| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Simon Potier | + 988 word(s) | 988 | 2020-10-19 11:23:35 | | | |
| 2 | Dean Liu | Meta information modification | 988 | 2020-11-03 06:31:45 | | | | |
| 3 | Dean Liu | -1 word(s) | 987 | 2020-11-05 09:53:34 | | | | |
| 4 | Dean Liu | + 618 word(s) | 1605 | 2020-11-09 09:50:23 | | | | |
| 5 | Dean Liu | Meta information modification | 1605 | 2020-11-09 10:12:59 | | |
Video Upload Options
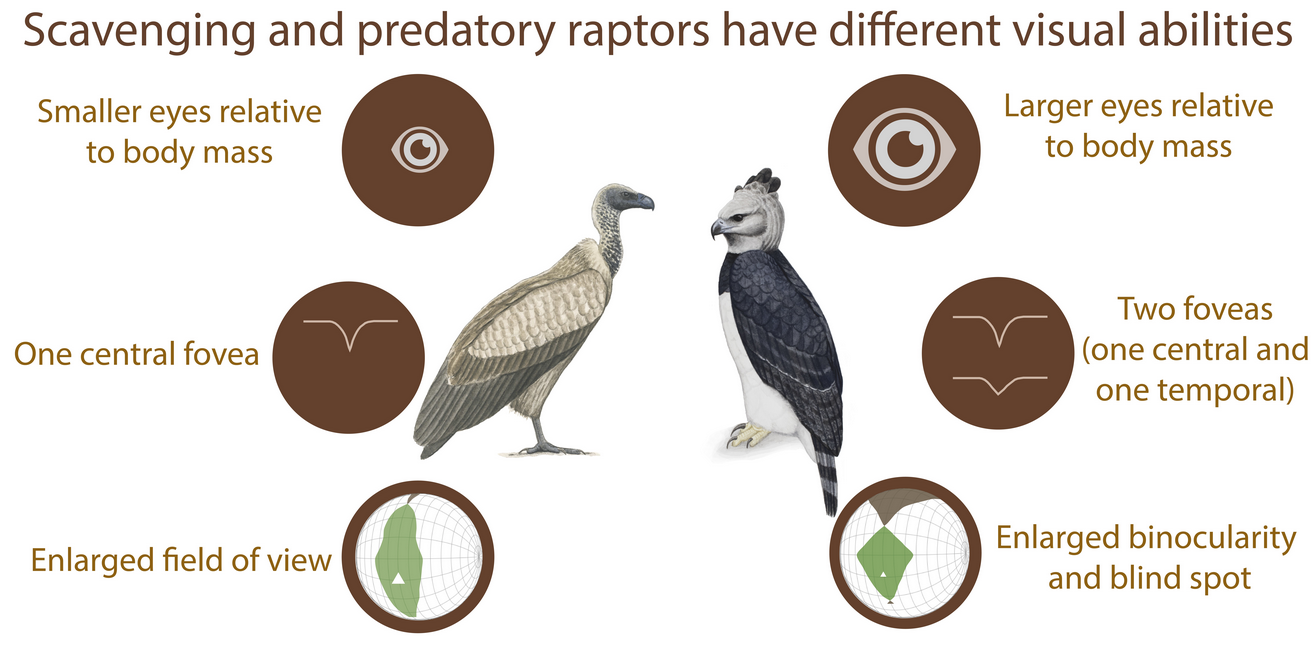
Ecological diversity among diurnal birds of prey, or raptors, is highlighted regarding their sensory abilities. While raptors are believed to forage primarily using sight, the sensory demands of scavengers and predators differ, as reflected in their visual systems. Here, I have reviewed the visual specialisations of predatory and scavenging diurnal raptors, focusing on (1) the anatomy of the eye and (2) the use of vision in foraging. Predators have larger eyes than scavengers relative to their body mass, potentially highlighting the higher importance of vision in these species. Scavengers possess one centrally positioned fovea that allows for the detection of carrion at a distance. In addition to the central fovea, predators have a second, temporally positioned fovea that views the frontal visual field, possibly for prey capture. Spatial resolution does not differ between predators and scavengers. In contrast, the organisation of the visual fields reflects important divergences, with enhanced binocularity in predators opposed to an enlarged field of view in scavengers. Predators also have a larger blind spot above the head. The diversity of visual system specializations according to the foraging ecology displayed by these birds suggests a complex interplay between visual anatomy and ecology, often unrelatedly of phylogeny.
