L.; Nakayasu, E.S.; Joffe, L.S.; Guimarães, A.J.; Sobreira, T.J.; Nosanchuk, J.D.; Cordero, R.J.; Frases, S.; Casadevall, A.; Almeida, I.C.; et al. Characterization of yeast extracellular vesicles: Evidence for the participation of different pathways of cellular traffic in vesicle biogenesis. PLoS ONE 2010, 5, e11113.
- Harding, C.; Heuser, J.; Stahl, P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J. Cell Biol. 1983, 97, 329–339.
- Pan, B.T.; Teng, K.; Wu, C.; Adam, M.; Johnstone, R.M. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J. Cell Biol. 1985, 101, 942–948.
- McConnell, R.E.; Higginbotham, J.N.; Shifrin, D.A., Jr; Tabb, D.L.; Coffey, R.J.; Tyska, M.J. The enterocyte microvillus is a vesicle-generating organelle. J. Cell Biol. 2009, 185, 1285–1298.
- Muralidharan-Chari, V.; Clancy, J.; Plou, C.; Romao, M.; Chavrier, P.; Raposo, G.; D’Souza-Schorey, C. ARF6-regulated shedding of tumor cell-derived plasma membrane microvesicles. Curr. Biol. 2009, 19, 1875–1885.
- Konoshenko, M.Y.; Lekchnov, E.A.; Vlassov, A.V.; Laktionov, P.P. Isolation of extracellular vesicles: General methodologies and latest trends. Biomed. Res. Int. 2018, 2018, 8545347.
- Böing, A.N.; van der Pol, E.; Grootemaat, A.E.; Coumans, F.A.; Sturk, A.; Nieuwland, R. Single-step isolation of extracellular vesicles by size-exclusion chromatography. J. Extracell. Vesicles 2014, 3, 23430.
- Deregibus, M.C.; Figliolini, F.; D’Antico, S.; Manzini, P.M.; Pasquino, C.; De Lena, M.; Tetta, C.; Brizzi, M.F.; Camussi, G. Charge-based precipitation of extracellular vesicles. Int. J. Mol. Med. 2016, 38, 1359–1366.
- Kenigsberg, S.; Wyse, B.A.; Librach, C.L.; da Silveira, J. Protocol for exosome isolation from small volume of ovarian follicular fluid: Evaluation of ultracentrifugation and commercial kits. Methods Mol. Biol. 2017, 1660, 321–341.
- Brennan, K.; Martin, K.; FitzGerald, S.P.; O’Sullivan, J.; Wu, Y.; Blanco, A.; Richardson, C.; Mc Gee, M.M. A comparison of methods for the isolation and separation of extracellular vesicles from protein and lipid particles in human serum. Sci. Rep. 2020, 10, 1039.
- Nakai, W.; Yoshida, T.; Diez, D.; Miyatake, Y.; Nishibu, T.; Imawaka, N.; Naruse, K.; Sadamura, Y.; Hanayama, R. A novel affinity-based method for the isolation of highly purified extracellular vesicles. Sci. Rep. 2016, 6, 33935.
- Sidhom, K.; Obi, P.O.; Saleem, A. A Review of exosomal isolation methods: Is size exclusion chromatography the best option? Int. J. Mol. Sci. 2020, 21, 6466.
- Zhang, H.; Lyden, D. Asymmetric-flow field-flow fractionation technology for exomere and small extracellular vesicle separation and characterization. Nat. Protoc. 2019, 14, 1027–1053.
- Zhang, Q.; Higginbotham, J.N.; Jeppesen, D.K.; Yang, Y.P.; Li, W.; McKinley, E.T.; Graves-Deal, R.; Ping, J.; Britain, C.M.; Dorsett, K.A.; et al. Transfer of functional cargo in exomeres. Cell Rep. 2019, 27, 940–954.
- Witwer, K.W.; Buzás, E.I.; Bemis, L.T.; Bora, A.; Lässer, C.; Lötvall, J.; Nolte-’t Hoen, E.N.; Piper, M.G.; Sivaraman, S.; Skog, J.; et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J. Extracell. Vesicles 2013, 2.
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750.
- Wu, Y.; Deng, W.; Klinke, D.J., 2nd. Exosomes: Improved methods to characterize their morphology, RNA content, and surface protein biomarkers. Nanoparticle Tracking Analysis (NTA) is commonly used to determine EV concentration and diameter. Analyst 2015, 140, 6631–6642.
- Cizmar, P.; Yuana, Y. Detection and characterization of extracellular vesicles by transmission and cryo-transmission electron microscopy. Methods Mol. Biol. 2017, 1660, 221–232.
- Szatanek, R.; Baj-Krzyworzeka, M.; Zimoch, J.; Lekka, M.; Siedlar, M.; Baran, J. The Methods of choice for extracellular vesicles (EVs) characterization. Int. J. Mol. Sci. 2017, 18, 1153.
- Kim, A.; Ng, W.B.; Bernt, W.; Cho, N.J. Validation of size estimation of nanoparticle tracking analysis on polydisperse macromolecule assembly. Sci. Rep. 2019, 9, 2639.
- Noble, J.M.; Roberts, L.M.; Vidavsky, N.; Chiou, A.E.; Fischbach, C.; Paszek, M.J.; Estroff, L.A.; Kourkoutis, L.F. Direct comparison of optical and electron microscopy methods for structural characterization of extracellular vesicles. J. Struct. Biol. 2020, 210, 107474.
- Menck, K.; Bleckmann, A.; Schulz, M.; Ries, L.; Binder, C. Isolation and characterization of microvesicles from peripheral blood. J. Vis. Exp. 2017, 119, 55057.
- Shao, H.; Im, H.; Castro, C.M.; Breakefield, X.; Weissleder, R.; Lee, H. New technologies for analysis of extracellular vesicles. Chem. Rev. 2018, 118, 1917–1950.
- Greening, D.W.; Simpson, R.J. Understanding extracellular vesicle diversity - current status. Expert Rev. Proteom. 2018, 15, 887–910.
- Claridge, B.; Kastaniegaard, K.; Stensballe, A.; Greening, D.W. Post-translational and transcriptional dynamics—Regulating extracellular vesicle biology. Expert Rev. Proteom. 2019, 16, 17–31.




