
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sara Isabel Pereira | + 1364 word(s) | 1364 | 2019-11-22 09:57:43 | | | |
| 2 | Nicole Yin | Meta information modification | 1364 | 2019-12-12 09:46:32 | | | | |
| 3 | Nicole Yin | + 2 word(s) | 1366 | 2020-07-30 11:53:13 | | |
Video Upload Options
Biopolymers derived from polysaccharides are a sustainable and environmentally friendly alternative to the synthetic counterparts available in the market. Due to their distinctive properties, the cyanobacterial extracellular polymeric substances (EPS), mainly composed of heteropolysaccharides, are a valid alternative to address several biotechnological and biomedical challenges. However, for the successful exploitation of cyanobacterial EPS, it is important to obtain polymers with high purity levels and exploit different strategies of functionalization to obtain EPS tailored for given applications. Considering all this, available approaches to isolate, purify and chemically modify cyanobacterial EPS are discussed.
1. Definition
Most cyanobacteria produce extracellular polymeric substances (EPS), mainly composed by heteropolysaccharides, with a distinctive set of industrially-desirable features including (i) strong anionic nature, (ii) presence of sulfate groups, (iii) high variety of possible structural conformations and (iv) amphiphilic behavior [1][2].
2. Introduction
In the last years, significant advances were made on the characterization of cyanobacterial EPS [2][3] and the validation of their biotechnological and biomedical potential [4][5][6][7][8][9][10]. However, the development of biomaterials based on these polymers has only recently started to be explored [11][12][13][14]. In this context, the repertoire of cyanobacterial EPS-based applications such as scaffolds, coatings, and drug carriers can be significantly expanded by optimizing their isolation/purification and obtaining designer biopolymers with specialized features.
3. Isolation and Purification of cyanobacterial EPS
The isolation and purification of the polymers must be cost effective, scalable, and easy to perform. It is also important to take into consideration that the methods selected influence the polymers’ yield and quality [15] and, thus, it may be necessary to adapt the protocols to the characteristics of the polymers and their final application [16]. One of the main aspects to consider is whether the EPS are attached to the cells or released to the culture medium (RPS). In the case of the EPS attached to the cells, detachment can be achieved using formaldehyde, glutaraldehyde, ethylenediaminetetraacetic acid (EDTA), sodium hydroxide, sonication, heating, cell washing with water, complexation, or ionic resins [15][17]. To select one of these methods, it is important to not only evaluate the yield, but also the levels of contamination of the polysaccharides with other cellular components. Regarding the isolation of RPS, the conventional methods based on precipitation techniques have been described recently (see [16]). Despite their efficiency, the costs and requirement of large amounts of precipitating agents led to the search of alternative techniques more suitable at the industrial scale, such as tangential ultrafiltration [17][18]. However, this methodology may need to be improved to minimize the problems of high viscosity of polymer solutions resulting in membrane clogging [17]. Tangential ultrafiltration can also be used to obtain a concentrated polymer solution before precipitation or spray-drying, increasing the efficiency of these processes.
After isolation of the EPS, contaminants such as inorganic salts, heavy metals, proteins, polyphenols, endotoxins, nucleic acids, or cell debris may still be present in the polymer solution. However, it is necessary to have polysaccharides with high purity levels to accurately determine their structure and composition and to obtain reproducible results for therapeutic applications [19]. Inorganic salts, monosaccharides, oligosaccharides and low molecular weight non-polar substances can be removed by dialysis. The choice of device, the molecular weight cut-off, and duration of the dialysis is very important to determine the success of this method. However, at an industrial scale, dialysis may not be a viable option. An alternative way to remove inorganic salts is through ion exchange resins, normally in the form of beads [20]. Removal of peptides and proteins can be achieved using different methods, including protease (e.g., pronase) treatment or the Sevag method (usually less efficient) [20][21]. Trichlorotrifluoroethane and trichloroacetic acids can also be used to remove proteins from the polysaccharide’s solution. However, it is necessary to consider that the first is highly volatile and, thus, has to be employed at 4 °C limiting its use, while the trichloroacetic acid is widely used but its acidity can damage the polymer structure [20][21]. The levels of polyphenol contaminants are usually reduced with charcoal washes and centrifugations, hydrogen peroxide method or functionalized resins with imidazole and pyridine [19][22]. The selection of the best purification methods depends on the characteristics of the polymers, the methods used for their isolation, and the envisaged application.
