
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Hazem Choukaife | + 1815 word(s) | 1815 | 2020-11-11 11:42:59 | | | |
| 2 | Peter Tang | -317 word(s) | 1498 | 2020-11-12 03:33:29 | | |
Video Upload Options
On this note, biodegradable polymeric nanocarrier is deemed to control the release of the drug, stabilize labile molecules from degradation and site-specific drug targeting, with the main aim of reducing the dosing frequency and prolonging the therapeutic outcomes. Thus, it is essential to select the appropriate biopolymer material, e.g., sodium alginate to formulate nanoparticles for controlled drug delivery. Alginate has attracted considerable interest in pharmaceutical and biomedical applications as a matrix material of nanocarriers due to its inherent biological properties, including good biocompatibility and biodegradability. Various techniques have been adopted to synthesize alginate nanoparticles in order to introduce more rational, coherent, efficient and cost-effective properties.
1. Introduction
Nanomaterials have recently received much attention as drug carriers. In particular, nanoparticle-based nanoparticles [1][2], lipids [3][4], magnetic nanoparticles [5][6] and liposomes [7] have been widely studied in drug delivery systems. Among all, polymeric nanoparticles have been widely investigated due to their unique physicochemical properties [8]. The natural and synthetic polymers are versatile materials and are preferred for many applications, including the pharmaceutical industry [9].
Naturally-derived polymers are superior to synthetic ones due to their biodegradability, biocompatibility and biological activity. Hydrogel-based natural polymers such as alginate, collagen and gelatin can be used to deliver hydrophilic drugs due to their ability to absorb large amounts of water while maintaining their structures. The water absorption ability is related to the hydrophilic groups such as –OH, –COOH, –CONH –SO3H and –CONH2 that are contained in the hydrogel matrices [10]. Besides, the chelating, biocompatible, immunogenic and mucoadhesive properties of alginate make it as an attractive polymer in drug and cell delivery systems [11][12]. Alginates are considered among the most biosynthesized polymers, where 70% of annual alginate production is allocated to pharmaceutical and biomedical applications and the remaining is used in the food industry [13][14].
After alginate evolution in the 1980s and their spread as microparticles for encapsulation purposes, several studies were carried out to synthesize nano-sized alginate particles [15][16]. Gelling properties of alginate and its remarkable processing ease have grown its importance in drug delivery, cell immobilization, food industry and research perspectives [17][18]. Furthermore, it is considered as an environmentally friendly polymer that can undergo recycling and degradation. Alginate nanomaterials represent a fast-developing field, particularly for the pharmaceutical and food industry as well as academia. The gelling property makes alginate polymer as one of the most frequently used in drug delivery [19].
The physicochemical properties of alginate, such as viscosity, thermo-stability, sol-gel transformation, pH-responsivity, as well as drug release can gain better insight into their potential applications. Many factors could impact the properties of alginate nanoparticles, such as alginate, surfactant and crosslinker concentrations, stirring time and speed as well as pH value [20].
2. Alginate Polymer
Over the last decades, researchers were extensively utilizing natural polymers, especially in the pharmaceutical [21] and food industry [22][23] due to their advantages such as biocompatibility, biodegradability and low cost [11]. Alginate is an anionic polymer that typically obtained from brown marine algae. It is an unbranched polysaccharide copolymer consisting of alternating of d-mannuronate (M) and l-guluronic (G) blocks linked together by 1,4-glycosidic linkages (Figure 1A). Divalent cations, such as Ba2+ and Ca2+, can quickly form so-called egg-box complexes with G block to create alginate hydrogel through gelation phenomenon (Figure 1B) [24]. Hydrogelling ability of alginate has broadened its applications in biomedical and pharmaceutical research to encapsulate proteins and drugs for controlled release and targeted delivery. Hydrogel alginate matrix is a pH responsive where it shrinks at low pH, thus, the payload is preserved for extended period of time. Conversely, it swells and releases the encapsulated drug at higher pH values, offering a great carrier for oral delivery.
