
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Alyssa Francavilla | + 4389 word(s) | 4389 | 2020-09-29 09:54:43 | | | |
| 2 | Camila Xu | -179 word(s) | 4210 | 2020-10-26 09:37:39 | | |
Video Upload Options
Anthocyanins are water-soluble phenolic pigments responsible for red, purple, blue, or even black colours in fruits, vegetables, grains, flowers, and other pigmented plant tissues. All anthocyanins share the same core structure, a flavylium ion, consisting of two aromatic ring structures linked by a three-carbon heterocyclic ring that contains oxygen. The anthocyanidin (aglycone form) is the core structure of the anthocyanin. The addition of a sugar side chain results in the glycosidic form of the anthocyanidin molecule, called an anthocyanin.
1. Introduction
Anthocyanidins, as well as their glycosylated and acyl glycosylated forms, can be found in nature [6,11]. Over 23 anthocyanidins and 500 different anthocyanins have been isolated from plants [7,12,13]. Anthocyanidins differ in their hydroxylation and methoxylation degree and pattern. The large diversity in anthocyanins does not only stem from the variability in the anthocyanidin core structure, they also differ in the nature and number of sugars attached to the core structure, as well as the nature and number of side chains attached to these sugar residues [6,9,14]. The most common sugar residues found in anthocyanins are glucose, xylose, rhamnose, arabinose, and galactose [6,9,14]. While monosaccharide functional groups are more common, di- and tri-saccharide groups are also found [14]. Sugars are most commonly linked to the aglycone core at position 3 (Figure 1) [9,14]. When multiple sugar groups are present, these additional sugar moieties are often linked to positions 5 and/or 7 of the aglycone core structure (Figure 1) [6,9,14]. The acylglycosidic form occurs when sugar groups are acylated with aliphatic, hydroxybenzoic, or hydroxycinnamic acids [9,15]. The most commonly found acids are malonic, acetic, and caffeic acids [14].

Figure 1. The structures of six common anthocyanins and one acylated anthocyanin, with the individual ring substituents listed below the flavylium ion backbone [9,16].
Despite the wide structural variations present in anthocyanin structures, six main anthocyanidin compounds are commonly found in food products [6,11]. About 50% of anthocyanins found in fruits and cereals are cyanidin derivatives, followed by pelargonidin (12%), delphinidin (12%), peonidin (12%), petunidin (7%), and malvidin (7%) derivatives [11]. The conjugated bonds of the anthocyanidin moiety are responsible for the molecule’s colour [8,11]. Anthocyanidin compounds vary in the degree of ring hydroxylation and methoxylation [6,8,13]. The level of hydroxylation/methoxylation generally influences the hue of the compound as well as the molecule’s stability, with increased hydroxylation increasing the anthocyanin’s blueness and reducing its stability [9]. Increased methoxylation, on the other hand, increases the anthocyanin’s redness and molecular stability [6,8,9,10].
In addition to the chemical structure of anthocyanins, the anthocyanin concentration, temperature, and pH, as well as exposure to light, oxygen, enzymes, ions, other flavonoid and phenolic compounds, ascorbic acid, and sulphites affect the stability of the anthocyanin molecules [9,10,17]. The anthocyanidin backbone of the molecule is highly reactive due to the electron-deficient flavylium cation structure [6,10,17]. This (deglycosylated) molecular structure is particularly vulnerable to nucleophilic attack by water; however, the glycosylated and acylated anthocyanins can also suffer from this degradation [6,9,10]. Anthocyanins are often partially degraded by the combined action of cellular and environmental factors [10,17]. One of the degradation patterns shows a breakdown from the quinoidal base to the flavylium ion, followed by a conversion to the carbinol pseudobase and chalcone, which is heat labile and easily cleaved [9,15]. The rate of this degradation is controlled significantly by temperature, with higher temperatures resulting in decreased stability [9,15]. Co-pigmentation also plays a large role in the stability of cereal anthocyanins, but may also impact the absorption of compounds during digestion [6,9,10,18,19]. The electron-deficient flavylium ion associates with co-pigments that are rich in electrons, having a net stabilising effect by protecting the flavylium cation from nucleophilic attack by water at position 2, and from other species (e.g., ascorbic acid, peroxides) at position 4 (Figure 1) [15]. Co-pigmentation can occur through a variety of interactions and with a range of co-pigments including other anthocyanins, aglycones, metals, or other phenolic compounds [6]. Acylated anthocyanins are also more stable than alternate forms, due to the protection from nucleophilic attacks conferred by the acyl groups [10,16]. The stability conferred by the acyl group is dictated by the location, type, and number of acyl groups that have been esterified to the anthocyanin [16]. While any of the anthocyanidin hydroxyl side groups can be esterified by organic acids under specific conditions, most commonly, acylation occurs on the glycosyl –OH groups [16]. Acyl groups can stack around the flavylium ring and protect it from degradation, due to the flexibility of the associated sugar groups [15,16]. Acyl groups have been found to negatively affect the absorption of anthocyanins after digestion [19].
