| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ashraf Madhoun | + 5811 word(s) | 5811 | 2020-09-10 08:36:13 | | | |
| 2 | Dean Liu | -2381 word(s) | 3430 | 2020-09-30 08:06:33 | | |
Video Upload Options
Wharton’s jelly-derived mesenchymal stem cells (WJ-MSCs) are a valuable tool in stem cell research due to their high proliferation rate, multi-lineage differentiation potential, and immunotolerance properties. However, fibroblast impurity during WJ-MSCs isolation is unavoidable because of morphological similarities and shared surface markers. Here, a proteomic approach was employed to identify specific proteins deferentially expressed by WJ-MSCs in comparison to those by neonatal foreskin and adult skin fibroblasts (NFFs and ASFs, respectively). EphA2, SLC25A4, and SOD2 were predominantly expressed by WJ-MSCs, while CDH2 and Talin2 were specific to NFFs and ASFs, respectively. Here, EphA2 was established as a potential surface-specific marker to distinguish WJ-MSCs from fibroblasts and for prospective use to prepare pure primary cultures of WJ-MSCs for prospective clinical use. Additionally, CDH2 could be used for a negative-selection isolation/depletion method to remove neonatal fibroblasts contaminating preparations of WJ-MSCs.
1. Introduction
Mesenchymal stem cells (MSCs) are a population of non-hematopoietic stem cells with multipotent properties that are not associated with teratoma formation [1][2]. Because of these properties, MSCs are an attractive alternative to embryonic stem cells for research and prospective clinical applications. The therapeutic potential of MSCs is not limited to their capacity to replace injured tissue cells. They also have a paracrine effect on the surrounding environment that modulates inflammation, reduces stress-induced apoptosis, and enhances revascularization[3]. MSCs have been isolated from various tissues of the human body[4][5][6], the bone marrow (BM) and adipose tissue (AT) are the main source for prospective clinical applications[7].
There are a number of limitations to the use of adult MSCs. For example, the procedure for the collection of BM-derived MSCs (BM-MSCs), which account for a small fraction of nucleated BM cells, is particularly invasive and restricted to the availability of suitable donors[8]. In addition, BM-MSCs have limited long-term proliferation and the differentiation potential is linked to the donor’s age[9]. On the other hand, AT-derived MSCs (AT-MSCs) are more abundant and the isolation procedure is less invasive. However, the expansion and differentiation of AT-MSCs are dependent on the age and health status of the donor[10]. Wharton’s jelly-derived MSCs (WJ-MSCs) continue to gain the interest of researchers as a promising alternative source of multipotent cells that do not require an invasive isolation procedure[11]. This unique population of cells is embedded within the gelatinous material of the umbilical cord, known as Wharton’s jelly[12][13]. Resembling adult MSCs, WJ-MSCs have the capacity of self-renewal and immuno-modular properties[1][14][16]. As multipotent cells, WJ-MSCs can be differentiated in vitro into a wide spectrum of cell types from the three germ layers or at least in part, express specific markers[17][18][19][20]. Indeed, current data are conflicting in regard to the ability of MSCs to generate terminal and functional cells from either germ layer due to the use of various isolation, proliferation, and differentiation protocols that are biased due to differences in the stimulus used to induce cell signaling[21][22][23][24] in addition to the existence of cell populations at different developmental stages.
Like MSCs, WJ-MSCs are not associated with teratoma formation upon transplantation, thus clinical applications of these cells are ethically accepted[25][26]. In fact, BM-MSCs and WJ-MSCs are believed to have a common ancestor. Nevertheless, we and others have shown differences in the differentiation potential as well as the transcriptomic and proteomic profiles[1][27][28].
