| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | V. P. Thinh NGUYEN | + 2987 word(s) | 2987 | 2020-10-12 11:56:14 |
Video Upload Options
Glucosinolates (GSLs) are secondary plant metabolites abundantly found in plant order Brassicales. GSLs are constituted by an S-β-d-glucopyrano unit anomerically connected to O-sulfated (Z)-thiohydroximate moiety. The side-chain of the O-sulfate thiohydroximate moiety, which is derived from a different amino acid, contributes to the diversity of natural GSL, with more than 130 structures identified and validated to this day. Both the structural diversity of GSL and their biological implication in plants have been biochemically studied. While intact GSLs are biologically inactive, various products, including isothiocyanates, nitriles, epithionitriles, and cyanides obtained through enzyme-catalyzed hydrolysis of GSLs, exhibit many different biological activities, among which several therapeutic benefits have been suggested.
1. Introduction
Amino acid-derived glucosinolates (GSLs), which are secondary plant metabolites constituted of a sulfate and thioglucose moiety, play important biological roles in the Brassicaceae family defense system, crops of great relevance to agriculture.[1] The coexistent thioglucosidase myrosinase (MYR) (EC 3.2.1.147) originally segregated within plants [2], will come in contact with GSL upon tissue disruption. Consequently, the enzymatic hydrolysis of GSL occurs to form glucose, and an unstable aglucone that undergoes degradation to afford a wide range of active components in response to environmental stresses (Figure 1). Along with the aforementioned role in the defense system, GSLs are likely involved in the survival system of the Brassicaceae family. In a study on Arabidopsis thaliana under abiotic stress (e.g., high salt), the overproduction of short-chain aliphatic GSL and underproduction of indolic GSL in leaves occurred [3], suggesting the adaptation of the plant in response to environmental stresses, and thus demonstrating the biological importance of GSLs in the Brassicaceae survival system, besides their prominent role involved in defense mechanism.
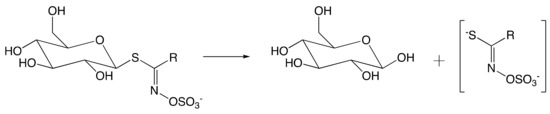
Figure 1. Hydrolysis of glucosinolate (GSL) by myrosinase (MYR) upon tissue disruption. (R = alkyl, aryl, indole).
2. Natural Occurrence of Glucosinolates
The abundant presence in Brassicaceae vegetables and condiments makes GSLs of interest to human society. To date, the therapeutic benefits of GSLs [4] have drawn more attention to this class of secondary metabolites, alongside with their original food purposes. Although several synthetic approaches have been documented [5], most natural GSLs reside in plants, with more than 130 different GSLs having been validated.[6][7]
GSL concentration is unequally distributed throughout the plant body. For instance, in Brassica napus, the GSL concentration in the seed is greater than that in leaves.[8] This variation appears to be more relevant in root vegetable crops (Moringacea family) than that in oilseed crops (Brassicaceae family). Moreover, the GSL profile varies depending on the tissue type. Although aliphatic GSLs predominate both in leaves and in seeds, indole GSLs are more abundant in leaves than in seeds.[9] This difference may be related to different functions of different parts of plants. A study of Troufflard et al. showing that A. thaliana accumulated more GSL in the roots than in the shoots in response to abiotic stress is clear evidence to support the last suggestion.[10] For further literature on plant response to abiotic stress involving GSL accumulation, we recommend the review by Martínez-Ballesta et al.[11]
Breeding approaches are often employed to obtain crops with low GSL content for food or feed purposes [26–28], while those with high GSL content remain of interest for non-food applications.[12][13][14] Therefore, the choice of species should be carefully considered with regard to the downstream purposes of raw materials. We also suggest that growth conditions should be highly regarded in order to adapt the chosen crops to their cultivating environment.
The occurrence of GSL varies among different species within the same order, as shown in Table 1. These variations even occur for the same crop depending on the years. For instance, Ishida et al. reported that the amount of GSLs in the same crops of Japanese radish varied between 2005 and 2009.[15] It is assumed that the accumulation of GSLs within plants highly depends on environmental factors such as the weather that undergoes slight changes through the years, thus directly impacting the GSL contents of the crops. Therefore, the GSL content of the same crops must be kept updated annually, or more frequently if needed.