Anthocyanin analysis is predicated on efficient extraction and identification. Multiple methods have been developed to achieve this [6,9,10]. Most commonly, crude solvent extraction methods are used on ground whole grains. Polar organic solvents (methanol, ethanol, or acetone) are combined with an acid (hydrochloric acid, formic acid, etc.) to create an acidic solvent [6,14]. Methanol and hydrochloric acid are often used due to their efficacy and minimal hazard level, and due to their status as common lab materials [14]. Acidification of the solvent allows for the anthocyanins to be extracted in a stable form, although it can result in partial hydrolysis of acyl moieties present [6,14]. This can be minimised with the use of weak acids [6,14]. Recent advances include the use of pressurised liquid extraction, supercritical fluid extraction, accelerated solvent extraction, and microwave-assisted solvent extraction [6,14,20]. Several anthocyanin quantification techniques exist that differ largely in their complexity. The spectrophotometric method first published by Hucl [21], wherein the anthocyanin concentration in an extract is measured at the wavelength of maximum cyanidin absorbance (i.e., 520–525 nm) and is corrected for molar absorptivity, is most commonly used. This simple method provides the total anthocyanin content (TAC) expressed as cyanidin-3-glucoside equivalents per weight of the starting material [21]. The differential pH method is also utilised for anthocyanin determination, and is predicated on the anthocyanin’s structural and absorption changes at different pH values, where the compound is red at pH 1.0, and turns colourless at pH 4.5 [22]. Alternatively, the TAC can also be derived from chromatographic methods used for identifying the different anthocyan (id) in species. High-performance liquid chromatography (HPLC), for example, is used with various detectors for this purpose. There is a strong correlation between the measured TAC results obtained with the spectrophotometric method and the HPLC-UV/Vis method [23]. UV/Vis detectors can be used for general anthocyanin determination in HPLC analysis, allowing for the identification (based on retention times) and quantification of the main anthocyanins and anthocyanidins (cyanidin, pelargonidin, etc.) but can present some difficulty when discriminating between different side chains [7,14]. The use of more specific detectors such as LC/MS (Liquid Chromatography-Mass Spectroscopy), and PDA-MS (Photo Diode Array-Mass Spectroscopy) can result in a more detailed understanding of the presence of side chains, including sugar moieties and acidic residues [6,14].
Anthocyanin analysis also includes a variety of assays that focus on the antioxidant potential of cereal anthocyanins. However, when utilising these methods, it can be difficult to distinguish the effect of the anthocyanins from that of other antioxidants (e.g., phenolic acids, coumarins, etc.) present in whole grains. Testing isolated extracts is one strategy to distinguish between anthocyanins and other phenolics. Some commonly utilised assays include DPPH-free radical assay, oxygen radical antioxidant capacity (ORAC), Trolox equivalent antioxidant capacity (TEAC), and ferric reducing antioxidant power (FRAP) analyses [24]. These are used alone, or in combination, to determine the antioxidant potential of the cereal grain in general, or the cereal anthocyanins in particular [24]. Differences in antioxidant potentials suggest that the reducing, radical scavenging, and iron chelating capacities of phenolic and flavonoid compounds vary depending on their structure [25].