MSCs are often employed in the field of regenerative medicine due to their immunomodulatory effects, which virtually erases the risk of immunorejection and eliminates the need for immunosuppressive therapy prior to transplantation[29][30]. The immunomodulatory capacity of MSCs is mediated through a cell contact-dependent mechanism and the secretion of paracrine factors[31][32][33]. Thus, crosstalk between MSCs and immune cells is sufficient to generate a homeostatic mechanism by which MSCs regulate the immune response. Although, MSCs from different sources have comparable influences on the immunophenotype[34][35], some minor differences exist. Relative to BM-MSCs, AT-MSCs reportedly have greater immunomodulatory potency due to high levels of cytokine secretion[36] and prevention of immunogenicity[35]. On the other hand, a recent report indicated that BM-MSCs possess higher immunomodulatory activities and secrete lower amounts of paracrine signaling molecules relative to AT-MSCs and WJ-MSCs[37]. These discrepancies have been attributed to differences in cultural conditions, variations in experimental setups, and, most importantly, the crosstalk between each type of MSCs and the targeted immune cell population[38]. Although further in-depth studies are needed to clarify the immunomodulatory effects of MSCs from different sources, the advantages of perinatal vs. adult MECs include the short prenatal lifespan, limited exposure to pro-aging factors, and less cellular/genetic damage that might affect cellular plasticity[39][40].
Fibroblasts, which are the most common somatic cell type, form structural frameworks and produce an extracellular matrix that supports the surrounding tissues. Fibroblasts are not terminally differentiated cells, but rather respond to stimuli that activate proliferation and differentiation potential, and also play important roles in wound healing, inflammation, angiogenesis, and tissue fibrosis[41]. Current methods for the isolation of MSC and WJ-MSC do not prevent fibroblast contamination, as these cell populations share a spindle-like morphology, expression of common surface antigens, and plastic adherence properties[42]. In addition to reducing the yield of these multipotent cell populations, fibroblast contamination may increase the risk of damage to MSCs and WJ-MSCs, resulting in senescence or cell death, reduced differentiation potential, or even tumorigenic transformation following transplantation[2][42].
Currently, there is no consensus on a single surface marker to differentiate WJ-MSCs from fibroblasts of various sources, which is essential for the isolation of pure and authentic populations of WJ-MSCs that can be introduced into damaged tissues or organs without passaging in tissue culture, for prospective clinical applications. In this study, the proteomic profiles of membrane-bound proteins extracted from WJ-MSCs, neonatal foreskin fibroblasts (NFFs), and adult skin fibroblasts (ASFs) were characterized using nanoscale liquid chromatography coupled to tandem mass spectrometry (Nano LC-MS/MS). Gene expression analysis at the transcriptional and protein levels indicated that ephrin type-A receptor 2 (EphA2) is a candidate surface-specific protein for the identification of WJ-MSCs, whereas cluster of differentiation (CD)H2 and Talin2 are markers for NFFs and ASFs, respectively.
2. Results
2.1. Analysis of Deferentially Expressed Proteins Detected by Nano LC-MS/MS
To illustrate differences in the proteomic patterns between deferent passages of WJ-MSCs, ASFs, and NFFs, membrane-fraction protein extracts were digested, and the generated peptides were detected by Nano LC-MS/MS. In total, 958, 866, and 813 proteins were shared among the different passages of WJ-MSCs, ASFs, and NFFs. Then, we compared the number of proteins shared among different cell-types and identified 905 proteins that were commonly expressed among WJ-MSCs, ASFs, and NFFs. Among the cell types, a total of 97 differentially expressed proteins were identified, including 56 that were unique to WJ-MSCs, 23 unique to NFFs, and 18 unique to ASFs. Moreover, 41 proteins were shared between WJ-MSCs and NFFs, 60 between WJ-MSCs and ASFs, and 23 between ASFs and NFFs.
