
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Taku Chibazakura | + 2250 word(s) | 2250 | 2020-10-15 07:47:09 | | | |
| 2 | Dean Liu | -842 word(s) | 1408 | 2020-11-05 10:06:05 | | |
Video Upload Options
NPM1 (nucleophosmin isoform 1) is a nucleolar and multifunctional protein. NPM1 is involved in ribosome assembly as ribonuclease, associated with centrosome duplication, and controls cellular apoptotic response by inhibiting ARF (alternative reading frame). In some cancers, mutant forms of NPM1 including the one fused to ALK, anaplastic lymphoma receptor tyrosine kinase, and NPM1c+ (NPM1 cytoplasmic positive) are known. Moreover, in this study, NPM1 has been identified as a novel factor interacting with a CDK inhibitor p27Kip1. Increased NPM1 expression in cancer cells suppresses p27 function and promotes cell proliferation of cancer cells in vitro and in vivo.
1.Introduction
Cell cycle progression is promoted by complexes of cyclins and cyclin-dependent kinases (CDKs) and inhibited by CDK inhibitors (CKIs). The cell cycle is tightly controlled by various interactions of these factors. Disturbance of these cell cycle regulatory mechanisms can lead to carcinogenesis and cancer progression, as well as cell death [1][2].
p27Kip1 is a member of CKIs and was first identified as an inhibitor of the cyclin E/CDK2 complex[3]. p27 inhibits CDK activities by binding to the cyclin/CDK complex via its N-terminal domain and blocking ATP binding of CDK [4]. A major regulatory mechanism of p27 function is controlling p27 protein levels through transcriptional, translational, and post-translational regulations[5][6][7][8][9][10]. Environmental factors such as TGF-β, serum starvation, and cell contact inhibition can increase p27 protein levels[11][12].
Clinically, p27 protein levels are often low in cancer cells and there is a negative correlation among p27 protein levels and malignancy of cancer in breast cancer, lung cancer, colorectal carcinomas, and gastric carcinomas [13][14][15][16]. Previous studies on p27-null mice have indicated that p27 deficiency can cause an increase in cell proliferation, body size, and weight[17][18][19]. In addition, p27-null mice have shown higher carcinogenicity in the intermediate robe of the pituitary gland and higher tumor induction by external factors such as γ-irradiation and carcinogens than p27 wild-type mice[20].
Recent studies have indicated that abnormal localization and degradation of p27, depending on phosphorylation, suppressed p27 function and promoted tumor cell proliferation[21]. For example, serine 10 phosphorylation by UHMK1, U2AF homology motif kinase 1, promotes p27 translocation from the nucleus to the cytoplasm depending on nuclear export protein CRM1, chromosomal maintenance 1, resulting in p27 inactivation and ovarian cancer cell proliferation[7][22]. Threonine 187 is phosphorylated by CDK1/2, which promotes binding of SCFSkp2 ubiquitin ligase complex to p27 and its polyubiquitination and degradation[23]. Threonine 157 and 198 are also phosphorylated by PKB/AKT1, which causes 14-3-3 binding to p27, resulting in maintenance of cytoplasmic localization and degradation of p27[24]. However, in human cancer cells, loss-of-function mutation or homozygous deletion of p27 gene is very rare[11].
On the basis of this information, normal expression level and localization are considered to be important for p27 function. However, some serious cancer patients show positive and normal expression of p27[25], raising a question whether or not p27 function is controlled quantitatively. In this study, authors focused on the antiproliferative function of p27 at normal or high expression levels in cancer cells and demonstrated that p27 did not function even under forced overexpression in some cancer cells. Thus, their experiments revealed qualitative, but not quantitative, suppression of p27 in cancer cells, and authors screened for factor(s) involved in such p27 functional suppression by proteomic screening and identified nucleophosmin isoform 1 (NPM1) as a novel p27-interacting factor.
2. Increased Expression of NPM1 Suppresses p27 Function In Vitro
NPM1 is an abundant nucleolar protein found in nucleolus in growing cells. Authors checked the expressions of NPM1 in normal cells and cancer cells and observed that the protein level of NPM1 is about two times higher in cancer cells than normal cells (Figure 3A).