2. Anthocyanins in Whole Grain Cereals
2.1. Wheat
Wheat (Triticum aestivum) is consumed by humans in large quantities globally, and is second only to rice as a cereal component in the human diet [7,26,27]. The non-anthocyanin-containing white and red wheat varieties are most commonly consumed, while blue, purple, and black wheat varieties are either not cultivated on a large scale or are predominantly grown as a speciality crop. Although wheat species have been studied to gain insight on the anthocyanin content, structure, and profile, the exact anthocyanin composition of these grains is still largely unknown [28]. This high level of uncertainty on coloured wheat anthocyanin structure and profile (i.e., the relative occurrence of each anthocyanin) is mainly due to large differences observed between wheat varieties and differences in extraction methods. Novel identification methods such as HPLC-MS have made strides in clarifying the presence and levels of the different anthocyanins in plants. These methods have even shed light on the prevalence of less common anthocyanins in plants.
Blue, purple, and black wheat species contain large quantities of anthocyanins in the outer kernel layers (Figure 2). In purple wheat, anthocyanins are localised in the pericarp, whereas in blue wheat they are found in the aleurone layer. In black (also referred to as “deep purple”) wheat, anthocyanins can be found in both the pericarp and the aleurone layer [29]. The distribution of anthocyanins in the outer kernel layers affects the extractability and stability of these compounds in the wheat grain. Purple wheat has, on average, a lower TAC than blue and black wheat varieties. It is theorised that, in blue and black varieties, the pigment location leads to an enhanced anthocyanin stability [30,31]. The superior colour stability stems from the protection that several layers of pericarp confer to the underlying aleurone layer [30]. Indeed, it has been shown that the pericarp of wheat kernels is often damaged during harvest or subsequent transport [32]. It has also been observed that anthocyanin pigments are more tightly bound in the pericarp than in the aleurone layer, therefore making them relatively less extractable from purple wheat varieties. Anthocyanin content and localisation can be determined from the genetic profile of the wheat [30]. The challenge of developing commercially relevant coloured wheat varieties can be mainly brought back to issues of limited harvest yield [30]. Blue aleurone genes have been linked to negative impacts on yield [30]. Finding a strategy to disrupt the link between anthocyanin production and yield would be beneficial for wheat breeders to develop a higher yield coloured wheat variety [30].
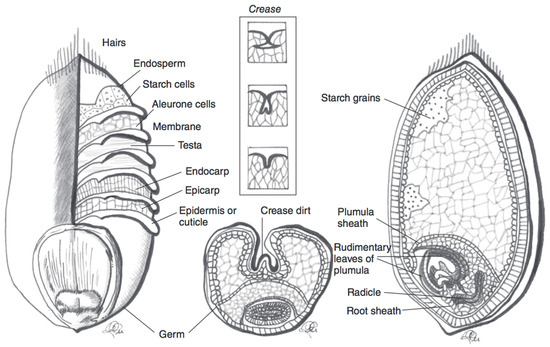
Figure 2. Schematic representation of a wheat kernel cross-section, with component parts. The pericarp is represented by the endocarp and esocarp in this illustration. Adapted from reference [27].
The anthocyanin compounds present in coloured wheat vary based on wheat variety, as well as growing conditions [33]. The influence of growing conditions and kernel maturity makes definitive anthocyanin identification and quantification challenging, even within one variety [33]. The bioaccumulation of phenolic compounds is not yet well understood [34,35]. Growth temperature and genotype have been found to correlate with total phenolic compounds [35]. Rain levels have also been demonstrated to affect TAC in grains [36]. Moreover, while identification methods have improved in sensitivity and specificity, there are still unidentified anthocyanins. Many studies have been performed to elucidate the identity and quantity of wheat anthocyanins. An overview of data, from a selection of studies, is presented in Table 1.