The presence of endotoxins is one of the major issues to be addressed before any biomaterial is consider safe to be used. Endotoxins are mainly due to the presence of LPS, with lipid A being responsible for most of the biological activity of these contaminants [23]. Endotoxins can significantly affect the biological effects of the polymers by eliciting a wide range of cellular responses that compromise cell viability [24][25]. Therefore, limits are imposed by regulatory entities ([26], pp. 171–175, 520–523) As an example, the food and drug administration (FDA) adopted the US Pharmacopoeia endotoxin reference standard, limiting the amount of endotoxins in eluates from medical devices to 0.5 Eurotoxin Units (EU)/mL [27]. Endotoxins are highly heat-stable and not easily destroyed by standard autoclave programs [28]. However, they can be removed by other techniques including ultrafiltration, two-phase extraction, and adsorption [23], although the efficiency of these methods depends on the characteristics of the polymer.
Depending on the application, it may be necessary to isolate fractions of the polymers with specific molecular weights. Fractionation is usually achieved by ultracentrifugation, with the added advantage of simultaneous elimination of contaminants [29]. Filtration and ultrafiltration are also popular alternatives, however, depending on the material of the filter membrane, the polysaccharides can be retained in the filter, decreasing the yield of the purification [30]. Other methods include affinity chromatography, gel chromatography, anion exchange chromatography, cellulose column chromatography, quaternary ammonium salt precipitation, graded precipitation methods, and preparative zone electrophoresis (reviewed in [20]).
4. Chemical functionalization of Cyanobacterial EPS
The development of polysaccharide-based biomaterials often requires the chemical functionalization of the polymers. In this context, the characteristics of cyanobacterial EPS, offer a vast range of opportunities for targeted modifications (Figure 1). Successful examples of these functionalization reactions have already been described for other bacterial EPS [31][32][33][34][35][36][37][38]. The hydroxyl groups present in hexoses, pentoses, deoxyhexoses, uronic acids, and aminosugars can act as nucleophiles in base-catalyzed esterification reactions in the presence of anhydrides, esters, or carboxylic acids (Figure 1A–C). This strategy has been successfully used to fabricate photocrosslinkable hydrogels based on dextran and hyaluronic acid [32][34][38]. Another approach consists of the oxidation of diols in the presence of sodium periodate to generate reactive aldehydes, which can further react with primary amines in reductive amination reactions (Figure 1D) to produce hydrogels [31][36]. Hydroxyl groups can also undergo free radical polymerization reactions to generate graft copolymers for drug delivery (Figure 1E), as previously demonstrated for xanthan gum [33]. The carboxylic groups present in uronic acid residues allow the polymers’ functionalization through esterification or carbodiimide reactions, with the latter being particularly interesting for bioconjugation (Figure 1F) [35][37]. On the other hand, free amino groups from glucosamine residues can react with anhydrides and carboxylic acids to form amides (Figure 1G,H), or with aldehydes to form Schiff bases, which can be further reduced to imines. Overall, these chemical modifications are valuable strategies to obtain designer polymers with improved properties suitable for the development of novel biomaterials.