Table 1. Occurrence of GSL in plants of order Brassicales. GSL concentration is expressed as a minimum–maximum in µmol/g of dry material.
|
Family |
Species |
Tissue |
GSL Content |
Reference |
|
Brassicaceae |
Camelina sativa |
Seed |
15.8–19.4 |
[16] |
|
Camelina rumelica subsp. rumelica |
Seed |
18.6–21.7 |
||
|
Camelina macrocarpa |
Seed |
8.0–19.1 |
||
|
Brassica napus |
Leaf Seed |
0.6–6.9 10.8–57.9 |
||
|
Brassica carinata A Braun |
Seed |
35–170 |
||
|
Brassica juncea |
Leaf |
4.3–129.9 |
[19] |
|
|
Seed |
15.7–127.6 |
|||
|
Brassica oleracea L. var capitata |
Leaf |
2.3–11.5 |
[20] |
|
|
Brassica oleracea L. var italica |
Floret |
8.2–19.5 |
[20] |
|
|
Brassica oleracea L. convar capitata var alba |
Petiole |
0.5–31.7 |
[21] |
|
|
Brassica rapa |
Leaf Seed |
17.3 39.4–81.3 |
[17] |
|
|
Arabidopsis thaliana |
Leaf |
5.0–30.7 |
[22] |
|
|
Raphannus sativus L. |
Root |
1.0–145.5 |
||
|
Moringacea |
Moringa oleifera Lam. |
Leaf Seed |
4.7–217 112–354.4 |
|
|
Moringa stenopetala L. |
Leaf Seed |
33.9–59.4 256–282 |
3. Structure and Classification of Glucosinolates
GSLs are anions composed of thiohydroxymates carrying an S-linked β-glucopyranosyl residue and an N-linked sulfate bearing an amino acid derived side-chain, which is referred to as the “R group” in the general structure Figure 1. This side-chain is subject to broad structural variation with associated biological functionalization associated.[6]
GSLs are frequently classified in three main families based on the nature of these amino acids, namely “aliphatic”, “aromatic”, and “indole”.[29] However, that classification is thought to be of little biological and chemical significance, according to the recent review by Blaževic et al.[6] The authors have then introduced a classification system based on amino acid precursors. In their review, they identify over 130 validated GSLs which were classified into nine panels from A to I depending on three main criteria: (1) amino acid precursor, (2) type of degradation product, either volatile or non-volatile isothiocyanates (ITC) or oxazolidine-2-thione; and, (3) presence and absence of an aromatic moiety in the GSL.
The proposed criteria offer a reliable system for GSL classification based on the chemical and biochemical properties of GSLs and their degradation product while conserving the information related to their amino acid precursor. The criterion concerning the presence or absence of an aromatic moiety in the GSL is meaningful as it allows the quick separation of a large amount of GSLs while using UV detectors. The usefulness of this criterion was demonstrated by the authors by separating GSLs of which Phe, Tyr, and Trp are precursors, from other non-aromatic groups. Moreover, further subgrouping within the aromatic group that separates indolic GSL from other phenylalkyl and less common aromatic GSLs appears to be of use.
4. Stability of Glucosinolates
4.1. Effects of Processing Methods on Glucosinolate Profile
Besides the chemical degradation involving MYR-catalyzed hydrolysis, the thermal degradation of GSLs is often mentioned.[30][31][32] As a result, GSL profiles of cooked brassica vegetables are altered at a different level depending on employed culinary techniques, such as cooking, steaming, and microwaving. The reduction of red cabbage (Brassica oleracea) indolic GSL during the cooking process was observed.[33] The content of glucobrassicin (Structure shown in Figure 8) and its homologs were drastically declined due to the cooking process performed under 120 °C. On the other hand, aliphatic GSLs appear to be more stable, with only a slight degradation has been observed under the same cooking conditions. The degradation became drastic for all GSL under canning conditions, whereas the process temperature exceeds 120 °C. The total amount of GSL has been reduced by over 70% under these harsh conditions. These observations are drawn from conclusions about the difference in thermal stabilities between aliphatic and indolic GSLs.
A study conducted by Song and Thornalley also reported the thermal degradation of GSL due to the domestic processing of Brassica vegetables, such as Brussel sprouts, broccoli, cauliflowers, and green cabbage.[34] Moreover, the effects of the cooking method, such as microwave, steam, and stir-fry, on GSL amounts of studied materials were investigated. The results showed that cooking by these cooking methods did not produce a significant loss of GSL, in contrast to boiling, which showed significant losses by leaching of GSL into cooking water at high temperatures.[30] Therefore, boiling Brassica should be avoided in order to preserve intact GSL in raw materials.
A recent study on the roasting process of rapeseed seed reported shows that industrial-scale post-harvest treatments, which are often necessary to produce higher quality oil-related products, also impact the GSL profile of plant materials.[32] Up to 29% of the original GSL amount in plant materials have been reduced during the roasting process. The results indicate that the industrial-scale roasting processes reduce the GSL amount of plant materials due to the thermal degradation, with up one-third of GSLs are degraded via thermal degradation.
Based on the information outlined above, we suggest that, with regards to downstream purposes, the selection of plant material should rely on the processing method. Although thermal treatments of plant materials, whereas the GSL content is often reduced, are beneficial for food and feed applications, these should be avoided in order to maintain the desired amount of GSL for non-food purposes. The review by Hanschen et al.[35] is highly recommended for further reading concerning the reactivity and stability of GSL and their breakdown products in food.