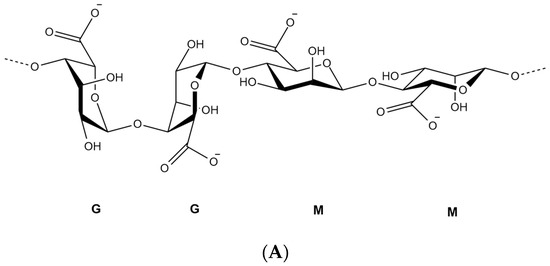
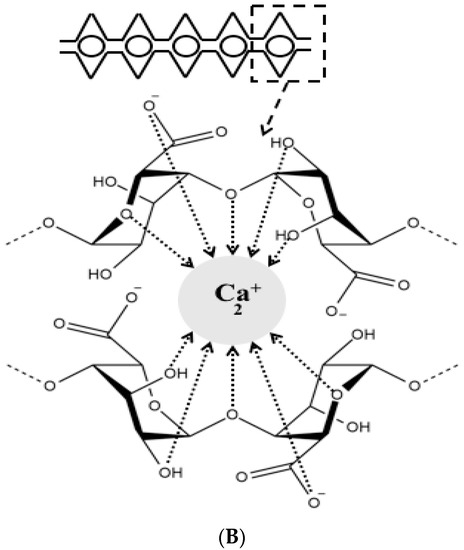
Figure 1. Chemical structure of alginate displaying the d-mannuronate (M) and l-guluronic (G) blocks (A), schematic representation of calcium binding to alginate to form an egg-box shape (B).
3. Sources of Alginates, Extraction, and Purification Methods
Alginates are unbranched polysaccharide, present in the c + ell walls of brown algae as well as some bacteria such as Azotobacter and Pseudomonas spp. [25][26]. Typically, the main sources of commercial alginates are from Laminaria hyperborea, Laminaria digitata, Macrocystis pyrifera, Ascophyllum nodosum, Eclonia maxima, Laminaria japonica, Lessonia nigrescens, Durvillea antarctica and Sargassum spp. [27]. Alginate chains are composed of units of β-d-mannuronic acid and α-l-guluronic acid of different arrangement depending on their natural source with pKa range from 3.38 to 3.65. Alginate is precipitated as an insoluble alginic acid in the low pH medium at room temperature [28]. Under alkaline extraction, alginates are purified with sodium hydroxide, sodium carbonate, or gelatinous aluminum hydroxide from the powdered brown algae. After purification, alginates are filtered and subsequently precipitated with Ca2+ ions/ethanol or by means of acidification [27][29].
Essentially, in order to avoid a brown discoloration of the final product, depigmentation of algae powder should take place prior to the extraction step. In addition, polyphenols are considered as another impurity that alter the rheological properties of alginate by forming strong dipolar forces with polysaccharides [30]. In order to render polyphenols insoluble during the extraction process, algae powder is soaked in formaldehyde or in a mixture of formaldehyde and ethanol [27]. Finally, polyphenolic levels in the final product are determined using fluorescence spectroscopy at 450 nm wavelength [31].
4. Alginate Nanoparticles
The remarkable properties of alginate enable the flexibility to synthesize various designs of particles such as nanoparticles and nanofibers. Nanosystem is a potential tool for controlling drug stability, delivery and release, which may improve drug bioavailability as well as expand the choices of drug administration routes [32]. Nanoparticles or ultrafine particles are defined as solid spheres of size range from 10 to 1000 nm [33]. Pharmaceutically, nanoparticles are made of biocompatible and biodegradable polymers of natural or synthetic origin, in which the pharmaceutical agent can be entrapped in or diffused into the particle matrix during the synthesis process [34]. Nanofibers, on the other hand, possess high surface area, controllable pore structure, diameter less than 1000 nm, and light weight compared to the conventional fibers [35][36]. The noticeable properties of nanofibers render them highly useful in biomedical [37] and drug delivery applications [38] as well as skin tissue engineering [39]. Figure 2 shows images of alginate nanoparticles/nanofibers produced by different fabrication approaches using scanning electron microscope (SEM).