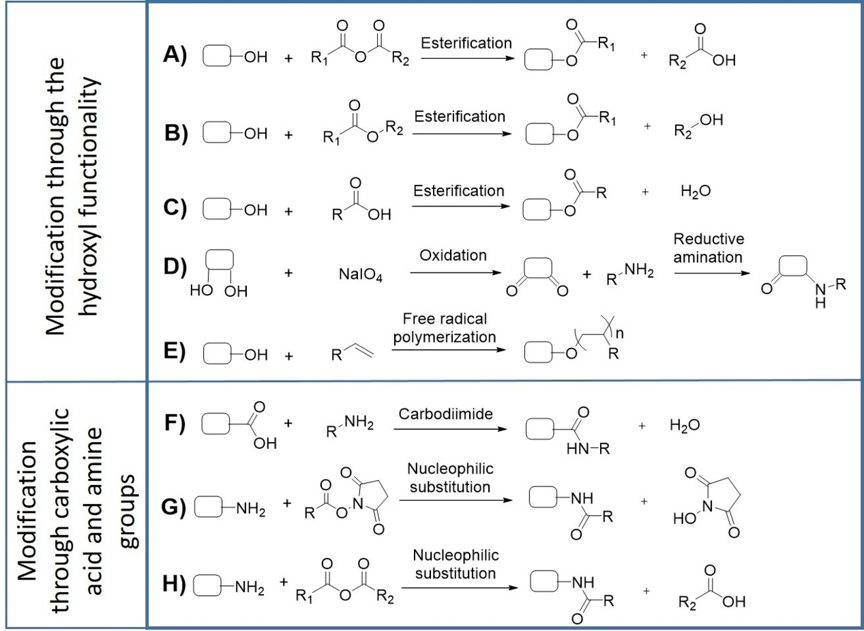
Figure 1. Representative strategies for functionalization of EPS by chemical modification. EPS can undergo esterification with anhydrides (A), esters (B), carboxylic acids (C), periodate-mediated oxidation followed by reductive amination (D), free radical polymerization with vinyl moiety (E), carbodiimide coupling (F) and nucleophilic substitution with esters and anhydrides (G,H), depending on the target functional group.
For their use in biomedical applications, the polymers and/or derived biomaterials have to be biocompatible, i.e., be able to “perform with an appropriate host response in a specific application” [39]. Biocompatibility is usually evaluated in vitro by accessing the effects that biopolymers or biomaterials have on living cells [40]. Several guidelines are described in international standard protocols, with the material’s toxicity (defined as cytotoxicity) being the most common and widely used parameter evaluated (ISO 10993-5) [41]. Depending on the application, biotolerability, i.e., “the ability to reside in the body for long periods of time with only low degrees of inflammatory reaction” is an important issue to consider. This property is particularly important for non-degrading or slow-degrading implant materials [39]. Other important biosafety tests include the evaluation of the mutagenic and carcinogenic potential [42][43].
References
- Pereira, S.; Zille, A.; Micheletti, E.; Moradas-Ferreira, P.; De Philippis, R.; Tamagnini, P.; Complexity of cyanobacterial exopolysaccharides: composition, structures, inducing factors and putative genes involved in their biosynthesis and assembly. FEMS Microbiol. Rev. 2009, 33, 917-941, 10.1111/j.1574-6976.2009.00183.x.
- Rossi, F.; De Philippis, R.. The Physiology of Microalgae.; Borowitzka, M. A.; Beardall, J.; Raven, J. A., Eds.; Springer International Publishing: Switzerland, 2016; pp. 565-590.
- Mota, R.; Guimaraes, R.; Buttel, Z.; Rossi, F.; Colica, G.; Silva, C. J.; Santos, C.; Gales, L.; Zille, A.; De Philippis, R.; et al.Pereira, S. B.Tamagnini, P. Production and characterization of extracellular carbohydrate polymer from Cyanothece sp. CCY 0110.. Carbohydr. Polym. 2013, 92, 1408-1415, 10.1016/j.carbpol.2012.10.070.
- De Philippis, R.; Colica, G.; Micheletti, E.; Exopolysaccharide-producing cyanobacteria in heavy metal removal from water: molecular basis and practical applicability of the biosorption process.. Appl. Microbiol. Biotechnol. 2011, 92, 697-708, 10.1007/s00253-011-3601-z.