1. Introduction
Understanding how animals extract information from their surrounding environment is essential to appreciate how they complete their daily tasks such as finding food, avoiding predators, attracting mates, and navigating their environment[1]. Among the sensory organs found in the animal kingdom, eyes can provide instantaneous information about the environment like no other[2]. While birds use a wide range of cues (ranging from the Earth’s magnetic field to sounds, odours, visual cues, and so on), vision appears to be a very important sensory modality in these animals, especially in the context of foraging[3]. In particular, birds of prey, or “raptors” (as defined below), are considered to forage primary using the visual sense ([4][5], but see [6]). Indeed, a number of species of raptors spot their prey/food at relatively high altitudes where acoustic and olfactory cues should be undetectable, and vision may be the only accessible cue available for prey/food detection in the absence of visual barriers. This apparent reliance on vision is underlined by the fact that some raptor species have the highest visual acuity (also called spatial resolution) found to date, for both achromatic ([7][8], reviewed in[5]) and chromatic [9] patterns.
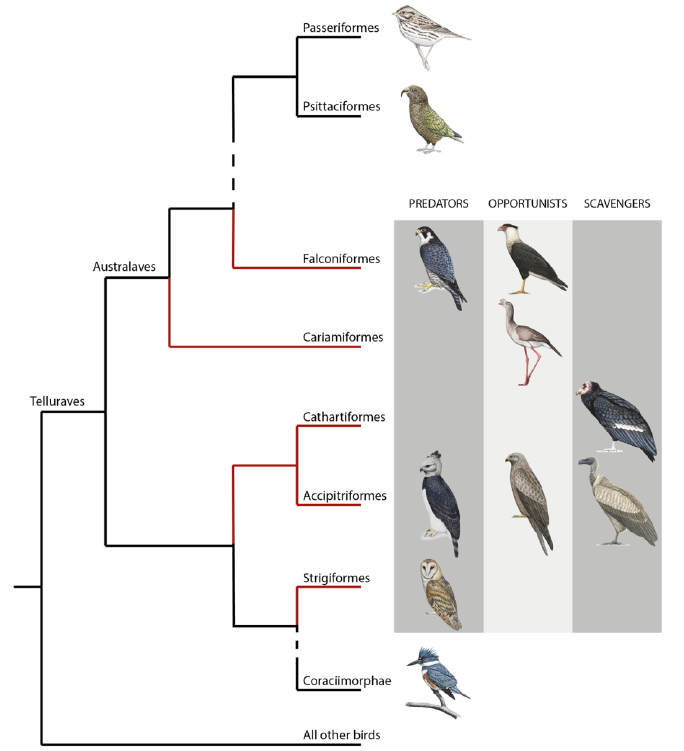
While precise terminology is essential in science, defining which species belong to “raptors” (or birds of prey) has been debated for decades [10]. Derived from the Latin word “rapere”, “raptor” means plunderer or ravisher. Recently, McClure et al. (2019) defined raptors as “all species within orders that evolved from a raptorial landbird lineage and in which most species maintained their raptorial lifestyle as derived from their common ancestor”[10]. This definition, based on phylogeny, ecology, and morphology, includes species in the orders of Accipitriformes (hawks, eagles, Old World vultures, kites), Cathartiformes (New World vultures), Strigiformes (owls), Falconiformes (falcons and caracaras), and the unexpected Cariamiformes (seriemas) (see Figure 1). Because they include species that do not belong to a monophyletic clade [11] and with important morphological and ecological differences, raptors may differ significantly in their sensory abilities.