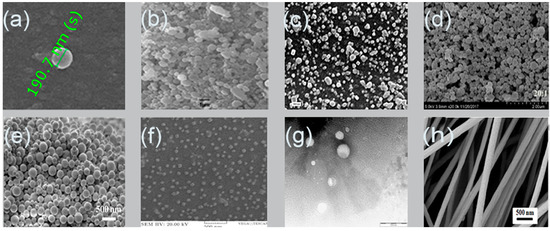
Figure 2. Scanning electron microscopy images of alginate nanoparticles prepared by: (a) emulsification/internal gelation, 1% w/w alginate, CaCO3:alginate mass ratios 0.1:1, pH 6 medium chain triglyceride (MCT) oil [40]; (b) nanospray dryer, spray cap 7 µm, flow rate 7 mL/min, drying gas flow of 110 L/min with relative flow rate 100%, inlet drying gas temperature 120 °C and outlet temperature 35 °C [41]; (c) polyelectrolyte complexation, nisin-loaded nanoparticles alginate 250 mg/mL and chitosan 250 mg/mL [42]; (d) evaporation method, zein-to-propylene glycol, alginate mass ratio 20:1 [43]; (e) layer-by-layer, paclitaxel, poly (lactic-co-glycolic acid) (PLGA) 10 mg/mL, alginate 5 mg/mL and chitosan 5 mg/mL [44]; (f) emulsification/external gelation, alginate 0.03% w/v and CaCl2 18 mM [45]; (g) electrospray DNA plasmid loaded alginate nanoparticles, flow rate 0.1 mL/h, voltage 12.5 kV, alginate 1% w/v, Tween 20 1% v/v, CaCl2 1.5% w/v, collector distance 4 cm and nozzle size 30 G [46]; (h) nanofiber produced by electrospinning, alginate 1.74% w/w, voltage 12 kV, needle 27 G, flow rate 0.6 mL/h and distance 12 cm [47].
The small size and large surface area of nanoparticles increase the dissolution rate and solubility of poorly soluble drugs. Nanoparticles can enhance the targetability of the encapsulant to specific sites of the body/tissue/cells, whether paracellularly or transcellularly [48]. The relationship between the rate of dissolution and particles size as well as surface area is explained by Noyes–Whitney Equation (1) as follows [49]:
where dc/dt = dissolution rate, D is the diffusion coefficient of the substance, A is the surface area of exposed solid, Cs and C represent the concentration of the dissolved substance at a given time t and the solubility concentration of the substance, respectively, and h is the thickness of the diffusion layer.
Also, nanoparticles enhance the solubility, dissolution rate and bioavailability of poorly soluble drugs by promoting the interaction propensity with the medium, owing to their large surface area [50]. Several researchers had developed and characterized nanoparticles-based natural polymers [51], lipids [52], polysaccharides [53] and synthetic biodegradable polymers over the last ten years [54]. Due to the unique physicochemical properties of alginate polymer among various natural polysaccharides, the ability to encapsulate foods, drugs and proteins into alginate nanoparticles has attracted the interest of many researchers [14]. Alginate-based nanocarrier seems to have all optimal requirements to be a successful drug delivery system due to its biodegradability, biocompatibility, protection effect of oral drugs against harsh gastrointestinal environment, controllable release, water solubility (avoiding the effect of noxious solvents during processing), availability and low cost [55]. Various studies have focused on enhancing the low intestinal penetration, gastrointestinal degradation and low bioavailability of orally administered insulin utilizing alginate nanoparticles as an oral drug delivery system [56][57]. On top of that, the application of alginate nanoparticles in cancer treatment has gained wide attention due to the ability to deliver anti-cancer therapeutics in sufficient manner at target site, promoting the bioavailability as well as reducing drug dosage and its side effects to the normal tissues [58][59]. Alginate nanoparticles have been also used for targeted antibiotic delivery applications without inducing resistant strains of bacteria [60][61]. The applications and fields that utilized alginate nanoparticles grew proportionally due to their useful properties and simple synthesis methods.