- Mota, R.; Rossi, F.; Andrenelli, L.; Pereira, S. B.; De Philippis, R.; Tamagnini, P.; Released polysaccharides (RPS) from Cyanothece sp. CCY 0110 as biosorbent for heavy metals bioremediation: interactions between metals and RPS binding sites.. Appl. Microbiol. Biotechnol. 2016, 100, 7765-7775, 10.1007/s00253-016-7602-9.
- Han, P.-p.; Sun, Y.; Wu, X.-y.; Yuan, Y.-j.; Dai, Y.-j.; Jia, S.-r.; Emulsifying, Flocculating, and Physicochemical Properties of Exopolysaccharide Produced by Cyanobacterium Nostoc flagelliforme.. Appl. Biochem. Biotechnol. 2014, 172, 36-49, 10.1007/s12010-013-0505-7.
- Hussein, M. H.; Abou-ElWafa, G. S.; Shaaban-Dessuuki, S. A.; Hassan, N. I.; Characterization and Antioxidant Activity of Exopolysaccharide Secreted by Nostoc carneum. Int. J. Pharmacol. 2015, 11, 32-439, 10.3923/ijp.2015.432.439.
- Dewi, I. C.; Falaise, C.; Hellio, C.; Bourgougnon, N.; Mouget, J.-L.. Microalgae in Health and Disease Prevention; Levine, I. A.; Fleurence, J., Eds.; Academic Press: London, 2018; pp. 235-26.
- Majdoub, H.; Ben Mansour, M.; Chaubet, F.; Roudesli, M. S.; Maaroufi, R. M.; Anticoagulant activity of a sulfated polysaccharide from the green alga Arthrospira platensis.. Biochim. Biophys. Acta 2009, 1790, 1377-1381, 10.1016/j.bbagen.2009.07.013.
- Flores, C.; Lima, R. T.; Adessi, A.; Sousa, A.; Pereira, S. B.; Granja, P. L.; De Philippis, R.; Soares, P.; Tamagnini, P.; Characterization and antitumor activity of the extracellular carbohydrate polymer from the cyanobacterium Synechocystis ΔsigF mutant.. Int. J. Biol. Macromol. 2019, 136, 1219-1227, 10.1016/j.ijbiomac.2019.06.152.
- Bellini, E.; Ciocci, M.; Savio, S.; Antonaroli, S.; Seliktar, D.; Melino, S.; Congestri, R.; Trichormus variabilis (Cyanobacteria) Biomass: From the Nutraceutical Products to Novel EPS-Cell/Protein Carrier Systems.. Mar. Drugs 2018, 16, 298, 10.3390/md16090298.
- Estevinho, B. N.; Mota, R.; Leite, J. P.; Tamagnini, P.; Gales, L.; Rocha, F.; Application of a cyanobacterial extracellular polymeric substance in the microencapsulation of vitamin B12.. Powder Technol. 2019, 343, 644-651, 10.1016/j.powtec.2018.11.079.
- Leite, J. P.; Mota, R.; Durão, J.; Neves, S. C.; Barrias, C. C.; Tamagnini, P.; Gales, L.; Cyanobacterium-Derived Extracellular Carbohydrate Polymer for the Controlled Delivery of Functional Proteins. Macromol. Biosci. 2017, 17, 1600206, 10.1002/mabi.201600206.
- Costa, B.; Mota, R.; Parreira, P.; Tamagnini, P.; L. Martins, M. C.; Costa, F.; Broad-Spectrum Anti-Adhesive Coating Based on an Extracellular Polymer from a Marine Cyanobacterium. Mar. Drugs 2019, 17, 243, 10.3390/md17040243.
- Bhunia, B.; Prasad Uday, U. S.; Oinam, G.; Mondal, A.; Bandyopadhyay, T. K.; Tiwari, O. N.; Characterization, genetic regulation and production of cyanobacterial exopolysaccharides and its applicability for heavy metal removal.. Carbohydr. Polym. 2018, 179, 228-243, 10.1016/j.carbpol.2017.09.091.
- Flores, C.; Tamagnini, P.; Looking Outwards: Isolation of Cyanobacterial Released Carbohydrate Polymers and Proteins.. JoVE 2019, 147, e59590, doi:10.3791/59590.