Ecological diversity among diurnal birds of prey, or raptors, is highlighted regarding their sensory abilities. While raptors are believed to forage primarily using sight, the sensory demands of scavengers and predators differ, as reflected in their visual systems. Here, I have reviewed the visual specialisations of predatory and scavenging diurnal raptors, focusing on (1) the anatomy of the eye and (2) the use of vision in foraging. Predators have larger eyes than scavengers relative to their body mass, potentially highlighting the higher importance of vision in these species. Scavengers possess one centrally positioned fovea that allows for the detection of carrion at a distance. In addition to the central fovea, predators have a second, temporally positioned fovea that views the frontal visual field, possibly for prey capture. Spatial resolution does not differ between predators and scavengers. In contrast, the organisation of the visual fields reflects important divergences, with enhanced binocularity in predators opposed to an enlarged field of view in scavengers. Predators also have a larger blind spot above the head. The diversity of visual system specializations according to the foraging ecology displayed by these birds suggests a complex interplay between visual anatomy and ecology, often unrelatedly of phylogeny.
Figure 1. Phylogeny of core landbirds modified from [10]. The red branches encompass the order considered as raptors. For each order belonging to raptors, the presence/absence of the three foraging diet categories (predators, opportunists and scavengers) is represented by a drawing of a chosen species. “Raptor” is a paraphyletic group where species mostly share the raptorial lifestyle passed down from their single common ancestor [10]. This assumes that raptorial lifestyle has been lost twice independently with the ancestor of both Coraciimorphae and Passeriformes/Psittaciformes clades. Coraciimorphae contains six orders: Coliiformes (mousebirds), Trogoniformes (trogons), Coraciiformes (roller, kingfishers, and bee-eaters), Piciformes (woodpeckers), Leptosomiformes (cuckoo-rollers), and Bucerotiformes (hoopoes and hornbills). Drawings from Bryce W. Robinson.
2. Anatomical Specialization of the Eye
In general, birds have big eyes in both relative (compared with body mass) and absolute terms [11], and raptors have relatively larger eyes than other birds[5][12]. Large eyes have long focal lengths and subsequently larger retinal images, and thus a potential for higher spatial resolving power in diurnal animals[2][13]. As a result, the relatively large eyes of raptors indicate the importance of the visual system for their daily life, especially because important costs are associated with increased eye size: (1) increased risk of being damaged, (2) mechanical and aerodynamic constraints[12], (3) higher metabolic and energetic costs[14], or (4) disability glare because of increased direct sunlight in the absence of adnexa (e.g., eye lids, eye brows[15]).
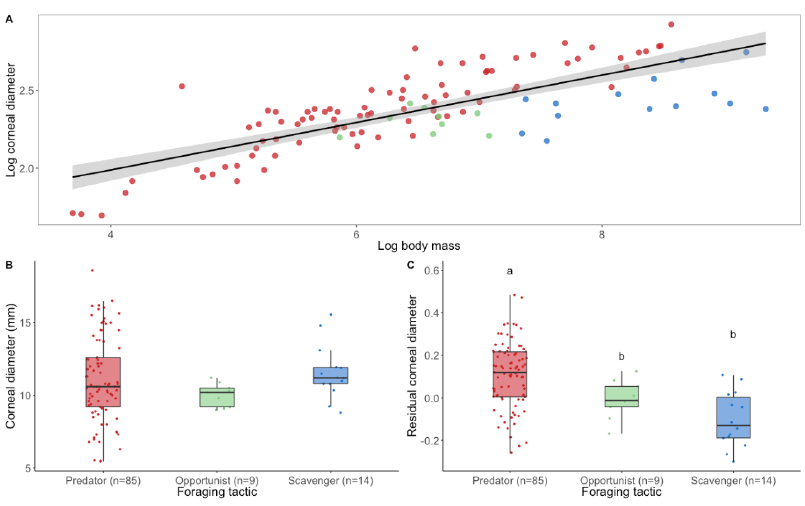
Interestingly, relative to their body mass, scavengers and opportunists have significantly smaller eyes than predators[16] (Figure 2A and Figure 3). This would suggest that scavengers and opportunists invest less in vision and potentially might not need as high spatial resolution of vision as predatory species. However, from behavioural and anatomical studies, there is no evidence for lower spatial resolution in scavengers, except for Cathartiformes, whose eyes are also smaller than those of other groups (but not significantly when controlling for phylogeny[5]). Because the neural structures compete for space in the brain[17] and larger eyes may require a greater proportion of brain space dedicated to vision, scavengers and opportunists may invest more in other sensory modalities. For instance, Cathartiformes have larger olfactory bulbs than other raptors[18], and most species in which olfactory abilities have been shown are scavengers (see [6] for a review). However, olfactory bulbs appear to be freer to vary in size irrespective of other sensory structures[19] and very little is known about olfactory abilities in raptors in general [6]. More investigations are needed to understand why scavengers have smaller eyes than predators.