References
- Jawahar, N.; Meyyanathan, S. Polymeric Nanoparticles for Drug Delivery and Targeting: A Comprehensive Review. Int. J. Heal. Allied Sci. 2012, 1, 217. https://doi.org/10.4103/2278-344x.107832.
- El-Say, K. M.; El-Sawy, H. S. Polymeric Nanoparticles: Promising Platform for Drug Delivery. Int. J. Pharm. 2017, 528, 675–691. https://doi.org/10.1016/j.ijpharm.2017.06.052.
- Witzigmann, D.; Kulkarni, J. A.; Leung, J.; Chen, S.; Cullis, P. R.; van der Meel, R. Lipid Nanoparticle Technology for Therapeutic Gene Regulation in the Liver. Adv. Drug Deliv. Rev. 2020. https://doi.org/10.1016/j.addr.2020.06.026.
- Poovi, G.; Damodharan, N. Lipid Nanoparticles: A Challenging Approach for Oral Delivery of BCS Class-II Drugs. Futur. J. Pharm. Sci. 2018, 4, 191–205. https://doi.org/10.1016/j.fjps.2018.04.001.
- Wu, K.; Su, D.; Liu, J.; Saha, R.; Wang, J. P. Magnetic Nanoparticles in Nanomedicine: A Review of Recent Advances. Nanotechnology 2019, 30, 502003. https://doi.org/10.1088/1361-6528/ab4241.
- Pansieri, J.; Gerstenmayer, M.; Lux, F.; Mériaux, S.; Tillement, O.; Forge, V.; Larrat, B.; Marquette, C. Magnetic Nanoparticles Applications for Amyloidosis Study and Detection: A Review. Nanomaterials 2018, 8, 1–19. https://doi.org/10.3390/nano8090740.
- Panahi, Y.; Farshbaf, M.; Mohammadhosseini, M.; Mirahadi, M.; Khalilov, R.; Saghfi, S.; Akbarzadeh, A. Recent Advances on Liposomal Nanoparticles: Synthesis, Characterization and Biomedical Applications. Artif. Cells, Nanomedicine, Biotechnol. 2017, 45, 788–799. https://doi.org/10.1080/21691401.2017.1282496.
- Rao, J. P.; Geckeler, K. E. Polymer Nanoparticles: Preparation Techniques and Size-Control Parameters. Prog. Polym. Sci. 2011, 36, 887–913. https://doi.org/10.1016/j.progpolymsci.2011.01.001.
- Liechty, W. B.; Kryscio, D. R.; Slaughter, B. V.; Peppas, N. A. Polymers for Drug Delivery Systems. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 149–173. https://doi.org/10.1146/annurev-chembioeng-073009-100847.
- Andrianov, A. K.; Payne, L. G. Polymeric Carriers for Oral Uptake of Microparticulates. Adv. Drug Deliv. Rev. 1998, 34, 155–170. https://doi.org/10.1016/S0169-409X(98)00038-6.
- Rinaudo, M. Main Properties and Current Applications of Some Polysaccharides as Biomaterials. Polym Int 2007, 57, 397–430.
- Sun, J.; Tan, H. Alginate-Based Biomaterials for Regenerative Medicine Applications. Materials (Basel). 2013, 6, 1285–1309. https://doi.org/10.3390/ma6041285.