Table 1. Selection of studies focusing on total anthocyanin content (TAC) in various wheat cultivars. The anthocyanin quantification method and assay used for antioxidant potential are also listed.
| Wheat Colour | Wheat Variety | TAC (mg/kg Kernel Weight) | TAC Measurement | Antioxidant Potential | Total Phenolic Content |
|---|---|---|---|---|---|
| Purple | Laval [28] | 96 ± 5 | Spectrophotometer | n.d. | n.d. |
| Purple | Laval 19 [31] | 10 | pH Differential | n.d. | n.d. |
| Purple | Charcoal [31] | 305 | pH Differential | n.d. | n.d. |
| Purple | Indigo [24] | 72 ± 1 | Spectrophotometer | 3959 µmol TE/100 g (ORAC) | 160 µmol FAE/100 g |
| Blue | Purendo [37] | 212 ± 3 | Spectrophotometer | n.d. | n.d. |
| Blue | Purendo [28] | 18 ± 4 | Spectrophotometer | n.d. | n.d |
| Blue | BVAL-214025 [38] | 74 ± 2 | Spectrophotometer | 5 mmol Fe2 + equiv/100 g (FRAP) | 163 mg FAE/100 g |
| Black | BW/2 PBW621-F5 [30] | 198 ± 9 | Spectrophotometer | n.d. | n.d. |
| Black | Black Wheat [30] | 186 ± 17 | Spectrophotometer | n.d. | n.d. |
| Black | UC 66049/Konini [31] | 56 | pH Differential | n.d. | n.d. |
The anthocyanin profile of black, blue, and purple wheat is complex and diverges between varieties. In Laval and Konini purple wheat varieties, Abdel-aal [28] found that the most abundant anthocyanin is cyanidin-3-glucoside (4 mg/g), followed by peonidin-3-glucoside (2 mg/g), cyanidin-malonyl-glucose (1 mg/g), and cyanidin-succinyl-glucose (1 mg/g), along with peonidin-malonyl-glucose, peonidin-succinyl-glucose, and peonidin-malonyl-succinyl-glucose. Purple wheat anthocyanins are more often acylated than the anthocyanins found in blue and black wheat [30]. It is unclear whether this observation is due to the location of anthocyanins (pericarp vs. aleurone), or to other unknown factors. The TAC of purple wheat has been found to range between 10 and 305 mg/kg dry kernel weight [24,28,31]. In one blue wheat variety, delphinidin-3-glucoside is the most prominent anthocyanin (57 mg/g), followed by delphinidin-3-rutinoside (41 mg/g), cyanidin-3-glucoside (20 mg/g), and cyanidin-3-rutinoside (17 mg/g), along with petunidin-3-glucoside, petunidin-3-rutinoside, and malvidin-rutinoside [28]. The TAC of blue wheat ranges between 17 and 211 mg/kg dry kernel weight [5,28,30,31,37,38]. Finally, black wheat has been less well characterised, possibly due to the limited availability of black wheat varieties. The black wheat TAC is thought to vary between 56 and 198 mg/kg dry wheat kernel, and an average of 120 ± 10 mg/kg dry kernel weight can be calculated [30,31]. Twenty-six distinct anthocyanin compounds were identified in a black wheat variety and two of its ancestral lines [30]. All six major anthocyanidins were represented (i.e., cyanidin, delphinidin, pelargonidin, petunidin, malvidin, and peonidin) with glucoside and rutinoside linkages [30]. Additionally, some anthocyanins had tri-saccharide linkages, such as rutinoside associated with a pentose sugar [30].
2.2. Barley
Barley (Hordeum vulgare) is another major cereal grain that is grown in temperate climates [27]. Barley is often used in fermentation processes as a sugar source. An example of this is during the production of alcoholic beverages [27]. Barley also serves as animal feed, and can be used as an edible grain (cooked berries). Barley cultivars are usually yellow to amber in colour, but the seeds can also take on more uncommon colours such as white, purple, blue, and black [27]. Both hulled and hull-less varieties of barley exist [27,39]. The fibrous hull is inedible, and is typically removed before consumption [27]. Dehulled (and hull-less) barley grains can then be further processed for human consumption. Barley seeds may be left as whole berries (pot barley), or the bran may be removed in a pearling process [39]. Both of these products can be further milled into a fine flour [39]. However, as is the case for other cereals, the anthocyanins in barley are localised in the outer kernel layers, and more specifically in the pericarp or aleurone cell layers for purple and blue barley, respectively (Figure 3) [38,40]. Therefore, similar to what is found for wheat, milling usually results in a fine flour that does not contain significant anthocyanin levels (after the removal of the bran layers). Purple and blue barley cultivars have a higher average anthocyanin content than their black counterparts [38]. This is likely due to the contribution of a melanin-based pigment to black barley pigmentation, rather than sole reliance on anthocyanins for colour [38].