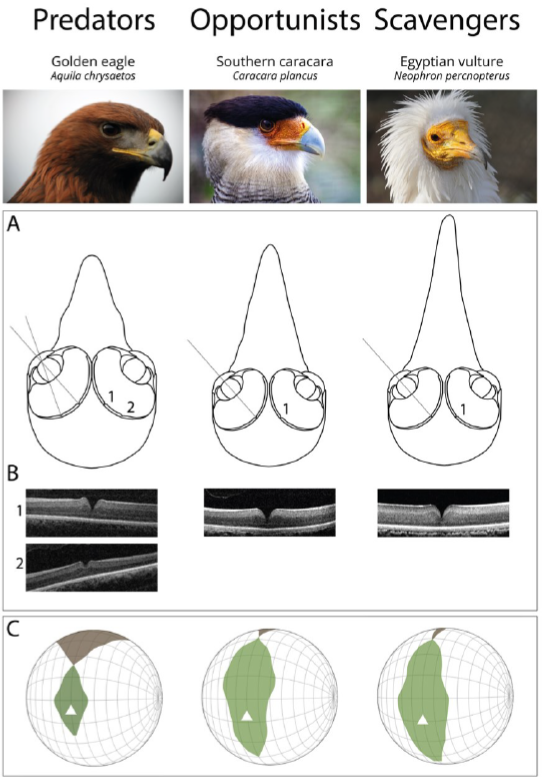
Figure 2. Functional differences of the visual system of raptors from different foraging tactics. (A) Schematic representation of frontal sections of the three chosen species (Golden eagle Aquila chrysaetos, Southern caracara Caracara plancus, and Egyptian vulture Neoprhon percnopterus) at the foveal plane. Fovea(s) and the centre of the pupil in each eye are on the plane. Grey lines represent the lines of sight of (1) the deep central fovea and (2) the shallow temporal fovea. Figures re-drawn from[20]. (B) Spectral domain optical coherence tomography (SD-OCT) images (B-scans) of the (1) central and (2) temporal fovea(s). Note that the Southern caracara and the Egyptian vulture lack temporal foveas. (C) Orthographic projection of retinal field boundaries of the eyes. A latitude and longitude coordinate system was used with the equator aligned vertically in the median sagittal plane (20 deg intervals in latitude and 10 deg intervals in longitude). The bird’s head is at the centre of the globe. Green areas represent the binocular sector, white areas represent the monocular sectors, and brown areas represent the blind sectors. Triangles: direction of bill projection. Figures modified from[21][22]. Photography of the species was free of right thanks to @myb777_photography for the Golden eagle, @wal_172619 for the Southern caracara, and @pixel_mixer for the Egyptian vulture.