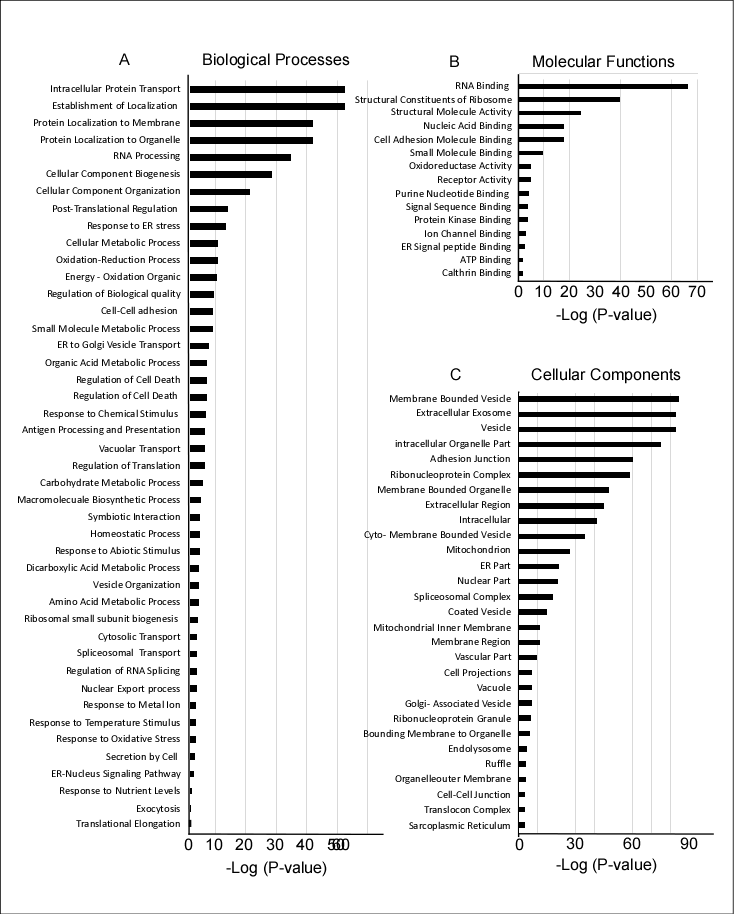
The identified proteins were first screened to identify all membrane-bound proteins that could be potential candidate cell surface markers. Of the initial 1126 screened proteins, 454 were found to have a transmembrane domain, and then categorized according to involvement in biological processes, molecular functions, and cellular localization using gene ontology (GO) enrichment methods (Figure 2). Of the proteins involved in biological processes, most had intracellular transport functions or targeted the cellular membranes and organelles. Several proteins were associated with RNA processing, cell biogenesis, and organization (Figure 2A). Of the proteins involved in molecular functions, most were either associated with RNA binding functions, cell signaling, or the ribosomal complex (Figure 2B). Most of the detected proteins were present in vesicles, membrane-bound organelles, or secretory exosomes (Figure 2C).
Figure 1. Pathways and gene ontology (GO) enrichment analysis of 1126 proteins expressed by WJ-MSCs, ASFs, and NFFs. The GO annotation enrichment score [−Log2 (p-value)] analysis of proteins involved in biological processes (A), molecular functions (B), and cellular components (C), as identified by MS and in proteomic analysis.
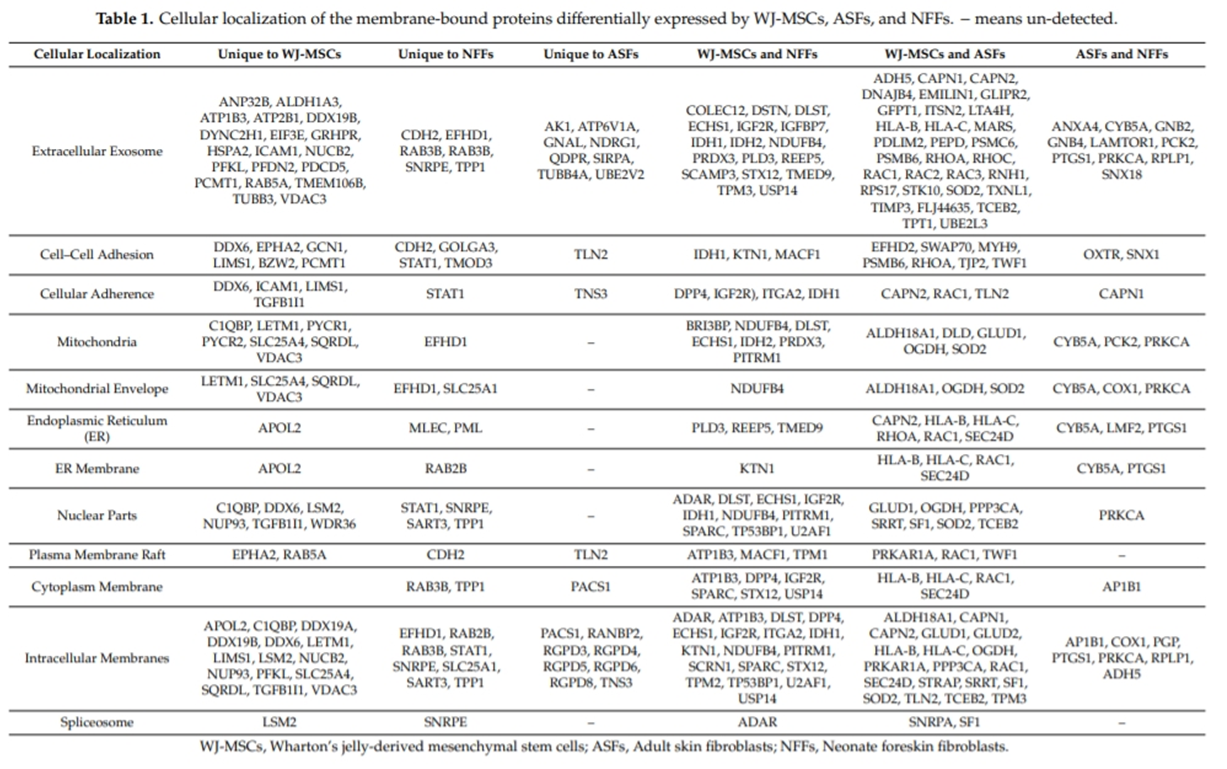