- Delattre, C.; Pierre, G.; Laroche, C.; Michaud, P.; Production, extraction and characterization of microalgal and cyanobacterial exopolysaccharides.. Biotechnol. Adv. 2016, 34, 1159-1179, 10.1016/j.biotechadv.2016.08.001.
- Patel, A. K.; Laroche, C.; Marcati, A.; Ursu, A. V.; Jubeau, S.; Marchal, L.; Petit, E.; Djelveh, G.; Michaud, P.; Separation and fractionation of exopolysaccharides from Porphyridium cruentum. . Bioresour. Technol. 2013, 145, 345-350, 10.1016/j.biortech.2012.12.038.
- Ghisalberti, E. L.. Bioactive Natural Products: Detection, Isolation, and Structural Determination; Colegate, S. M.; Molyneux, R., Eds.; CRC Press: Boca Raton, USA, 2007; pp. 11-76.
- Shi, L.; Bioactivities, isolation and purification methods of polysaccharides from natural products: A review.. Int. J. Biol. Macromol. 2016, 92, 37-48, 10.1016/j.ijbiomac.2016.06.100.
- Chaplin, M. F.; Kennedy, J. F.. Carbohydrate Analysis: A Practical Approach; Oxford University Press: Oxford, U.K., 1994; pp. 1-244.
- Silva, M.; Castellanos, L.; Ottens, M.; Capture and Purification of Polyphenols Using Functionalized Hydrophobic Resins. . Ind. Eng. Chem. Res. 2018, 57, 5359-5369., 10.1021/acs.iecr.7b05071.
- Petsch, D.; Anspach, F. B.; Endotoxin removal from protein solutions.. J. Biotechnol. 2000, 76, 97-119, 10.1016/S0168-1656(99)00185-6.
- Gao, B.; Tsan, M.-F.; Endotoxin Contamination in Recombinant Human Heat Shock Protein 70 (Hsp70) Preparation Is Responsible for the Induction of Tumor Necrosis Factor α Release by Murine Macrophages.. J. Biol. Chem. 2003, 278, 174-179, 10.1074/jbc.M208742200.
- Suri, R. M.; Austyn, J. M.; Bacterial lipopolysaccharide contamination of commercial collagen preparations may mediate dendritic cell maturation in culture.. J. Immunol. Methods 1998, 214, 149-163, 10.1016/S0022-1759(98)00048-9.
- Commission, E. P., European Pharmacopoeia 7.0. In Council Of Europe: European Directorate for the Quality of Medicines and Healthcare, Strasbourg, France, 2010; pp 171–175, 520–523.
- Guidance for Industry Pyrogen and Endotoxins Testing: Questions and Answers; U.S. Department of Health and Human Services Food and Drug Administration: Washington, DC, USA, 2012.
- Gorbet, M. B.; Sefton, M. V.; Endotoxin: The uninvited guest. . Biomaterials 2005, 26, 6811-6817, 10.1016/j.biomaterials.2005.04.063.
- Zhang, Z.-P.; Shen, C.-C.; Gao, F.-L.; Wei, H.; Ren, D.-F.; Lu, J.; Isolation, Purification and Structural Characterization of Two Novel Water-Soluble Polysaccharides from Anredera cordifolia.. Molecules 2017, 22, 1276, 10.3390/molecules22081276.
- Li, J.; Chase, H. A.; Applications of membrane techniques for purification of natural products. . Biotechnol. Lett. 2010, 32, 601-608, 10.1007/s10529-009-0199-7.
- Azzam, T.; Eliyahu, H.; Makovitzki, A.; Linial, M.; Domb, A. J.; Hydrophobized dextran-spermine conjugate as potential vector for in vitro gene transfection. . J. Control. Release 2004, 96, 309-323, 10.1016/j.jconrel.2004.01.022.