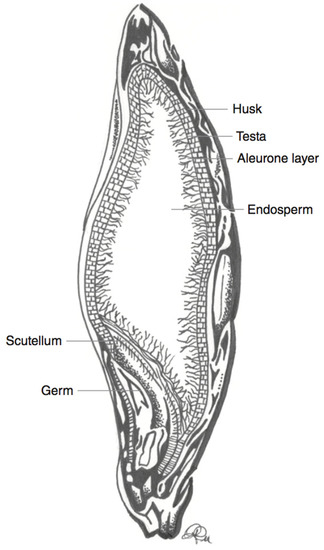
Figure 3. Schematic representation of a barley kernel cross-section, with component parts. Adapted from reference [27].
As with wheat, growing conditions and genotype influence the type and quantity of anthocyanins in the grain (Table 2) [33].
Table 2. Selection of studies focusing on total anthocyanin content (TAC) in various barley cultivars. The anthocyanin quantification method and assay used for antioxidant potential are also listed.
| Barley Colour | Barley Variety | TAC (mg/kg Kernel Weight) | TAC Measurement | Antioxidant Potential | Total Phenolic Content |
|---|---|---|---|---|---|
| Purple | CI-1248 [39] | 573 ± 26 | HPLC | 3937 µmol TE/100 g (ORAC) | n.d. |
| Purple | Yu 5904-088 [40] | 679 ± 19 | Spectrophotometer | 78% (DPPH) | n.d. |
| Blue | Kompolti korai 1 [41] | 84 ± 3 | Spectrophotometer | n.d. | n.d. |
| Blue | Ubamer [40] | 77 ± 2 | Spectrophotometer | 35% (DPPH) | n.d. |
| Blue | Tankard [28] | 345 ± 1 | Spectrophotometer | n.d. | n.d. |
| Black | Peru-35 [39] | 0 | HPLC | 5430 µmol TE/100 g (ORAC) | n.d. |
| Black | Black [40] | 8 ± 1 | Spectrophotometer | 29% (DPPH) | n.d. |
HPLC: High-performance liquid chromatography.
The anthocyanin profile and quantity of barley vary between studies. In black barley, the TAC varied from 0 to 77 ± 17 mg/kg of kernel [38,39,40]. The anthocyanin types that were found in the black barley samples were dissimilar between varieties. Using LC-MS, peonidin derivatives were identified as the main anthocyanins [40]. Conversely, using HPLC-UV/Vis, delphinidin-3-glucoside was identified as the main anthocyanin in another purple variety, followed by peonidin-3-glucoside and malvidin-3-glucoside [39]. In blue barley, using LC-MS—similarly to black cultivars—peonidin derivatives were identified as the dominant anthocyanins [40]. In another study, LC-MS resolved cyanidin-3-glucoside and petunidin-3-glucoside, which were found to be the most prevalent anthocyanins [41]. Conversely, using HPLC, petunidin-based anthocyanins were not detected, while cyanidin-3-glucoside and delphinidin-3-glucoside were [41,42]. Blue barley has a TAC between 35 and 84 mg/kg dry kernel weight, while purple barley has anthocyanin contents between 573 and 679 mg/kg dry kernel weight [28,38,39,40,41,42]. In purple barley, a more complex anthocyanin profile was found. Using LC-MS, cyanidin-3-glucoside, pelargonidin-3-glucoside, peonidin-3-glucoside, cyanidin-3-(6″ succinyl) glucoside, and peonidin-3-(6″ succinyl) glucoside, as well as various other cyanidin and peonidin derivatives, are found [39,42]. HPLC-UV/Vis analysis confirmed this anthocyanin profile, identifying peaks for cyanidin, pelargonidin, and peonidin-3-glucoside [42]. Hulled barley cultivars had lower anthocyanin contents than their hull-less counterparts, across all three coloured varieties [42]. However, the hull fraction was found to also contain anthocyanins [42]. As such, a portion of the total anthocyanins would be lost upon processing/dehulling [42].