Figure 3. The eye size of raptors according to their foraging tactic. (A) Logarithmic relationship (black line) and 95% confidence level interval (grey shade) between corneal diameter (a proxy for eye size) and body mass in raptors (estimate = 0.14 ± 0.01, t = 9.48, p < 0.001). (B) Corneal diameter and (C) residual corneal diameter calculated from corneal diameter scaled to body weight in relation to foraging tactics. Differences among foraging tactic were tested using phylogenetic linear regression. The phylogenetic relationships among 130 species were estimated using a consensus tree based on 100 randomly selected trees from www.BirdTree.org[11]using Ericson tree distribution. Data were analysed on R 4.0.0 using ggplot2[23], phylolm[24], phytools [25], caper[26], lmtest[27], ggpubr[28], and plyr[29]. Edge lengths were obtained by computing the mean edge length for each edge in the consensus tree. Model selection based on AICc and likelihood ratio test (lrtest function from lmtest package [27]) showed no differences for corneal diameter among foraging tactics (Chisq = 0.52, p = 0.77). By contrast, a significant difference was found for residual corneal diameter among foraging tactics (Chisq = 24.47, p < 0.001). Predators have significantly larger eyes compared with their body mass than opportunists (estimate = −0.11 ± 0.05, t = −2.20, p = 0.03) and scavengers (estimate = −0.21 ± 0.04, t = −4.90, p < 0.001). Scavengers and opportunists do not differ (estimate = −0.09 ± 0.06, t = −1.50, p = 0.14). Dots represent species. Different colours represent different foraging tactics (red = predator, green = opportunist, blue = scavenger). Different letters represent significant difference. Body mass were taken from [30] and corneal diameters from [[19],[31][32][33]]. (Boxplots: black lines represent the median, coloured boxes represent the interquartile (IQR) range from 25th (Q1) to 75th (Q3) percentile, whiskers represent Q1 − 1.5 *IQR and Q3 + 1.5 *IQR). Note: for foraging tactics classification, please refer to Supplementary Table S1.
References
- Stevens, M. Sensory Ecology, Behaviour, and Evolution; Oxford University Press: Oxford, UK, 2013.
- Land, M.F.; Nilsson, D.-E. Animal Eyes; Oxford University Press: Oxford, UK, 2012.
- Martin, G.R. The Sensory Ecology of Birds; Oxford University Press: Oxford, UK, 2017.
- Mitkus, M.; Potier, S.; Martin, G.R.; Duriez, O.; Kelber, A. Raptor vision. In Oxford Research Encyclopedia of Neuroscience; Oxford University Press: Oxford, UK, 2018.
- Potier, S.; Mitkus, M.; Kelber, A. Visual adaptations of diurnal and nocturnal raptors. Semin. Cell Dev. Biol. 2020, in press.
- Potier, S. Olfaction in raptors. Zool. J. Linn. Soc. 2019, 189, 713–721.
- Reymond, L. Spatial visual acuity of the eagle Aquila audax: A behavioural, optical and anatomical investigation. Vis. Res. 1985, 25, 1477–1491.
- Fischer, A.B. Laboruntersuchungen und freilandbeobachtungen zum sehvermögen und verhalten von altweltgeiern. Zool. Jahrb. Syst. Ökol. Geogr. Tiere 1968, 96, 81–132.
- Potier, S.; Mitkus, M.; Kelber, A. High resolution of colour vision, but low contrast sensitivity in a diurnal raptor. Proc. R. Soc. B Biol. Sci. 2018, 285, 20181036.
- McClure, C.J.W.; Schulwitz, S.E.; Anderson, D.L.; Robinson, B.W.; Mojica, E.L.; Therrien, J.-F.; Oleyar, M.D.; Johnson, J. Commentary: Defining Raptors and Birds of Prey. J. Raptor Res. 2019, 53, 419–430.
- Jetz, W.; Thomas, G.H.; Joy, J.B.; Hartmann, K.; Mooers, A.O. The global diversity of birds in space and time. Nature 2012, 491, 444–448.
- Walls, G.L. The Vertebrate Eye and Its Adaptive Radiation; Cranbrook Institute of Science : Bloomfield Hills, MI, USA ; Cranbrook Press: Toowoomba City, QLD, Australia, 1942.
- Brooke, M.L.; Hanley, S.; Laughlin, S.B. The scaling of eye size with body mass in birds. Proc. R. Soc. B Biol. Sci. 1999, 266, 405–412.