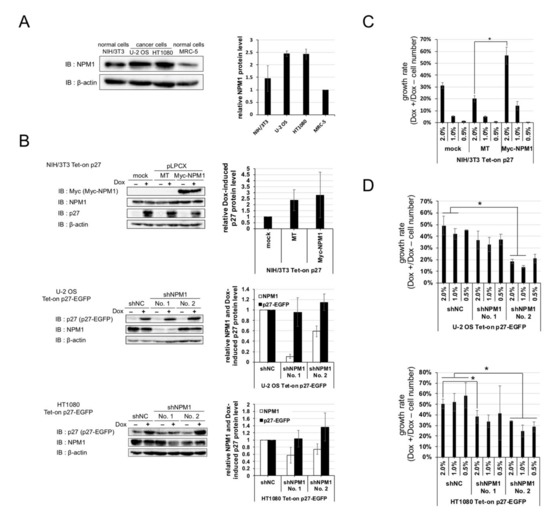
Figure 3. Nucleophosmin isoform 1 (NPM1) suppresses the function of p27 in vitro. (A) (left) Expressions of NPM1 protein in human cancer cell lines (U-2 OS and HT1080) and normal cell lines (murine NIH/3T3 and human MRC-5) were detected by Western blot analysis using antibodies against NPM1 and control β-actin. (right) The relative expression levels of NPM1 (normalized with β-actin) in three cell lines are plotted (MRC-5 = 1); (B) (left) Expressions of NPM1 and p27 in NIH/3T3 cells carrying Dox-inducible p27 and overexpressing NPM1 (top) and U-2 OS and HT1080 cells carrying Dox-inducible p27 and expressing NPM1 shRNAs (middle and bottom) were detected by Western blot analysis using antibodies against p27, NPM1, and control β-actin (mock, no vector; MT, Myc-tag only expression vector; Myc-NPM1, Myc-tagged NPM1 expression vector; and shNC, negative control shRNA). The relative expression levels of p27 and/or NPM1 (normalized with β-actin) in the three cell lines are plotted in the right (mock or shNC = 1); (C) Proliferation assay for NIH/3T3 Tet-on p27 cells with or without NPM1 overexpression. The cells were seeded at low densities (1%, 3 × 103 cells per 35 mm dish), cultured for one week with or without 10 µg/mL Dox, and counted. Growth rate is the division of the Dox+ cell number by the Dox–; (D) Proliferation assays for U-2 OS (top) and HT1080 (bottom) Tet-on p27-EGFP cells with or without NPM1 knockdown. * 0.01 < p < 0.05.
Thus, authors predicted that increased expression of NPM1 in cancer cells is involved in suppression of p27 function. To verify the effect of increased expression of NPM1 on p27 function, normal cells carrying Dox-inducible p27 and NPM1 stable overexpression constructs and cancer cells carrying Dox-inducible p27 and NPM1 stable knockdown constructs were established (Figure 3B), and their growth rates were compared. NPM1-overexpressing normal cells showed a higher growth rate than the mock-expressing normal cells upon p27 induction (Figure 3C). Conversely, NPM1 knocked down cancer cells showed a lower growth rate than the control cancer cells upon p27 induction (Figure 3D). The cell numbers, after one week of cultivation with and without Dox, showed that overexpression or NPM1 knockdown did not have significant effect on proliferation of all the cell lines in the absence of Dox. These results suggest that increased expression of NPM1 in cancer cells suppresses p27 function in vitro.
3. NPM1 Suppresses p27 Function in Xenografted Tumors in Mouse
Next, to verify the effect of NPM1 on p27 function in more physiological settings, mouse xenograft model experiments were carried out. The HT1080 cells knocked down for NPM1 and carrying Dox-inducible p27 were transplanted into the back of BALB/c-nu nude mice and the tumor growth was monitored with or without Dox feeding. The tumors derived from the negative control cells showed a steady growth, with or without p27 induction. By contrast, the growth of tumors derived from NPM1 knockdown cells was markedly suppressed upon p27 induction as compared with no p27 induction (Figure 4A(#)). Importantly, authors observed that the combination of p27 induction and NPM1 knockdown significantly suppressed tumor growth (Figure 4A(**)).