- Salleh, S. N.; Fairus, A. A. H.; Zahary, M. N.; Raj, N. B.; Jalil, A. M. M. Unravelling the Effects of Soluble Dietary Fibre Supplementation on Energy Intake and Perceived Satiety in Healthy Adults: Evidence from Systematic Review and Meta-Analysis of Randomised-Controlled Trials. Foods 2019, 8, 15. https://doi.org/10.3390/foods8010015.
- Pawar, S. N.; Edgar, K. J. Alginate Derivatization: A Review of Chemistry, Properties and Applications. Biomaterials 2012, 33, 3279–3305. https://doi.org/10.1016/j.biomaterials.2012.01.007.
- Lim, Franklin, and A. M. S. Microencapsulated Islets as Bioartificial Endocrine Pancreas. Science (80-. ). 1980, 210, 908–910.
- Paques, J. P.; Van Der Linden, E.; Van Rijn, C. J. M.; Sagis, L. M. C. Preparation Methods of Alginate Nanoparticles. Adv. Colloid Interface Sci. 2014, 209, 163–171. https://doi.org/10.1016/j.cis.2014.03.009.
- Ismail, I., Fauzi, N. H. M., Baki, M. Z., & Hoon, H. L. Effects of Different Drying Methods and Hydrocolloids on Quality Properties of Semi-Dried Catfish Jerky. Malaysian J. Appl. Sci. 2016, 2, 11–18.
- Lee, K. Y.; Mooney, D. J. Alginate: Properties and Biomedical Applications. Prog. Polym. Sci. 2012, 37, 106–126. https://doi.org/10.1016/j.progpolymsci.2011.06.003.
- Ching, S. H.; Bansal, N.; Bhandari, B. Alginate Gel Particles–A Review of Production Techniques and Physical Properties. Crit. Rev. Food Sci. Nutr. 2017, 57, 1133–1152. https://doi.org/10.1080/10408398.2014.965773.
- Loquercio, A.; Castell-Perez, E.; Gomes, C.; Moreira, R. G. Preparation of Chitosan-Alginate Nanoparticles for Trans-Cinnamaldehyde Entrapment. J. Food Sci. 2015, 80, N2305–N2315. https://doi.org/10.1111/1750-3841.12997.
- Spadari, C. de C.; Lopes, L. B.; Ishida, K. Potential Use of Alginate-Based Carriers as Antifungal Delivery System. Front. Microbiol. 2017, 8, 97. https://doi.org/10.3389/fmicb.2017.00097.
- Aisyah, N.; Muhammad, N.; Hashim, H.; Huda, N. Improving the Texture of Sardine Surimi Using Duck Feet Gelatin. J. Agrobiotech 2017, 8, 25–32.
- Tang, Y.; Lan, X.; Liang, C.; Zhong, Z.; Xie, R.; Zhou, Y.; Miao, X.; Wang, H.; Wang, W. Honey Loaded Alginate/PVA Nanofibrous Membrane as Potential Bioactive Wound Dressing. Carbohydr. Polym. 2019, 219, 113–120. https://doi.org/10.1016/j.carbpol.2019.05.004.
- Tavakoli, J.; Laisak, E.; Gao, M.; Tang, Y. AIEgen Quantitatively Monitoring the Release of Ca2+ during Swelling and Degradation Process in Alginate Hydrogels. Mater. Sci. Eng. 2019, 104, 109951. https://doi.org/10.1016/j.msec.2019.109951.
- Al-kafaween, M.; Hilmi, A. M.; … N. J.-J. J. of; 2020, U. Effects of Trigona Honey on the Gene Expression Profile of Pseudomonas Aeruginosa ATCC 10145 and Streptococcus Pyogenes ATCC 19615. Jordan J. Biol. Sci. 2020, 13, 133–138.
- Rehm, B. H. A.; Valla, S. Bacterial Alginates: Biosynthesis and Applications. Appl. Microbiol. Biotechnol. 1997, 48, 281–288. https://doi.org/10.1007/s002530051051.
