
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Vered Padler-Karavani | + 2055 word(s) | 2055 | 2020-10-10 08:03:17 | | | |
| 2 | Dean Liu | -1033 word(s) | 1022 | 2020-10-20 11:59:33 | | |
Video Upload Options
We generated a platform for designing optimized functional therapeutic antibodies against cancer glycans. The target tumor-associated carbohydrate antigen is commonly expressed in colon and pancreatic cancers. We developed a system for selection of potent antibodies by yeast surface display against this carbohydrate antigen, then showed that elite clones have potent affinity, specificity, cancer cell binding, and therapeutic efficacy. These tools have broad utility for manipulating and engineering antibodies against carbohydrate antigens, and provide major innovative avenues of research in the field of cancer therapy and diagnostics.
1. Introduction
Cancer is a leading cause of death worldwide and selective targeting by therapeutic monoclonal antibodies (mAbs) shows increasing success in modern oncology, mostly targeting proteins[1][2][3][4]. Cell surface glycosylation expression pattern is altered on cancer cells, leading to abnormal tumor-associated carbohydrate antigens (TACA) that are selectively and abundantly expressed on cancer cells[5][6]. These potentially valuable cancer cell surface targets are poorly immunogenic, hindering functional TACA-based cancer vaccines or immunotherapies, thus far [7][8][9]. Anti-carbohydrate antibodies typically have much lower affinities than antibodies recognizing proteins or peptide antigens (by 3–5 orders of magnitude), further complicated by carbohydrates large diversity in linkage types and modifications[10][11].
In 2018, The Nobel Prize in Chemistry was awarded to Frances H Arnold for inventing the directed evolution of enzymes, conjointly with George P. Smith and Sir Gregory P. Winter for their discoveries on phage display of peptides and antibodies. Yeast surface display (YSD) is one of the leading antibody engineering technologies to date, for both isolating novel antibodies and for directed evolution by in vitro affinity maturation of selected clones[12][13][14][15][16], allowing to identify mAb leads with good specificity and affinity. This system takes advantage of the agglutinin mating proteins (Aga1p and Aga2p) that are normally expressed on the yeast cell surface. These are expressed at 104–105 copies per cell, where Aga1p domain is anchored to the yeast cell wall and Aga2p is covalently attached to Aga1p through disulfide bonds[15][16]. In YSD, an antibody fragment is fused to the Aga2p allowing its cell surface presentation in accordance with the expression of the agglutinin proteins [18]. Most commonly, single chain fragment variable (scFv) or Fab antibody fragments are used in YSD, with surface expression and antigen binding monitored by flow cytometer allowing a very efficient sorting of large libraries according to antigen binding[18]. This system had mostly been employed against protein antigens[19][20].
Sialic acids (Sias) are acidic sugars found in vertebrates, topping cell surfaces glycans and glycoconjugates. Their expression patterns are altered on cancer cells[21][22][23], correlating with advanced stage, progression, and/or metastasis[24][25][26]. Thus, sialylated-TACA are promising targets for cancer therapy[27]. These include sialyl Lewis a (SLea) found on pancreas, colorectal, stomach and liver cancers that suffer very short five-year survival rates [28][29]. The SLea tetrasaccharide Neu5Acα2−3Galβ1−3(Fucα1−4)GlcNAcβ1-R, namely CA19−9 antigen, is a cancer-associated marker widely used in clinical practice[30][31][32][33]. It is the only FDA-approved test for pancreatic cancer, but is also used in colorectal, gastric, or biliary cancers. It is utilized to monitor response to therapy; however, it is not useful for early detection, diagnosis, or therapy[33][34][35]. Different antibodies are used to measure CA19-9; however, there is great variability between measured outcomes[36][37]. Taken together, these findings suggest that antibodies of greater specificity and affinity against SLea carbohydrate antigen could potentially serve as better cancer theranostic tools. Importantly, such potent antibodies should recognize their glycan target in the right context as presented on cancer cells or shed from them. As a proof of concept for development of efficient cancer therapeutics targeting glycosylation, we cloned the most commonly used antibody against SLea (1116-NS-19-9)[38] into YSD platform that was used to obtain specific anti-SLea antibodies of high affinity and potency in cancer cells binding and cytotoxicity.