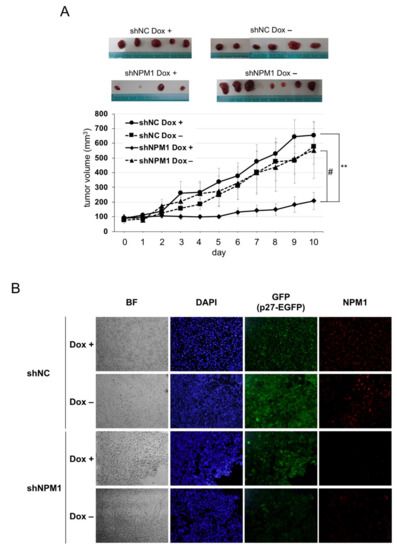
Figure 4. NPM1 suppresses p27 function in mouse xenograft model. (A) Control shRNA (NC)- and shNPM1 (No. 2)-introduced HT1080 Tet-on p27-EGFP cells were injected subcutaneously into the back of BALB/c-nu nude mice. One week after injection, mice were fed with Dox (200 µg/mL) or only solvent and the size of tumor was measured every day. (top) Samples of the tumors excised from four groups of xenografted mice. (bottom) The tumor volume was calculated as follows: volume (mm3) = (length, mm) × (width, mm)2 × 0.523. ** 0.005 < p < 0.01 and # 0.05 < p < 0.1; (B) Immunofluorescence analysis of p27 and NPM1 in tumors. The tumors excised from each group of xenografts were subjected to immunofluorescence analysis using anti-NPM1 antibody (red). Nuclei were stained with DAPI. BF, bright field.
Finally, to check whether p27 expression was induced by Dox and NPM1 was knocked down in tumors, immunofluorescence analysis was carried out (Figure 4B). After the tumors were excised from each nude mouse, their cryosections were subjected to immunofluorescence analysis. On the one hand, p27-EGFP (green) was detected in the nuclei of Dox + tumor cells, while some background whole cell signals were observed in Dox– cells (especially in negative control shRNA Dox– cells). On the other hand, NPM1 (red) was detected remarkably weaker in tumors derived from NPM1 knockdown cells than in those from negative control cells.
These results strongly suggest that the increased expression of NPM1 suppresses p27 function in vivo, as well as in vitro. Author's findings have an impact on the conventional understanding of p27, as a novel mechanism for its functional regulation, and also, might contribute to cancer prevention, as well as cancer treatment, because a lot of tissue cells maintain high p27 protein expression in our body.
References
- Bartek, J.; Lukas, J. DNA damage checkpoints: From initiation to recovery or adaptation. Curr. Opin. Cell Biol. 2007, 19, 238–245.
- Jackson, S.P.; Bartek, J. The DNA-damage response in human biology and disease. Nature 2009, 461, 1071–1078.
- Polyak, K.; Lee, M.H.; Erdjument-Bromage, H.; Koff, A.; Roberts, J.M.; Tempst, P.; Massagué, J. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell 1994, 78, 59–66.
- Russo, A.A.; Jeffrey, P.D.; Patten, A.K.; Massagué, J.; Pavletich, N.P. Crystal structure of the p27Kip1 cyclin-dependent-kinase inhibitor bound to the cyclin A-Cdk2 complex. Nature 1996, 382, 325–331.
- Bagui, T.K.; Cui, D.; Roy, S.; Mohapatra, S.; Shor, A.C.; Ma, L.; Pledger, W.J. Inhibition of p27Kip1 gene transcription by mitogens. Cell Cycle 2009, 8, 115–124.
- Carrano, A.C.; Eytan, E.; Hershko, A.; Pagano, M. SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nat. Cell Biol. 1999, 1, 193–199.
- Boehm, M.; Yoshimoto, T.; Crook, M.F.; Nallamshetty, S.; True, A.; Nabel, G.J.; Nabel, E.G. A growth factor-dependent nuclear kinase phosphorylates p27(Kip1) and regulates cell cycle progression. EMBO J. 2002, 21, 3390–3401.
- Shin, M.H.; Mavila, N.; Wang, W.H.; Alvarez, S.V.; Hall, M.C.; Andrisani, O.M. Time-dependent activation of Phox2a by the cyclic AMP pathway modulates onset and duration of p27Kip1 transcription. Mol. Cell. Biol. 2009, 29, 4878–4890.
- Trabosh, V.A.; Divito, K.A.; D Aguda, B.; Simbulan-Rosenthal, C.M.; Rosenthal, D.S. Sequestration of E12/E47 and suppression of p27KIP1 play a role in Id2-induced proliferation and tumorigenesis. Carcinogenesis 2009, 30, 1252–1259.
- Hengst, L.; Reed, S.I. Translational control of p27Kip1 accumulation during the cell cycle. Science 1996, 271, 1861–1864.
- Lee, J.; Kim, S.S. The function of p27 KIP1 during tumor development. Exp. Mol. Med. 2009, 41, 765–771.