- Kim, S.-H.; Chu, C.-C.; Synthesis and characterization of dextran–methacrylate hydrogels and structural study by SEM. . J. Biomed. Mater. Res. 2000, 49, 517-527, 10.1002/(sici)1097-4636(20000315)49:4<517::Aid-jbm10>3.0.Co;2-8.
- Kumar, A.; Deepak; Sharma, S.; Srivastava, A.; Kumar, R.; Synthesis of xanthan gum graft copolymer and its application for controlled release of highly water soluble Levofloxacin drug in aqueous medium.. Carbohydr. Polym. 2017, 171, 211-219, 10.1016/j.carbpol.2017.05.010.
- Oudshoorn, M. H. M.; Rissmann, R.; Bouwstra, J. A.; Hennink, W. E.; Synthesis of methacrylated hyaluronic acid with tailored degree of substitution. . Polymer 2007, 48, 1915-1920, 10.1016/j.polymer.2007.01.068.
- Palma, S. I. C. J.; Rodrigues, C. A. V.; Carvalho, A.; Morales, M. D.; Freitas, F.; Fernandes, A. R.; Cabral, J. M. S.; Roque, A. C. A.; A value-added exopolysaccharide as a coating agent for MRI nanoprobes. . Nanoscale 2015, 7, 14272-14283, 10.1039/c5nr01979f.
- Tang, Y. J.; Sun, J.; Fan, H. S.; Zhang, X. D.; An improved complex gel of modified gellan gum and carboxymethyl chitosan for chondrocytes encapsulation.. Carbohydr. Polym. 2012, 88, 46-53, 10.1016/j.carbpol.2011.11.058.
- Theilacker, C.; Coleman, F. T.; Mueschenborn, S.; Llosa, N.; Grout, M.; Pier, G. B.; Construction and characterization of a Pseudomonas aeruginosa mucoid exopolysaccharide-alginate conjugate vaccine. . Infect. Immun. 2003, 71, 3875-3884, 10.1128/Iai.71.7.3875-3884.2003.
- van Dijk-Wolthuis, W. N. E.; Franssen, O.; Talsma, H.; van Steenbergen, M. J.; Kettenes-van den Bosch, J. J.; Hennink, W. E.; Synthesis, Characterization, and Polymerization of Glycidyl Methacrylate Derivatized Dextran. . Macromolecules 1995, 28, 6317-6322, 10.1021/ma00122a044.
- Ratner, B. D.; The Biocompatibility Manifesto: Biocompatibility for the Twenty-first Century. . J. Cardiovasc. Transl. Res. 2011, 4, 523-527, 10.1007/s12265-011-9287-x.
- Sousa, A.; Neves, S. C.; Gonçalves, I. C.; Barrias, C. C.. Characterization of Polymeric Biomaterials; Tanzi, M. C.; Farè, S., Eds.; Woodhead Publishing: U.K., 2017; pp. 285-315.
- Biological evaluation of medical devices. In Part 5: tests for in vitro cytotoxicity, International Organization for Standardization: 2009; Vol. ISO 10993-5, p 34.
- Doak, S. H.; Manshian, B.; Jenkins, G. J. S.; Singh, N.; In vitro genotoxicity testing strategy for nanomaterials and the adaptation of current OECD guidelines. Mutat. Res. . Genet. Toxicol. Environ. Mutagen. 2012, 745, 104-111, doi.org/10.1016/j.mrgentox.2011.09.013.
- Cowie, H.; Magdolenova, Z.; Saunders, M.; Drlickova, M.; Carreira, S. C.; Kenzaoi, B. H.; Gombau, L.; Guadagnini, R.; Lorenzo, Y.; Walker, L.; et al.Fjellsbo, L. M.Huk, A.Rinna, A.Tran, L.Volkovova, K.Boland, S.Juillerat-Jeanneret, L.Marano, F.Collins, A. R.Dusinska, M. Suitability of human and mammalian cells of different origin for the assessment of genotoxicity of metal and polymeric engineered nanoparticles. . Nanotoxicology 2015, 9, 57-65, 10.3109/17435390.2014.940407.