4.2. Degradation of Glucosinolates in Solution
The stability of GSL and desGSL from Moringa oleifera in solution was investigated with the presence and absence of buffer.[25] The GSL extracted from plant materials, either desulfated or intact, were dissolved in ultra-pure water and stored at room temperature or −20 °C. After nine days of storage, the GSL profile of the extracts was analyzed. The results showed that GSLs were stable at low temperatures with little isomeric conversion or degradation of GSLs having occurred. On the other hand, a GSL solution stored at room temperature showed conversion among acetylated GSL isomers. Furthermore, the degradation of GSLs has been reported to be up to 32% of the original total amount of GSL. At room temperature, buffered solutions of GSL appear to be more stable than those in water solution, with a reduction of 20% of the total amount of GSLs being recorded within nine days. There was no significant difference between unbuffered and buffered GSL stored at low temperatures. Based on this information, storing GSL in buffer solutions at low temperatures (at −20 °C, in preference) is suggested to safely conserve the original GSL profile in extract when GSL is required to be stored in solution instead of stable solid salt form.
5. Biological Activities of Glucosinolates
5.1. Mechanism of Myrosinase
GSL play an important role in the defense mechanism of Brassica plants. Upon tissue disruption, catabolites released by MYR-catalyzed hydrolysis are frequently responsible for the toxicity of the parent GSL, which, in contrast, are biologically inactive.[36][37] This mechanism of prevention against herbivory feeding suggested the main function of GSLs in plant defense systems.[38]
The intact GSLs are stored separately from the thioglycosidase MYR. The latter catalyzes the hydrolysis of GSL upon plant tissue disruption. As described in Figure 1, an unstable aglucone moiety has been released alongside with the glucose during hydrolysis. The aglucone moiety then undergoes further transformation to yield a number of metabolites.
MYR belongs to the Glycosidase family (EC 3.2.1.). Although it catalyzes S-glycosylation, the deduced amino acid sequences of MYR reveal strong similarities with several O-glycosidases.[39] Furthermore, MYR displays a retaining mechanism that is similar to that of family 1-O-glycosidases.[40] In order to elucidate the mechanism of MYR, Burmeister et al. have studied the crystallographic structure of MYR.[39][41]
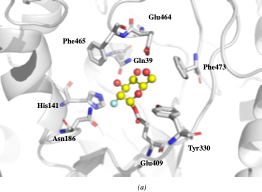
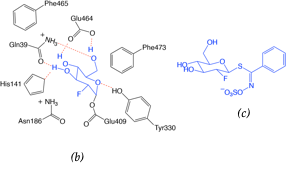
The crystallographic structure was generated by soaking the MYR crystals in 2-deoxy-2-fluoroglucosinolate (2FG) (Structure shown in Figure 2c). The results clearly showed that the 2-fluoroglucose moiety, released from the substrate upon myrosinase attack, is covalently bound to Glu409 within the active site (Figure 2a). The crystallization of 2FG-MYR complex confirmed MYRs as retaining glycosyl hydrolases.
Figure 2. Overview of the active site of Sinapis alba Myrosinase showing interactions between residues and the 2-deoxy-2-fluoroglucosinolate (2FG) as substrate (Protein Data Bank accession number 1E70, resolution: 1.65 Å).[41] Red dashed lines show hydrogen bonding interactions between the substrate and MYR residues within the active site. (a) Representation of the active site of Sinapis alba Myrosinase generated using PyMol. (b) Chemical structure representation of the MYR-2FG. (c) Structure of 2-deoxy-2-fluoroglucosinolate.
Like most retaining glycosyl hydrolases, MYRs follow a conventional two-step mechanism: (1) the formation of covalent substrate-enzyme intermediate; and (2) the release of glucose via hydrolysis of the previously formed intermediate. The mechanism of glucose hydrolysis is described in Figure 3. The glycosylation begins with the introduction of GSL into the active site of MYR. The residue Glu406 then binds to the glucose moiety of the substrate at the anomeric position, releasing aglucone moiety.
Ascorbic acid was identified as a coenzyme of MYR for the first time by Ettlinger et al.[42] Although it has been proved to be nonessential for the catalyzed hydrolysis of GSL[41], the presence of ascorbic acid enhances up to 400-fold the glycosylation of MYR [42]. The ultimate step consists in the release of both ascorbic acid and glucose from the active site to yield the enzyme in its native conformation.
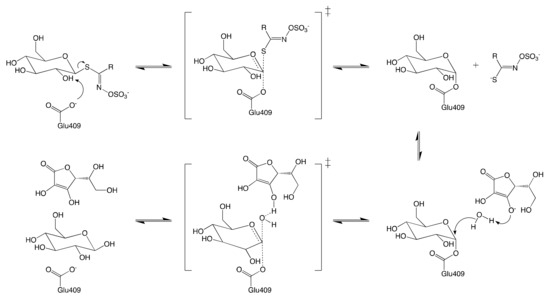
Figure 3. Schematic reaction mechanism of MYR in the presence of ascorbic acid.