2. Cloned Antibodies Are Effective at Cancer Cells Binding and Cytotoxicity
Cancer cell binding is critical for antibody therapeutic and diagnostic applications. Hence, cancer cell binding was examined to further evaluate antibody clones potency in the natural context. SLea expression was determined by native antibody and found positive in some cell lines (WiDr, Capan2, BxPC-3), while negative in others (MCF-7, MDA-MB-231) (Figure 1). Thus, we followed up investigation on antigen-positive cells. We compared the binding of native and RA9-23 antibody clones to the SLea-positive cancer cell lines WiDr and Capan2. RA9-23 showed higher binding efficiency compared to the native antibody clone in both cell lines, at various concentrations (Figure 1), and with high specificity since binding was reduced dramatically after removal of sialic acids from the cell surface by a sialidase treatment (Figure 1). Thus, RA9-23 antibody clone has high affinity against nanoparticle multivalent-glycans, and this is also reflected in the whole cell context, despite their heterogenous glycan expression patterns. It would be interesting to examine these novel antibody variants on patient samples in comparison to the native antibody. We next examined whether this improved cancer cell binding also translates into cancer cell killing. Antibodies of IgG1 isotype can facilitate cell killing by complement recruitment. Hence, complement-dependent cytotoxicity (CDC) was evaluated showing that RA9-23 clone has higher cytotoxicity in both WiDr and Capan2 cancer cell lines compared to the native clone (Figure 1). These results exemplify the potential of this improved antibody clone for both detection of SLea-positive tumors, and as cancer therapeutics.
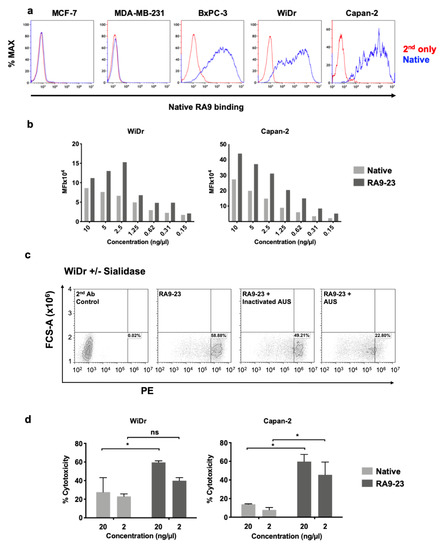
Figure 1. Antibody binding and cytotoxicity against cancer cells. (a) Binding of native antibody was examined by FACS against different cancer cell lines at 10 ng/μL (blue), compared to secondary antibody control (red). (b) Binding of native and RA9-23 IgGs to SLea-expressing cancer cells (WiDr and Capan2) was examined by FACS at various concentrations (10–0.15 ng/μL). (c) Specificity of binding to cells was demonstrated by treatment of cells with Arthrobacter Ureafaciens Sialidase (AUS) that abrogated binding of RA9-23 IgG to SLea-expressing WiDr cells, in comparison to direct binding of the antibody or its binding to cells treated with heat-inactivated AUS. (d) Complement-dependent cytotoxicity (CDC) of native and RA9-23 IgGs was examined. WiDr and Capan2 target cells were incubated with antibodies at concentrations 20 ng/μL and 2 ng/μL, then rabbit complement was added. Cytotoxicity was determined by LDH detection kit (representative of 2 independent experiments; 2-way ANOVA, * p < 0.05).
References
- Mellman, I.; Coukos, G.; Dranoff, G. Cancer immunotherapy comes of age. Nature 2011, 480, 480–489.
- Scott, A.M.; Allison, J.P.; Wolchok, J.D. Monoclonal antibodies in cancer therapy. Cancer Immun. 2012, 12, 14.
- Sliwkowski, M.X.; Mellman, I. Antibody therapeutics in cancer. Science 2013, 341, 1192–1198.
- Ankri, C.; Cohen, C.J. Out of the bitter came forth sweet: Activating CD28-dependent co-stimulation via PD-1 ligands. Oncoimmunology 2014, 3, e27399.