Quantitative MS was performed to identify proteins with differential expression patterns in different cell types. These proteins were compared and classified based on their cellular localization, as well as involvement in biological processes and molecular functions (Tables 1 and 2). Many of the identified proteins were common among different cell types, while others were specific to a particular cell type. For example, proteins involved in plasma membrane rafts and cell–cell junctions were specific to a particular cell type, including EphA2 in WJ-MSCs, the cytoskeletal anchoring protein Talin2 (TLN2) in ASFs, and neuronal (N)-cadherin (CDH2/CD325) in NFFs (Table 1, Cytoplasmic membrane). In the mitochondria, the adenosine di/triphosphate (ADP/ATP) translocase 1/solute carrier family 25 member 4 (SLC25A4) and voltage-dependent anion-selective channel protein VDAC3 appear to be specific to WJ-MSCs, while mitochondrial superoxide dismutase (SOD2) was identified in both WJ-MSCs and ASFs. The integrin alpha subunit ITGA2 (CD49b) was common to both WJ-MSCs and NFFs, while lipase maturation factor (LMF2) was detected specific to fibroblasts (both ASFs and NFFs).
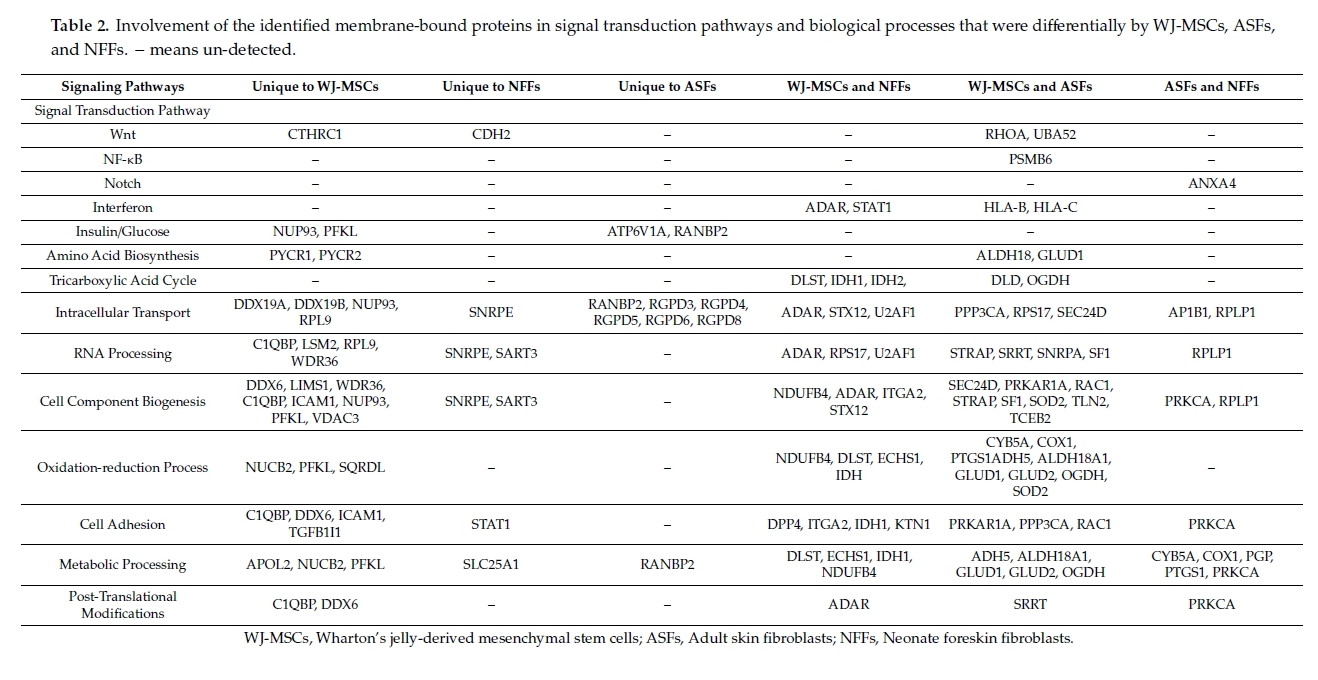
In addition, the dataset contains shared and cell type-specific proteins within many functional classes, thereby revealing important differences in the protein profiles of specific cell types (Table 2). For example, signaling proteins known to be involved in the tricarboxylic acid cycle were detected in all of the cell types studied. On the other hand, the notch