5.1.1. Hypothetical Recognition Role of Sulfate Group
Although represented as a characteristic of GSL, the sulfate group in the aglucone moiety exhibits an unclear function towards MYRs. Nonetheless, the distorted conformation of GSLs due to the interaction of the sulfate group with the amino acid side-chain of the myrosinase within its active site has been mentioned.[41] Based on these results, it was hypothesized that myrosinase recognizes glucosinolate substrates via the sulfate group.
Attempts to rationalize the recognitive function of the sulfate group have been conducted based on the feeding pattern of crucifer specialist insects. The investigation on Plutella xylostella larvae feeding pattern devised by Ratzka et al. suggested that the removal of the sulfate group renders GSLs invisible to MYR.[43] Furthermore, a number of articles have been published emphasizing the importance of the removal of the sulfate group of GSL which allows specialist insects to feed on crucifer plants.[43][44][45]
These observations are strong proof supporting our hypothesis regarding the recognition role of the sulfate group within the defense system in crucifer plants. However, there is, to date, no further research article investigating the sulfate group of GSLs since the publication of the crystal structure of Sinapis alba MYR by Burmeister et al.[39][41] Further investigation of the substrate recognition mechanism of MYRs will undoubtedly confirm the role of the sulfate group.
5.1.2. Reconfiguration of Unstable Aglucone
As described previously, an unstable aglucone moiety of GSL is released alongside with a glucose unit upon MYR-catalyzed hydrolysis. A number of biologically active compounds are next obtained via the reconfiguration of unstable aglucone.[30] ITC, the most studied among GSL catabolites, is obtained via a spontaneous Lossen rearrangement of the corresponding aglucone under physiological conditions (Figure 4).
An additional range of bioactive non-ITC catabolites from MYR-catalyzed hydrolysis were also identified.[30][43] Sinigrin is the only known GSL that can form ITC alongside other products such as nitriles, epithionitriles, and thiocyanates (Figure 4). Their formation is regulated by the prerequisite allyl structure of the aglucone and the presence of protein specifiers.[46] It is noteworthy that these catabolites are as well obtained in low-yield in vitro at low pH in the presence of ferrous ions in spite of the absence of specifier-proteins.[43] These findings draw conclusions about the pH dependence of catabolite formation due to the reconfiguration of GSL aglucones.[6]
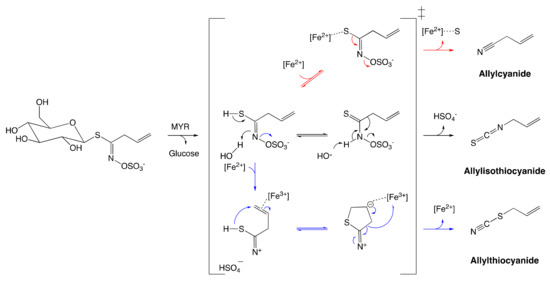
Figure 4. Reconfiguration of unstable allylglucosinolate aglucone upon myrosinase-catalyzed hydrolysis. The black arrow pathway shows the formation of allylisothiocyanates employing spontaneous Lossen arrangement. The Blue arrow pathway shows the formation of allylthiocyanate assisted by protein specifier. The red arrow pathway indicates the formation of allylcyanide assisted by protein specifier. The figure was adapted from Eisenschmidt-Bönn et al.[46]
5.2. Biological Activities of Glucosinolates and Their Catabolites
Negative effects of GSL on domestic animals have been documented by Tripathi and Mishra in their review.[47] These effects usually occur upon the assimilation of GSLs at high concentrations. Among relevant symptoms, reduction of feed intake, which causes growth depression, and induction iodine deficiency are often reported.[48][49] Moreover, high GSL diets eventually result in higher mortality in pigs, rats, and rabbits.[47] As such, an intake limit of GSL should be defined d in order to avoid the occurrence of unexpected negative effects.
To the best of our knowledge, there is no clear evidence in the literature indicating the negative effect of GSL on human health upon assimilation. In contrast, GSL catabolites such as ITC and nitrile have been proved to provide attractive therapeutic effects such as the induction of phase II enzymes.[50] The augmentation of tissue levels of the phase II detoxification enzymes is associated with decreased susceptibility to chemical carcinogenesis.[51] In their study, Munday and Munday observed an increase in the phase II detoxification enzymes, such as quinone reductase and glutathione S-transferase in rat tissues by daily oral-assimilating of different ITC compounds.[50] The authors, therefore, suggested that chemoprotective effects are common in ITC.