- Sanders, D.S.; Kerr, M.A. Lewis blood group and CEA related antigens; coexpressed cell-cell adhesion molecules with roles in the biological progression and dissemination of tumours. Mol. Pathol. 1999, 52, 174–178.
- Werther, J.L.; Tatematsu, M.; Klein, R.; Kurihara, M.; Kumagai, K.; Llorens, P.; Neto, J.G.; Bodian, C.; Pertsemlidis, D.; Yamachika, T.; et al. Sialosyl-Tn antigen as a marker of gastric cancer progression: An international study. Int. J. Cancer 1996, 69, 193–199.
- Heimburg-Molinaro, J.; Lum, M.; Vijay, G.; Jain, M.; Almogren, A.; Rittenhouse-Olson, K. Cancer vaccines and carbohydrate epitopes. Vaccine 2011, 29, 8802–8826.
- Yin, Z.; Huang, X. Recent Development in Carbohydrate Based Anti-cancer Vaccines. J. Carbohydr. Chem. 2012, 31, 143–186.
- Guo, Z.; Wang, Q. Recent development in carbohydrate-based cancer vaccines. Curr. Opin. Chem. Biol. 2009, 13, 608–617.
- Dingjan, T.; Spendlove, I.; Durrant, L.G.; Scott, A.M.; Yuriev, E.; Ramsland, P.A. Structural biology of antibody recognition of carbohydrate epitopes and potential uses for targeted cancer immunotherapies. Mol. Immunol. 2015, 67, 75–88.
- Haji-Ghassemi, O.; Blackler, R.J.; Martin Young, N.; Evans, S.V. Antibody recognition of carbohydrate epitopes†. Glycobiology 2015, 25, 920–952.
- Feldhaus, M.J.; Siegel, R.W. Yeast display of antibody fragments: A discovery and characterization platform. J. Immunol. Methods 2004, 290, 69–80.
- Hoogenboom, H.R. Selecting and screening recombinant antibody libraries. Nat. Biotechnol. 2005, 23, 1105–1116.
- Doerner, A.; Rhiel, L.; Zielonka, S.; Kolmar, H. Therapeutic antibody engineering by high efficiency cell screening. FEBS Lett. 2014, 588, 278–287.
- Chao, G.; Lau, W.L.; Hackel, B.J.; Sazinsky, S.L.; Lippow, S.M.; Wittrup, K.D. Isolating and engineering human antibodies using yeast surface display. Nat. Protoc. 2006, 1, 755–768.
- Angelini, A.; Chen, T.F.; de Picciotto, S.; Yang, N.J.; Tzeng, A.; Santos, M.S.; Van Deventer, J.A.; Traxlmayr, M.W.; Wittrup, K.D. Protein Engineering and Selection Using Yeast Surface Display. Methods Mol. Biol. 2015, 1319, 3–36.
- Cherf, G.M.; Cochran, J.R. Applications of Yeast Surface Display for Protein Engineering. Methods Mol. Biol. 2015, 1319, 155–175.
- Sheehan, J.; Marasco, W.A. Phage and Yeast Display. Microbiol. Spectr. 2015, 3, AID-0028.
- Boder, E.T.; Raeeszadeh-Sarmazdeh, M.; Price, J.V. Engineering antibodies by yeast display. Arch. Biochem. Biophys. 2012, 526, 99–106.
- Pepper, L.R.; Cho, Y.K.; Boder, E.T.; Shusta, E.V. A decade of yeast surface display technology: Where are we now. Comb. Chem. High Throughput Screen 2008, 11, 127–134.
- Padler-Karavani, V. Aiming at the sweet side of cancer: Aberrant glycosylation as possible target for personalized-medicine. Cancer Lett. 2014, 352, 102–112.
- Zanetta, J.-P.; Staedel, C.; Kuchler, S.; Zaepfel, M.; Meyer, A.; Vincendon, G. Malignant transformation in hepatocytes is associated with the general increase of glycoprotein ligands specifically binding to the endogenous lectin CSL. Carbohydr. Res. 1991, 213, 117–126.
- Kim, Y.J.; Varki, A. Perspectives on the significance of altered glycosylation of glycoproteins in cancer. Glycoconj. J. 1997, 14, 569–576.