- Draget KI, Smidsrød O, S. G. Alginates from Algae. Biol. Chem. Biotechnol. Appl. 2005, 6.
- Simsek-Ege, F. A.; Bond, G. M.; Stringer, J. Polyelectrolye Complex Formation between Alginate and Chitosan as a Function of PH. J. Appl. Polym. Sci. 2003, 88, 346–351. https://doi.org/10.1002/app.11989.
- Skjåk‐Bræk, G.; Murano, E.; Paoletti, S. Alginate as Immobilization Material. II: Determination of Polyphenol Contaminants by Fluorescence Spectroscopy, and Evaluation of Methods for Their Removal. Biotechnol. Bioeng. 1989, 33, 90–94. https://doi.org/10.1002/bit.260330112.
- Wijesinghe, W. A. J. P.; Jeon, Y. J. Enzyme-Assistant Extraction (EAE) of Bioactive Components: A Useful Approach for Recovery of Industrially Important Metabolites from Seaweeds: A Review. Fitoterapia 2012, 83, 6–12. https://doi.org/10.1016/j.fitote.2011.10.016.
- Sharma, A.; Gupta, M. N. Three Phase Partitioning of Carbohydrate Polymers: Separation and Purification of Alginates. Carbohydr. Polym. 2002, 48, 391–395. https://doi.org/10.1016/S0144-8617(01)00313-7.
- Gigliobianco, M. R.; Casadidio, C.; Censi, R.; Di Martino, P. Nanocrystals of Poorly Soluble Drugs: Drug Bioavailability and Physicochemical Stability. Pharmaceutics 2018, 10, 134. https://doi.org/10.3390/pharmaceutics10030134.
- Hamidi, M.; Azadi, A.; Rafiei, P. Hydrogel Nanoparticles in Drug Delivery. Adv. Drug Deliv. Rev. 2008, 60, 1638–1649. https://doi.org/10.1016/j.addr.2008.08.002.
- Jeevanandam, J.; Barhoum, A.; Chan, Y. S.; Dufresne, A.; Danquah, M. K. Review on Nanoparticles and Nanostructured Materials: History, Sources, Toxicity and Regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. https://doi.org/10.3762/bjnano.9.98.
- Wei, Q.; Tao, D.; Xu, Y. Functional Nanofibers and Their Applications; 2012. https://doi.org/10.1533/9780857095640.1.1.
- Cai, Y.; Wei, Q.; Huang, F. Processing of Composite Functional Nanofibers. In Functional Nanofibers and their Applications; Woodhead Publishing Limited: Sawston, UK, 2012. https://doi.org/10.1533/9780857095640.1.38.
- Al-Enizi, A. M.; Zagho, M. M.; Elzatahry, A. A. Polymer-Based Electrospun Nanofibers for Biomedical Applications. Nanomaterials 2018, 8, 1–22. https://doi.org/10.3390/nano8040259.
- Han, X.; Huo, P.; Ding, Z.; Kumar, P.; Liu, B. Preparation of Lutein-Loaded PVA/Sodium Alginate Nanofibers and Investigation of Its Release Behavior. Pharmaceutics 2019, 11, 1–11. https://doi.org/10.3390/pharmaceutics11090449.
- Li, Wan-Ju, Cato T. Laurencin, Edward J. Caterson, R. S. T. and F. K. K. Electrospun Nanofibrous Structure: A Novel Scaffold for Tissue Engineering. J. Biomed. Mater. Res. 2001, 60, 613–621.
- Paques, J. P.; Sagis, L. M. C.; van Rijn, C. J. M.; van der Linden, E. Nanospheres of Alginate Prepared through w/o Emulsification and Internal Gelation with Nanoparticles of CaCO3. Food Hydrocoll. 2014, 40, 182–188. https://doi.org/10.1016/j.foodhyd.2014.02.024.