- Kiltie, R.A. Scaling of visual acuity with body size in mammals and birds. Funct. Ecol. 2000, 14, 226–234.
- Niven, J.E.; Laughlin, S.B. Energy limitation as a selective pressure on the evolution of sensory systems. J. Exp. Biol. 2008, 211, 1792–1804.
- Martin, G.R. Visual fields and their functions in birds. J. Ornithol. 2007, 148, 547–562.
- Potier, S.; Mitkus, M.; Bonadonna, F.; Duriez, O.; Isard, P.-F.; Dulaurent, T.; Mentek, M.; Kelber, A. Eye size, fovea, and foraging ecology in accipitriform raptors. Brain Behav. Evol. 2017, 90, 232–242.
- Zelenitsky, D.K.; Therrien, F.; Ridgely, R.C.; McGee, A.R.; Witmer, L.M. Evolution of olfaction in non-avian theropod dinosaurs and birds. Proc. R. Soc. B Biol. Sci. 2011, 279, 3625–3634.
- Bang, B.G.; Cobb, S. The size of the olfactory bulb in 108 species of birds. Auk 1968, 85, 55–61.
- Corfield, J.R.; Price, K.; Iwaniuk, A.N.; Gutiérrez-Ibáñez, C.; Birkhead, T.; Wylie, D.R. Diversity in olfactory bulb size in birds reflects allometry, ecology, and phylogeny. Front. Neuroanat. 2015, 9, 102.
- Tucker, V.A. The deep fovea, sideways vision and spiral flight paths in raptors. J. Exp. Biol. 2000, 203, 3745–3754.
- Potier, S.; Bonadonna, F.; Martin, G.R.; Isard, P.-F.; Dulaurent, T.; Mentek, M.; Duriez, O. Visual configuration of two species of Falconidae with different foraging ecologies. Ibis 2017, 160, 54–61.
- Potier, S.; Duriez, O.; Cunningham, G.B.; Bonhomme, V.; O’Rourke, C.; Fernández-Juricic, E.; Bonadonna, F. Visual field shape and foraging ecology in diurnal raptors. J. Exp. Biol. 2018, 221, jeb177295.
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis. Version 3.3.0; Springer-Verlag: New York, NY, USA, 2016.
- Ho, L.S.T.; Ane, C. phylolm: A linera-time algorithm for Gaussian and non-Gaussian trait evolution models. Syst. Biol. 2014, 63, 63–408.
- Revell, L.J. phytools: An R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 2012, 3, 217–223.
- Orme, D.; Freckleton, R.; Thomas, G.; Petzoldt, T.; Fritz, S.; Isaac, N.; Pearse, W. The caper: Comparative Analysis of Phylogenetics and Evolution in R. Available online: https://cran.r-project.org/web/packages/caper/vignettes/ (accessed on 24 September 2020).
- Zeileis, A.; Hothorn, T. lmtest: Diagnostic Checking in Regression Relationships. Available online: http://pkg.cs.ovgu.de/LNF/i386/5.10/R/LNFr-lmtest/reloc/R-2.10/library/lmtest/ (accessed on 24 September 2020).
- Kassambara, A. ggpubr: “ggplot2” based publication ready plots. R Package Version 0.2.5. 2020. Available online: https://CRAN.R-project.org/package=ggpubr (accessed on 24 September 2020).
- Wickham, H. plyr: The split-apply-combine strategy for data analysis. J. Stat. Soft. 2011, 40, 1–29.
- Dunning, J.B., Jr. CRC Handbook of Avian Body Masses; CRC Press: Boca Raton, FL, USA, 2007.
- Hwang, J.; Kang, S.; Seok, S.; Ahmed, S.; Yeon, S. Ophtalmic findings in cinereous vultures (Aegypius monachus). Vet. Ophthalmol. 2020, 23, 314–324.
- Ritland, S.M. The Allometry of the Vertebrate Eye; Department of Biology, University of Chicago: Chicago, IL, USA, 1984.