2.3. Maize
Maize (Zea mays) is widely consumed in Mexico and Central America [27]. The major use of maize in the human diet, however, involves the isolation of corn starch and its use as a refined food ingredient [27,43]. The classification of maize into subspecies is primarily based on the amount and type of starch in the kernel [27,43]. The maize subspecies include dent, waxy, and sweet corn species, among others. There also exists great genetic diversity in maize kernel colour [43,44]. Black, blue, pink, red, purple, and brown maize varieties exist [43]. In purple maize kernels, as with wheat and barley, anthocyanins are found in the pericarp, while in blue varieties, anthocyanins are found in the aleurone layer. In black and dark red kernels, anthocyanins are found in both the aleurone and pericarp layers (Figure 4) [44]. A selection of the current data for TAC in various coloured maize species is outlined in Table 3.
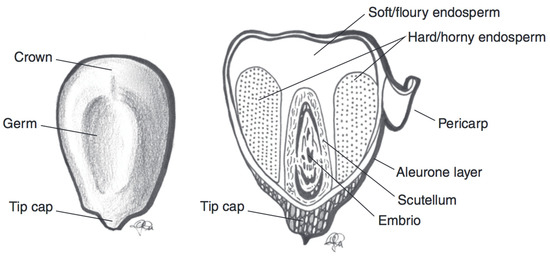
Figure 4. Schematic representation of a maize kernel cross-section, with component parts. Adapted from reference [27].
Table 3. Selection of studies focusing on total anthocyanin content (TAC) in various maize cultivars. The anthocyanin quantification method and assay used for antioxidant potential are also listed.
| Maize Colour | Maize Variety | TAC (mg/kg Kernel Weight) | TAC Measurement | Antioxidant Potential | Total Phenolic Content |
|---|---|---|---|---|---|
| Purple | KKU-WX211003 [45] | 89 ± 1 | pH Differential | 14 μmol TE/g DW (DPPH) | 7 mg GAE/g DW |
| Purple | AREQ516540TL [46] | 850 ± 6 | Spectrophotometer | 92% (ABTS) | 3400 mg GAE/100g |
| Purple-black | KKU-WX111031 [45] | 1439 ± 8 | pH Differential | 22 μmol TE/g DW (DPPH) | 20 mg GAE/g DW |
| Purple-yellow | KKU-WX211004 [45] | 27 ± 1 | pH Differential | 12 μmol TE/g DW (DPPH) | 6 mg GAE/g DW |
| Red-yellow | ZPL-5 [47] | 3 ± 0 | HPLC | 23 mmol TE/kg (TEAC) | 6011 GAE/kg DM |
| Dark red | ZPP-1 selfed [47] | 696 ± 3 | HPLC | 27 mmol TE/kg (TEAC) | 6115 GAE/kg DM |
| Light blue | ZPP-2 selfed [47] | 379 ± 5 | HPLC | 36 mmol TE/kg (TEAC) | 10529 GAE/kg DM |
| Blue | Blue [46] | 100 ± 2 | Spectrophotometer | 63% (ABTS) | 343 mg GAE/100 g |
| Black | Black [46] | 77 ± 2 | Spectrophotometer | 60% (ABTS) | 457 mg GAE/100 g |
| Black | Negro normal OLI 04 PV [46] | 120 ± 2 | Spectrophotometer | 85% (ABTS) | 544 mg GAE/100 g |
Variations in anthocyanin profile can be observed between different maize colours, as well as between waxy and other maize subspecies. When testing ornamental maize varieties using LC/MS, a very complex anthocyanin profile was resolved [28]. Upon in-depth analysis of the anthocyanin profile in Shaman Blue, Cutie Pink, purple, Scarlet Red, and Fiesta maize varieties, a minimum of 15 distinct anthocyanins were identified (Fiesta) [28]. The Scarlet Red maize variety even had 27 distinct anthocyanin peaks [28]. All of the tested varieties had cyanidin-3-glucoside as the main anthocyanin [28]. Most of the anthocyanins in the coloured maize varieties were glycosylated and some were acylated (purple and scarlet) [28]. However, a substantial amount of aglycones (cyanidin, peonidin, pelargonidin) was also found [28]. The aglycones found could have been present in the corn prior to extraction and quantification, or were produced due to anthocyanin degradation during processing. In the waxy corn varieties tested, the anthocyanin profile is somewhat less intricate, with only ~7 to 10 anthocyanin compounds identified [45]. In all varieties, cyanidin derivatives were predominant [45]. Acylated cyanidins, including cyanidin-3-(6″ malonyl glucoside) and cyanidin-3-(3″,6″-dimalonyl glucoside), were the majority of these, making up between 46–84% of the total anthocyanins in waxy corn [45]. In general, coloured maize varieties were found to contain between 27 to 1439 mg anthocyanins/kg of dry kernel [28,44,45,46,47]. When classified according to the different coloured varieties, the average TAC (in mg/kg dry kernel weight) was between 99 to 379 (blue), 26.5 to 1439 (purple), 76.2 to 120 (black), and 2.5 to 696 (pink/red) [28,44,45,46,47]. The differences in TAC reported in these studies may be due to various factors, including differences between genotypes, developmental stages, growing conditions, and quantification and extraction methods [33]. Milk stage maize kernels were consistently shown to have lower TAC than mature kernels, indicating that anthocyanins are formed or deposited in the kernel during maturation [48]. Therefore, slight differences in maturation level at harvest can have a large impact on the anthocyanin content [48].
2.4. Rice
Rice is the most consumed cereal grain in Asia [27]. Rice is either consumed in a polished form (the bran layer is removed), as a milled flour, or eaten as a whole grain (with the bran layer intact). Colloquially, rice with its bran layer intact is referred to as brown rice [4,27]. However, the terminology “brown” refers to the presence of the darker coloured bran rather than the hue of the kernel [4,27]. The USDA National Small Grains Collection classifies rice into seven different colour groups: purple, variable purple, red, brown, speckled brown, light brown, and white coloured rice [49]. The most widely cultivated and consumed rice is light brown in colour [4,27]. However, the coloured rice varieties classified as red and purple have been the focus of increased interest due to potential health benefits. In coloured rice kernels, anthocyanins are also believed to accumulate in the outer layers of the kernels. However, studying the exact localisation of anthocyanins in rice kernels is hampered by the difficulty of separating the outer layers of a rice kernel [49]. After polishing, Shao [50] found that only 3% of TAC was retained in the kernels relative to the whole kernels. It is hypothesised that anthocyanins are localised similarly in rice kernels to other cereal grains; with anthocyanins in the pericarp of purple varieties, the aleurone of blue varieties, and with anthocyanins in both locations of black varieties (Figure 5). A selection of some reports on the TAC in rice is displayed in Table 4.
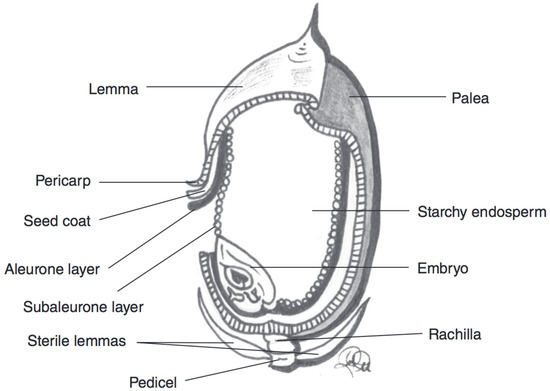
Figure 5. Schematic representation of a rice kernel cross-section, with component parts. Adapted from reference [27].