GSL catabolites are potent inhibitors of bacterial activity].[4] Although intact GSL was usually bio-inactive [47], allyl ITCs exhibit antimicrobial activities. By studying the effect of allyl ITCs on Staphylococcus aureus, a methicillin-resistant bacterium that causes purulent skin and soft tissue infections, Dias et al. concluded that these molecules issued from catalyzed-hydrolysis GSL possess strong antimicrobial activity against these specific bacteria.[52]
Biofumigation is a process where plants are used as natural “pesticides” to reduce soil-borne pests and pathogens. Biofumigation properties of GSL and their breakdown products have been investigated by Haschen et al.[53] In their study, the cultivation of Brassica juncea produced a significant amount of GSL and their hydrolysis products, such as ITC and nitrile, and released them into the cultivating soil. Consequently, the inhibition of bacterial community growth that cannot support the effects of breakdown products of GSLs has been observed. These results confirmed the fumigation properties of GSLs and their breakdown products
In other circumstances, GSLs are catalytically hydrolyzed in vivo by supplementary proteins known as specifier proteins.[54] These latter promote the formation of non-ITC catabolites such as nitriles, epithionitriles, and thiocyanates, of which biological roles have been reviewed.[46] The coexistence of specifier proteins, along with MYR suggests the adaptation of the plant to circumvent the presence of natural enemies. For instance, favoring the production of simple nitriles over ITC upon herbivore damage enables better defense of A. thaliana against the specialist herbivore.[55]
References
- Ute Wittstock; Barbara Ann Halkier; Cytochrome P450 CYP79A2 fromArabidopsis thalianaL. Catalyzes the Conversion of l-Phenylalanine to Phenylacetaldoxime in the Biosynthesis of Benzylglucosinolate. Journal of Biological Chemistry 2000, 275, 14659-14666, 10.1074/jbc.275.19.14659.
- Birgit Hafeld Borgen; Ole Petter Thangstad; Ishita Ahuja; John Trevor Rossiter; Atle M. Bones; Removing the mustard oil bomb from seeds: transgenic ablation of myrosin cells in oilseed rape (Brassica napus) produces MINELESS seeds. Journal of Experimental Botany 2010, 61, 1683-1697, 10.1093/jxb/erq039.
- Mcarmen Emartinez-Ballesta; Diego A. Moreno; Diego Ecastejon; Cristina Eochando; Piero A. Morandini; Micaela Carvajal; The impact of the absence of aliphatic glucosinolates on water transport under salt stress in Arabidopsis thaliana. Frontiers in Plant Science 2015, 6, 524, 10.3389/fpls.2015.00524.
- Anisha Mazumder; Anupma Dwivedi; Johan Du Plessis; Sinigrin and Its Therapeutic Benefits. Molecules 2016, 21, 416, 10.3390/molecules21040416.
- Patrick Rollin; Arnaud Tatibouët; Glucosinolates: The synthetic approach. Comptes Rendus Chimie 2011, 14, 194-210, 10.1016/j.crci.2010.05.002.
- Ivica Blažević; Sabine Montaut; Franko Burčul; Carl Erik Olsen; Meike Burow; Patrick Rollin; Niels Agerbirk; Glucosinolate structural diversity, identification, chemical synthesis and metabolism in plants. Phytochemistry 2020, 169, 112100, 10.1016/j.phytochem.2019.112100.
- Niels Agerbirk; Carl Erik Olsen; Glucosinolate structures in evolution. Phytochemistry 2012, 77, 16-45, 10.1016/j.phytochem.2012.02.005.
- Sheng Liu; Huibin Huang; Xinqi Yi; Yuanyuan Zhang; Qingyong Yang; Chunyu Zhang; Chuchuan Fan; Yongming Zhou; Dissection of genetic architecture for glucosinolate accumulations in leaves and seeds of Brassica napus by genome‐wide association study. Plant Biotechnology Journal 2019, 18, 1472-1484, 10.1111/pbi.13314.
- Pablo Velasco; Pilar Soengas; Marta Vilar; Maria Elena Cartea; Mercedes Del Rio; Comparison of Glucosinolate Profiles in Leaf and Seed Tissues of Different Brassica napus Crops. Journal of the American Society for Horticultural Science 2008, 133, 551-558, 10.21273/jashs.133.4.551.
- Stephanie Troufflard; William Mullen; Tony R Larson; Ian A. Graham; Alan Crozier; A. Amtmann; Patrick Armengaud; Potassium deficiency induces the biosynthesis of oxylipins and glucosinolates in Arabidopsis thaliana. BMC Plant Biology 2010, 10, 172-172, 10.1186/1471-2229-10-172.
- Mcarmen Emartinez-Ballesta; Diego A. Moreno; Micaela Carvajal; The Physiological Importance of Glucosinolates on Plant Response to Abiotic Stress in Brassica. International Journal of Molecular Sciences 2013, 14, 11607-11625, 10.3390/ijms140611607.