- Polyak, K.; Kato, J.Y.; Solomon, M.J.; Sherr, C.J.; Massague, J.; Roberts, J.M.; Koff, A. p27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-beta and contact inhibition to cell cycle arrest. Genes Dev. 1994, 8, 9–22.
- Loda, M.; Cukor, B.; Tam, S.W.; Lavin, P.; Fiorentino, M.; Draetta, G.F.; Jessup, J.M.; Pagano, M. Increased proteasome-dependent degradation of the cyclin-dependent kinase inhibitor p27 in aggressive colorectal carcinomas. Nat. Med. 1997, 3, 231–234.
- Porter, P.L.; Malone, K.E.; Heagerty, P.J.; Alexander, G.M.; Gatti, L.A.; Firpo, E.J.; Daling, J.R.; Roberts, J.M. Expression of cell-cycle regulators p27Kip1 and cyclin E, alone and in combination, correlate with survival in young breast cancer patients. Nat. Med. 1997, 3, 222–225.
- Yasui, W.; Kudo, Y.; Semba, S.; Yokozaki, H.; Tahara, E. Reduced expression of cyclin-dependent kinase inhibitor p27Kip1 is associated with advanced stage and invasiveness of gastric carcinomas. Jpn. J. Cancer Res. 1997, 88, 625–629.
- Yatabe, Y.; Masuda, A.; Koshikawa, T.; Nakamura, S.; Kuroishi, T.; Osada, H.; Takahashi, T.; Mitsudomi, T. p27KIP1 in human lung cancers: differential changes in small cell and non-small cell carcinomas. Cancer Res. 1998, 58, 1042–1047.
- Nakayama, K.; Ishida, N.; Shirane, M.; Inomata, A.; Inoue, T.; Shishido, N.; Horii, I.; Loh, D.Y. Mice lacking p27(Kip1) display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell 1996, 85, 707–720.
- Fero, M.L.; Rivkin, M.; Tasch, M.; Porter, P.; Carow, C.E.; Firpo, E.; Polyak, K.; Tsai, L.H.; Broudy, V.; Perlmutter, R.M.; et al. A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis, and female sterility in p27(Kip1)-deficient mice. Cell 1996, 85, 733–744.
- Kiyokawa, H.; Kineman, R.D.; Manova-Todorova, K.O.; Soares, V.C.; Hoffman, E.S.; Ono, M.; Khanam, D.; Hayday, A.C.; Frohman, L.A.; Koff, A. Enhanced growth of mice lacking the cyclin-dependent kinase inhibitor function of p27(Kip1). Cell 1996, 85, 721–732.
- Fero, M.L.; Randel, E.; Gurley, K.E.; Roberts, J.M.; Kemp, C.J. The murine gene p27Kip1 is haplo-insufficient for tumour suppression. Nature 1998, 396, 177–180.
- He, W.; Wang, X.; Chen, L.; Guan, X. A crosstalk imbalance between p27(Kip1) and its interacting molecules enhances breast carcinogenesis. Cancer Biother. Radiopharm. 2012, 27, 399–402.
- Wang, Y.; Xiang, J.; Ji, F.; Deng, Y.; Tang, C.; Yang, S.; Xi, Q.; Liu, R.; Di, W. Knockdown of CRM1 inhibits the nuclear export of p27(Kip1) phosphorylated at serine 10 and plays a role in the pathogenesis of epithelial ovarian cancer. Cancer Lett. 2014, 343, 6–13.
- Tsvetkov, L.M.; Yeh, K.H.; Lee, S.J.; Sun, H.; Zhang, H. p27(Kip1) ubiquitination and degradation is regulated by the SCF(Skp2) complex through phosphorylated Thr187 in p27. Curr. Biol. 1999, 9, 661–664.
- Fujita, N.; Sato, S.; Katayama, K.; Tsuruo, T. Akt-dependent phosphorylation of p27Kip1 promotes binding to 14-3-3 and cytoplasmic localization. J. Biol. Chem. 2002, 277, 28706–28713.
- Masciullo, V.; Sgambato, A.; Pacilio, C.; Pucci, B.; Ferrandina, G.; Palazzo, J.; Carbone, A.; Cittadini, A.; Mancuso, S.; Scambia, G.; et al. Frequent loss of expression of the cyclin-dependent kinase inhibitor p27 in epithelial ovarian cancer. Cancer Res. 1999, 59, 3790–3794.