Table 4. Selection of studies focusing on total anthocyanin content (TAC) in various rice cultivars. The anthocyanin quantification method and assay used for antioxidant potential are also listed.
| Rice Colour | Rice Variety | TAC (mg/kg Kernel Weight) | TAC Measurement | Antioxidant Potential | Total Phenolic Content |
|---|---|---|---|---|---|
| Purple | GPNO 20,175 [49] | 68 ± 29 | Spectrophotometer | 98 µmol TE/g bran (ORAC) | 6 mg GAE/g bran |
| Purple | Hung Hsien Ju PI 16,097 [49] | 151 ± 25 | Spectrophotometer | 174 µmol TE/g bran (ORAC) | 12 mg GAE/g bran |
| Purple | Hung Tsan [49] | 199 ± 36 | Spectrophotometer | 147 µmol TE/g bran (ORAC) | 11 mg GAE/g bran |
| Purple | IAC600 [51] | 4700 | Spectrophotometer | 101 µmol TE/g (ORAC) | 6 mg GAE/g |
| Red | SB7 [50] | 0 | pH Differential | 75 µmol TE/g (ORAC) | 1 mg GAE/g |
| Red | Red [28] | 94 ± 1 | Spectrophotometer | n.d. | n.d. |
| Black | Asamurasaki [52] | 1400 ± 41 | HPLC | n.d. | n.d. |
| Black | Asamurasaki [53] | 474 | HPLC | 65 mmol TE/g DW (ORAC) | n.d. |
| Black | Okunomurasaki [53] | 80 | HPLC | 55 mmol TE/g DW (ORAC) | n.d. |
| Black | Longjin 01 [54] | 5101 ± 79 | pH Differential | 1138 mmol TE/g DW (ORAC) | 4766 mg GAE/100 g DW |
In agreement with the other grains, rice varieties display a large variability in their anthocyanin profiles and contents, with kernel colour and type (glutinous vs. non-glutinous) playing the largest role. Additionally, the method used to analyse the profile contributes to the specificity of the results. Again, growing and harvesting conditions impact the TAC substantially [33]. Over a two-year period, the same variety of rice was grown, harvested, and tested twice, and the results showed variance in the contents of individual and total anthocyanins [49]. Overall, anthocyanin content decreased in the second year, with disproportionate decreases in some anthocyanins compared to others [49]. Changes in environment, weather patterns, and soil characteristics could have a role in this observed difference [33]. In red rice, low amounts of anthocyanins are detected, between 0 to 93.5 mg anthocyanins/kg dry kernel [28,50,53,55]. The colour of red rice, however, is not caused by elevated levels of anthocyanins in the kernel, but is rather due to higher levels of proanthocyanidins. Proanthocyanidins are conjugated tannin structures [56]. Chen [53] was able to identify malvidin-3-glucoside in red rice extracts. However, other studies had no conclusive results as to the anthocyanin profile, indicating that if any anthocyanins are present, they exist in low levels. Conversely, purple rice has significantly higher levels of anthocyanins than what was found in red rice, with an average between 68 to 4700 mg anthocyanins/kg dry kernel [49,56]. With HPLC-PDA, cyanidin-3-glucoside, peonidin-3-glucoside, cyanidin-3-galactoside, and cyanidin-3-rutinoside were identified, as well as other assorted cyanidin and peonidin derivatives [49,56]. Finally, black rice has the highest recorded TAC of all the coloured rice samples studied, with an average between 79.5 to 5101 mg anthocyanins/kg dry kernel weight [28,50,52,53,54,55]. The main anthocyanins in black rice are cyanidin-3-glucoside and peonidin-3-glucoside. These two anthocyanins were identified and quantified through HPLC methods [49,50,53,56]. Other studies [50,56] have also found the presence of malvidin and cyanidin-3-rutinoside. With HPLC-PDA-MS, a greater diversity of anthocyanins was identified. The detected anthocyanins included (in order of prevalence) cyanidin-3,5-diglucoside, cyanidin-3-glucoside, cyanidin-3-(6″-p-coumaryl) glucoside, pelargonidin-3-glucoside, peonidin-3-glucoside, peonidin-3-(6″-p-coumaryl) glucoside, and cyanidin-3-arabidoside [52]. However, inter-subspecies variation in anthocyanin profile exists. When comparing black rice japonica and indica subspecies, for example, the cyanidin-3-glucoside:cyanidin-3-rutinoside:peonidin-3-glucoside ratio changed depending on subspecies as well as rice type (glutinous vs. non-glutinous) [54]. The average cyanidin-3-glucoside content of japonica subspecies was found to be 45% higher than the levels detected in indica subspecies, and 37% higher in non-glutinous black rice types than in the glutinous varieties [54].