- Shehata, T. M.; Ibrahima, M. M. BÜCHI Nano Spray Dryer B-90: A Promising Technology for the Production of Metformin Hydrochloride-Loaded Alginate–Gelatin Nanoparticles. Drug Dev. Ind. Pharm. 2019, 45, 1907–1914. https://doi.org/10.1080/03639045.2019.1680992.
- Zohri, M.; Alavidjeh, M. S.; Haririan, I.; Ardestani, M. S.; Ebrahimi, S. E. S.; Sani, H. T.; Sadjadi, S. K. A Comparative Study Between the Antibacterial Effect of Nisin and Nisin-Loaded Chitosan/Alginate Nanoparticles on the Growth of Staphylococcus Aureus in Raw and Pasteurized Milk Samples. Probiotics Antimicrob. Proteins 2010, 2, 258–266. https://doi.org/10.1007/s12602-010-9047-2.
- Dai, L.; Zhan, X.; Wei, Y.; Sun, C.; Mao, L.; McClements, D. J.; Gao, Y. Composite Zein - Propylene Glycol Alginate Particles Prepared Using Solvent Evaporation: Characterization and Application as Pickering Emulsion Stabilizers. Food Hydrocoll. 2018, 85, 281–290. https://doi.org/10.1016/j.foodhyd.2018.07.013.
- Wang, F.; Yuan, J.; Zhang, Q.; Yang, S.; Jiang, S.; Huang, C. PTX-Loaded Three-Layer PLGA/CS/ALG Nanoparticle Based on Layer-by-Layer Method for Cancer Therapy. J. Biomater. Sci. Polym. Ed. 2018, 29, 1566–1578. https://doi.org/10.1080/09205063.2018.1475941.
- Daemi, H.; Barikani, M. Synthesis and Characterization of Calcium Alginate Nanoparticles, Sodium Homopolymannuronate Salt and Its Calcium Nanoparticles. Sci. Iran. 2012, 19, 2023–2028. https://doi.org/10.1016/j.scient.2012.10.005.
- Alallam, B.; Altahhan, S.; Taher, M.; Mohd Nasir, M. H.; Doolaanea, A. A. Electrosprayed Alginate Nanoparticles as CRISPR Plasmid DNA Delivery Carrier: Preparation, Optimization, and Characterization. Pharmaceuticals 2020, 13, 158. https://doi.org/10.3390/ph13080158.
- Zhang, J.; Wang, X. X.; Zhang, B.; Ramakrishna, S.; Yu, M.; Ma, J. W.; Long, Y. Z. In Situ Assembly of Well-Dispersed Ag Nanoparticles throughout Electrospun Alginate Nanofibers for Monitoring Human Breath - Smart Fabrics. ACS Appl. Mater. Interfaces 2018, 10, 19863–19870. https://doi.org/10.1021/acsami.8b01718.
- Jong, W. H. De; Paul, J. B. Drug Delivery and Nanoparticles : Applications and Hazards. Int. J. Nanomedicine 2008, 3, 133–149.
- Dizaj, S. M.; Vazifehasl, Z.; Salatin, S.; Adibkia, K.; Javadzadeh, Y. Nanosizing of Drugs: Effect on Dissolution Rate. Res. Pharm. Sci. 2015, 10, 95–108.
- Alshora, D. H.; Ibrahim, M. A.; Alanazi, F. K. Nanotechnology from Particle Size Reduction to Enhancing Aqueous Solubility; Elsevier Inc., 2016. https://doi.org/10.1016/B978-0-323-42861-3.00006-6.
- Hassani, A.; Mahmood, S.; Enezei, H. H.; Hussain, S. A.; Hamad, H. A.; Aldoghachi, A. F.; Hagar, A.; Doolaanea, A. A.; Ibrahim, W. N. Formulation, Characterization and Biological Activity Screening of Sodium Alginate-Gum Arabic Nanoparticles Loaded with Curcumin. Molecules 2020, 25, 2244. https://doi.org/10.3390/molecules25092244.