- A. Marquez-Lema; José M. Fernández-Martínez; Begoña Pérez-Vich; Leonardo Velasco; Transgressive segregation for reduced glucosinolate content in Brassica carinata A. Braun. Plant Breeding 2006, 125, 400-402, 10.1111/j.1439-0523.2006.01240.x.
- A. Márquez‐Lema; José M. Fernández-Martínez; Begoña Pérez-Vich; Leonardo Velasco; Inheritance of very high glucosinolate content in Ethiopian mustard seeds. Plant Breeding 2009, 128, 278-281, 10.1111/j.1439-0523.2008.01563.x.
- Gwen M. Chodur; Mark E. Olson; Kristina L. Wade; Katherine K. Stephenson; Wasif Nouman; Garima; Jed W. Fahey; Wild and domesticated Moringa oleifera differ in taste, glucosinolate composition, and antioxidant potential, but not myrosinase activity or protein content. Scientific Reports 2018, 8, 7995, 10.1038/s41598-018-26059-3.
- Masahiko Ishida; Masayasu Nagata; Takayoshi Ohara; Tomohiro Kakizaki; Katunori Hatakeyama; Takeshi Nishio; Small variation of glucosinolate composition in Japanese cultivars of radish (Raphanus sativus L.) requires simple quantitative analysis for breeding of glucosinolate component. Breeding Science 2012, 62, 63-70, 10.1270/jsbbs.62.63.
- Lisa Amyot; Tim McDowell; Sara L. Martin; Justin Renaud; Margaret Y. Gruber; Abdelali Hannoufa; Assessment of Antinutritional Compounds and Chemotaxonomic Relationships between Camelina sativa and Its Wild Relatives. Journal of Agricultural and Food Chemistry 2018, 67, 796-806, 10.1021/acs.jafc.8b04724.
- M. Moshgani; E. Kolvoort; T. J. De Jong; Pronounced effects of slug herbivory on seedling recruitment of Brassica cultivars and accessions, especially those with low levels of aliphatic glucosinolates. Basic and Applied Ecology 2014, 15, 607-615, 10.1016/j.baae.2014.08.011.
- B. Martina Baaij; Hye Kyong Kim; Katharina Grosser; Anja Worrich; T. J. De Jong; Slug herbivory on hybrids of the crop Brassica napus and its wild relative B. rapa. Basic and Applied Ecology 2018, 31, 52-60, 10.1016/j.baae.2018.06.001.
- Shilpa Gupta; Manjeet K Sangha; Gurpreet Kaur; Amarjeet K Atwal; Shashi Banga; Variability for Leaf and Seed Glucosinolate Contents and Profiles in a Germplasm Collection of the Brassica juncea. Biochemistry & Analytical Biochemistry 2012, 1, 1, 10.4172/2161-1009.1000120.
- Liyang Wei; Changhong Liu; Huanhuan Zheng; L. Zheng; Melatonin treatment affects the glucoraphanin-sulforaphane system in postharvest fresh-cut broccoli (Brassica oleracea L.). Food Chemistry 2020, 307, 125562, 10.1016/j.foodchem.2019.125562.
- Alexander Döring; Bernd Ulber; Performance of cabbage stem flea beetle larvae (Psylliodes chrysocephala ) in brassicaceous plants and the effect of glucosinolate profiles. Entomologia Experimentalis et Applicata 2020, 168, 200, 10.1111/eea.12891.
- Francisco R. Badenes-Perez; Michael Reichelt; Jonathan Gershenzon; David G. Heckel; Interaction of glucosinolate content of Arabidopsis thaliana mutant lines and feeding and oviposition by generalist and specialist lepidopterans. Phytochemistry 2013, 86, 36-43, 10.1016/j.phytochem.2012.11.006.
- Gibum Yi; Sooyeon Lim; Won Byoung Chae; Jeong Eun Park; Hye Rang Park; Eun Jin Lee; Jin Hoe Huh; Root Glucosinolate Profiles for Screening of Radish (Raphanus sativusL.) Genetic Resources. Journal of Agricultural and Food Chemistry 2015, 64, 61-70, 10.1021/acs.jafc.5b04575.
- Olivia Naa Ayorkor Tetteh; Christian Ulrichs; Susanne Huyskens-Keil; Inga Mewis; Newton Kwaku Amaglo; Ibok Nsa Oduro; Charles Adarkwah; Daniel Obeng-Ofori; Nadja Förster; Effects of harvest techniques and drying methods on the stability of glucosinolates in Moringa oleifera leaves during post-harvest. Scientia Horticulturae 2019, 246, 998-1004, 10.1016/j.scienta.2018.11.089.
- Nadja Förster; Christian Ulrichs; Monika Schreiner; Carsten T. Müller; Inga Mewis; Development of a reliable extraction and quantification method for glucosinolates in Moringa oleifera. Food Chemistry 2015, 166, 456-464, 10.1016/j.foodchem.2014.06.043.