- Brooks, S.A.; Carter, T.M.; Royle, L.; Harvey, D.J.; Fry, S.A.; Kinch, C.; Dwek, R.A.; Rudd, P.M. Altered glycosylation of proteins in cancer: What is the potential for new anti-tumour strategies. Anticancer Agents Med. Chem. 2008, 8, 2–21.
- Hedlund, M.; Ng, E.; Varki, A.; Varki, N.M. Alpha 2–6-linked sialic acids on N-glycans modulate carcinoma differentiation in vivo. Cancer Res. 2008, 68, 388–394.
- Fuster, M.M.; Esko, J.D. The sweet and sour of cancer: Glycans as novel therapeutic targets. Nat. Rev. Cancer 2005, 5, 526–542.
- Padler-Karavani, V.; Hurtado-Ziola, N.; Pu, M.; Yu, H.; Huang, S.; Muthana, S.; Chokhawala, H.A.; Cao, H.; Secrest, P.; Friedmann-Morvinski, D.; et al. Human xeno-autoantibodies against a non-human sialic acid serve as novel serum biomarkers and immunotherapeutics in cancer. Cancer Res. 2011, 71, 3352–3363.
- Ugorski, M.; Laskowska, A. Sialyl Lewis(a): A tumor-associated carbohydrate antigen involved in adhesion and metastatic potential of cancer cells. Acta Biochim. Pol. 2002, 49, 303–311.
- Borentain, P.; Carmona, S.; Mathieu, S.; Jouve, E.; El-Battari, A.; Gérolami, R. Inhibition of E-selectin expression on the surface of endothelial cells inhibits hepatocellular carcinoma growth by preventing tumor angiogenesis. Cancer Chemother. Pharm. 2016, 77, 847–856.
- Ballehaninna, U.K.; Chamberlain, R.S. The clinical utility of serum CA 19–9 in the diagnosis, prognosis and management of pancreatic adenocarcinoma: An evidence based appraisal. J. Gastrointest Oncol. 2012, 3, 105–119.
- Kirwan, A.; Utratna, M.; O’Dwyer, M.E.; Joshi, L.; Kilcoyne, M. Glycosylation-Based Serum Biomarkers for Cancer Diagnostics and Prognostics. BioMed Res. Int. 2015, 2015, 490531.
- Pinho, S.S.; Reis, C.A. Glycosylation in cancer: Mechanisms and clinical implications. Nat. Rev. Cancer 2015, 15, 540–555.
- Huang, Z.; Liu, F. Diagnostic value of serum carbohydrate antigen 19–9 in pancreatic cancer: A meta-analysis. Tumour Biol. 2014, 35, 7459–7465.
- Bauer, T.M.; Bekaii-Saab, T.S.; Li, X.; Villalona-Calero, M.A.; Philip, P.A.; Shields, A.F.; Zalupski, M.M.; Hammad, N.; El-Rayes, B.F. CA19–9 for the prediction of efficacy of chemotherapy in patients with advanced pancreas cancer: A pooled analysis of six prospective trials. J. Clin. Oncol. 2011, 29, 4071.
- Passerini, R.; Cassatella, M.C.; Boveri, S.; Salvatici, M.; Radice, D.; Zorzino, L.; Galli, C.; Sandri, M.T. The pitfalls of CA19–9: Routine testing and comparison of two automated immunoassays in a reference oncology center. Am J. Clin. Pathol. 2012, 138, 281–287.
- La’ulu, S.L.; Roberts, W.L. Performance characteristics of five automated CA 19–9 assays. Am. J. Clin. Pathol. 2007, 127, 436–440.
- Tang, H.; Singh, S.; Partyka, K.; Kletter, D.; Hsueh, P.; Yadav, J.; Ensink, E.; Bern, M.; Hostetter, G.; Hartman, D.; et al. Glycan motif profiling reveals plasma sialyl-lewis x elevations in pancreatic cancers that are negative for sialyl-lewis A. Mol. Cell Proteom. 2015, 14, 1323–1333.
- Koprowski, H.; Steplewski, Z.; Mitchell, K.; Herlyn, M.; Herlyn, D.; Fuhrer, P. Colorectal carcinoma antigens detected by hybridoma antibodies. Somat. Cell Genet. 1979, 5, 957–971.