- Elena Ortega, Santos Blanco, Adolfina Ruiz, M. Á.; Peinado, S. P. and M. E. M. LIPID NANOPARTICLES FOR THE TRANSPORT OF DRUGS LIKE DOPAMINE THROUGH THE BLOOD–BRAIN BARRIER. Beilstein J. Nanotechnol. 2020, 1, 79. https://doi.org/10.3762/bxiv.2020.79.v1.
- Thomas, D.; KurienThomas, K.; Latha, M. S. Preparation and Evaluation of Alginate Nanoparticles Prepared by Green Method for Drug Delivery Applications. Int. J. Biol. Macromol. 2020, 154, 888–895. https://doi.org/10.1016/j.ijbiomac.2020.03.167.
- Roces, C. B.; Christensen, D.; Perrie, Y. Translating the Fabrication of Protein-Loaded Poly(Lactic-Co-Glycolic Acid) Nanoparticles from Bench to Scale-Independent Production Using Microfluidics. Drug Deliv. Transl. Res. 2020, 10, 582–593. https://doi.org/10.1007/s13346-019-00699-y.
- Ciofani, G.; Raffa, V.; Menciassi, A.; Dario, P. Alginate and Chitosan Particles as Drug Delivery System for Cell Therapy. Biomed. Microdevices 2008, 10, 131–140. https://doi.org/10.1007/s10544-007-9118-7.
- Li, M.; Sun, Y.; Ma, C.; Hua, Y.; Zhang, L.; Shen, J. Design and Investigation of Penetrating Mechanism of Octaarginine-Modified Alginate Nanoparticles for Improving Intestinal Insulin Delivery. J. Pharm. Sci. 2020, in press. https://doi.org/10.1016/j.xphs.2020.07.004.
- Alfatama, M.; Lim, L. Y.; Wong, T. W. Alginate-C18 Conjugate Nanoparticles Loaded in Tripolyphosphate-Cross-Linked Chitosan-Oleic Acid Conjugate-Coated Calcium Alginate Beads as Oral Insulin Carrier. Mol. Pharm. 2018, 15, 3369–3382. https://doi.org/10.1021/acs.molpharmaceut.8b00391.
- Sorasitthiyanukarn, F. N.; Muangnoi, C.; Ratnatilaka Na Bhuket, P.; Rojsitthisak, P.; Rojsitthisak, P. Chitosan/Alginate Nanoparticles as a Promising Approach for Oral Delivery of Curcumin Diglutaric Acid for Cancer Treatment. Mater. Sci. Eng. 2018, 93, 178–190. https://doi.org/10.1016/j.msec.2018.07.069.
- Markeb, A. A.; El-Maali, N. A.; Sayed, D. M.; Osama, A.; Abdel-Malek, M. A. Y.; Zaki, A. H.; Elwanis, M. E. A.; Driscoll, J. J. Synthesis, Structural Characterization, and Preclinical Efficacy of a Novel Paclitaxel-Loaded Alginate Nanoparticle for Breast Cancer Treatment. Int. J. Breast Cancer 2016, 2016. https://doi.org/10.1155/2016/7549372.
- Baek, S.; Joo, S. H.; Toborek, M. Treatment of Antibiotic-Resistant Bacteria by Encapsulation of ZnO Nanoparticles in an Alginate Biopolymer: Insights into Treatment Mechanisms. J. Hazard. Mater. 2019, 373, 122–130. https://doi.org/10.1016/j.jhazmat.2019.03.072.
- Scolari, I. R.; Páez, P. L.; Musri, M. M.; Petiti, J. P.; Torres, A.; Granero, G. E. Rifampicin Loaded in Alginate/Chitosan Nanoparticles as a Promising Pulmonary Carrier against Staphylococcus Aureus. Drug Deliv. Transl. Res. 2020, 10, 1403–1417. https://doi.org/10.1007/s13346-019-00705-3.