- Rui Chen; Xiu-Juan Wang; Yao-Yuan Zhang; Yan Xing; Liu Yang; He Ni; Hai-Hang Li; Simultaneous extraction and separation of oil, proteins, and glucosinolates from Moringa oleifera seeds. Food Chemistry 2019, 300, 125162, 10.1016/j.foodchem.2019.125162.
- Yalemtsehay Mekonnen; Birgit Dräger; Glucosinolates inMoringa stenopetala. Planta Medica 2003, 69, 380-382, 10.1055/s-2003-38881.
- Richard N. Bennett; Fred A. Mellon; Nikolaus Foidl; John H. Pratt; M. Susan Dupont; Lionel Perkins; Paul A. Kroon; Profiling Glucosinolates and Phenolics in Vegetative and Reproductive Tissues of the Multi-Purpose TreesMoringa oleiferaL. (Horseradish Tree) andMoringa stenopetalaL.. Journal of Agricultural and Food Chemistry 2003, 51, 3546-3553, 10.1021/jf0211480.
- Ida E. Sønderby; Fernando Geu-Flores; Barbara A. Halkier; Biosynthesis of glucosinolates – gene discovery and beyond. Trends in Plant Science 2010, 15, 283-290, 10.1016/j.tplants.2010.02.005.
- Francisco J. Barba; Nooshin Nikmaram; Shahin Roohinejad; Anissa Khelfa; Zhenzhou Zhu; Mohamed Koubaa; Bioavailability of Glucosinolates and Their Breakdown Products: Impact of Processing. Frontiers in Nutrition 2016, 3, 24, 10.3389/fnut.2016.00024.
- Franziska S. Hanschen; Domestic boiling and salad preparation habits affect glucosinolate degradation in red cabbage (Brassica oleracea var. capitata f. rubra). Food Chemistry 2020, 321, 126694, 10.1016/j.foodchem.2020.126694.
- Bingyu Jing; Rui Guo; Mengzhu Wang; Lingyan Zhang; Xiuzhu Yu; Influence of seed roasting on the quality of glucosinolate content and flavor in virgin rapeseed oil. LWT - Food Science and Technology 2020, 126, 109301, 10.1016/j.lwt.2020.109301.
- Kirsten Oerlemans; Diane M. Barrett; Carme Bosch Suades; Ruud Verkerk; Matthijs Dekker; Thermal degradation of glucosinolates in red cabbage. Food Chemistry 2006, 95, 19-29, 10.1016/j.foodchem.2004.12.013.
- Lijiang Song; Paul J. Thornalley; Effect of storage, processing and cooking on glucosinolate content of Brassica vegetables. Food and Chemical Toxicology 2007, 45, 216-224, 10.1016/j.fct.2006.07.021.
- Franziska S. Hanschen; Evelyn Lamy; Monika Schreiner; Sascha Rohn; Reactivity and Stability of Glucosinolates and Their Breakdown Products in Foods. Angewandte Chemie International Edition 2014, 53, 11430-11450, 10.1002/anie.201402639.
- L. Lazzeri; Giovanna Curto; Onofrio Leoni; Elisabetta Dallavalle; Effects of Glucosinolates and Their Enzymatic Hydrolysis Products via Myrosinase on the Root-knot NematodeMeloidogyne incognita(Kofoid et White) Chitw.. Journal of Agricultural and Food Chemistry 2004, 52, 6703-6707, 10.1021/jf030776u.
- S. Buskov; B. Serra; E. Rosa; H. Sørensen; J. C. Sørensen; Effects of Intact Glucosinolates and Products Produced from Glucosinolates in Myrosinase-Catalyzed Hydrolysis on the Potato Cyst Nematode (Globodera rostochiensisCv. Woll). Journal of Agricultural and Food Chemistry 2002, 50, 690-695, 10.1021/jf010470s.
- Barbara Ann Halkier; Jonathan Gershenzon; BIOLOGY AND BIOCHEMISTRY OF GLUCOSINOLATES. Annual Review of Plant Biology 2006, 57, 303-333, 10.1146/annurev.arplant.57.032905.105228.
- Wim P. Burmeister; S. Cottaz; Hugues Driguez; Renato Iori; Sandro Palmieri; Bernard Henrissat; The crystal structures of Sinapis alba myrosinase and a covalent glycosyl-enzyme intermediate provide insights into the substrate recognition and active-site machinery of an S-glycosidase.. Structure 1997, 5, 663-676, 10.1016/s0969-2126(97)00221-9.
- S. Cottaz; Bernard Henrissat; Hugues Driguez; Mechanism-Based Inhibition and Stereochemistry of Glucosinolate Hydrolysis by Myrosinase†. Biochemistry 1996, 35, 15256-15259, 10.1021/bi9622480.
- Wim P. Burmeister; S. Cottaz; Patrick Rollin; Andrea Vasella; Bernard Henrissat; High Resolution X-ray Crystallography Shows That Ascorbate Is a Cofactor for Myrosinase and Substitutes for the Function of the Catalytic Base. Journal of Biological Chemistry 2000, 275, 39385-39393, 10.1074/jbc.m006796200.
- M. G. Ettlinger; G. P. Dateo; B. W. Harrison; T. J. Mabry; C. P. Thompson; VITAMIN C AS A COENZYME: THE HYDROLYSIS OF MUSTARD OIL GLUCOSIDES. Proceedings of the National Academy of Sciences 1961, 47, 1875-1880, 10.1073/pnas.47.12.1875.
- Ute Wittstock; Meike Burow; Glucosinolate Breakdown in Arabidopsis: Mechanism, Regulation and Biological Significance. The Arabidopsis Book 2010, 8, e0134, 10.1199/tab.0134.
- Andreas Ratzka; Heiko Vogel; Daniel J. Kliebenstein; Thomas Mitchell-Olds; Juergen Kroymann; Disarming the mustard oil bomb. Proceedings of the National Academy of Sciences 2002, 99, 11223-11228, 10.1073/pnas.172112899.
- Kimberly L. Falk; Jonathan Gershenzon; The Desert Locust, Schistocerca gregaria, Detoxifies the Glucosinolates of Schouwia purpurea by Desulfation. Journal of Chemical Ecology 2007, 33, 1542-1555, 10.1007/s10886-007-9331-0.
- Daniela Eisenschmidt‐Bönn; Nicola Schneegans; Anita Backenköhler; Ute Wittstock; Wolfgang Brandt; Structural diversification during glucosinolate breakdown: mechanisms of thiocyanate, epithionitrile and simple nitrile formation.. The Plant Journal 2019, 99, 329-343, 10.1111/tpj.14327.
- M.K. Tripathi; A.S. Mishra; Glucosinolates in animal nutrition: A review. Animal Feed Science and Technology 2007, 132, 1-27, 10.1016/j.anifeedsci.2006.03.003.
- A. Aumaitre; D. Bourdon; J. Peiniau; J.P.B. Freire; Effect of graded levels of raw and processed rapeseed on feed digestibility and nutrient utilization in young pigs. Animal Feed Science and Technology 1989, 24, 275-287, 10.1016/0377-8401(89)90149-1.
- Nicolas Mabon; S.N.M Mandiki; G DeRycke; J.-L Bister; J.-P Wathelet; M Marlier; R Paquay; Chemical changes and influences of rapeseed antinutritional factors on lamb physiology and performance. 3. Antinutritional factors in plasma and organs. Animal Feed Science and Technology 2000, 85, 111-120, 10.1016/s0377-8401(00)00122-x.
- Rex Munday; Christine M. Munday; Induction of Phase II Detoxification Enzymes in Rats by Plant-Derived Isothiocyanates: Comparison of Allyl Isothiocyanate with Sulforaphane and Related Compounds. Journal of Agricultural and Food Chemistry 2004, 52, 1867-1871, 10.1021/jf030549s.
- Thomas W. Kensler; Chemoprevention by Inducers of Carcinogen Detoxication Enzymes. Environmental Health Perspectives 1997, 105, 965, 10.2307/3433311.
- Carla Dias; Alfredo Aires; Maria J. Saavedra; Antimicrobial Activity of Isothiocyanates from Cruciferous Plants against Methicillin-Resistant Staphylococcus aureus (MRSA). International Journal of Molecular Sciences 2014, 15, 19552-19561, 10.3390/ijms151119552.
- Franziska S. Hanschen; Bunlong Yim; Traud Winkelmann; Kornelia Smalla; Monika Schreiner; Degradation of Biofumigant Isothiocyanates and Allyl Glucosinolate in Soil and Their Effects on the Microbial Community Composition. PLOS ONE 2015, 10, e0132931, 10.1371/journal.pone.0132931.
- Jennifer C Kuchernig; Meike Burow; Ute Wittstock; Evolution of specifier proteins in glucosinolate-containing plants. BMC Evolutionary Biology 2012, 12, 127, 10.1186/1471-2148-12-127.
- Roland Mumm; Meike Burow; Gabriella Bukovinszkine’Kiss; Efthymia Kazantzidou; Ute Wittstock; Marcel Dicke; Jonathan Gershenzon; Formation of Simple Nitriles upon Glucosinolate Hydrolysis Affects Direct and Indirect Defense Against the Specialist Herbivore, Pieris rapae. Journal of Chemical Ecology 2008, 34, 1311-1321, 10.1007/s10886-008-9534-z.
- Ida E. Sønderby; Fernando Geu-Flores; Barbara A. Halkier; Biosynthesis of glucosinolates – gene discovery and beyond. Trends in Plant Science 2010, 15, 283-290, 10.1016/j.tplants.2010.02.005.