signaling protein ANXA4 was specific to fibroblasts. The Wnt signaling molecules CTHRC1 and CDH2 were only detected in WJ-MSCs and NFFs respectively, whereas Ras homolog family member A (RHOA) and ubiquitin A-52 residue ribosomal protein fusion product 1 (UBA52) were present in both WJ-MSCs and ASFs. Similarly, the studied cell types had notable differentially expressed proteins involved in other biological mechanisms, including metabolic and oxidation-reduction, cell adherence, cell component transportation, and biogenesis (Table 2). Thus, the proteomic dataset provides an important resource of cell-surface proteins present on WJ-MSCs that could be used in future functional studies.
2.2. Quantitative RT-PCR Analysis of Gene Products (Proteins) Identified by Mass spectrometry (MS) Screening
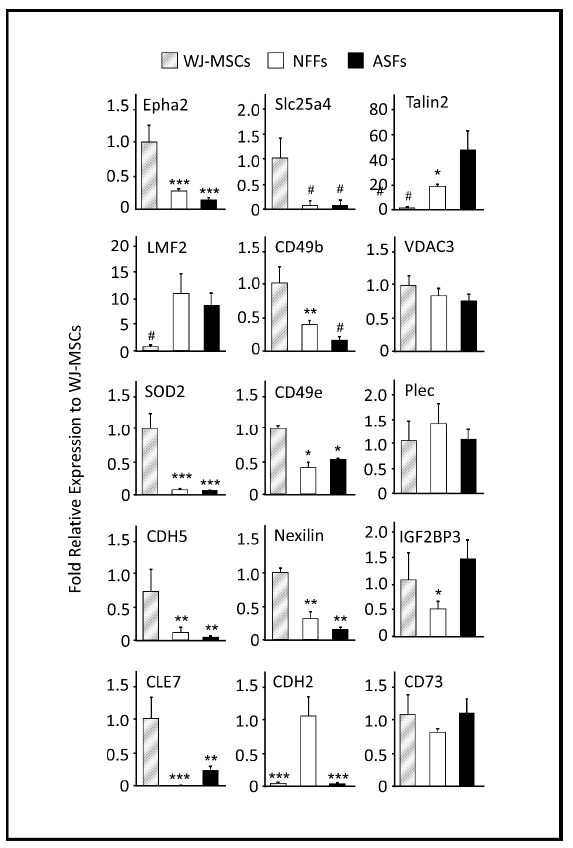
While generally a good indicator of protein translation in the cells, the mRNA level is not always correlated with the presence of the encoded protein[43]. Thus, to verify whether the relative expression levels of the proteins detected via MS in different cell types could have been predicted by the mRNA levels; quantitative reverse transcription polymerase chain reaction (qRT-PCR) analysis was performed to analyze the gene expression (Figure 3). The expression pattern of CD73, a well-known marker of WJ-MSCs was also analyzed, which showed comparable expression levels in both WJ-MSCs and fibroblasts (Figure 3).
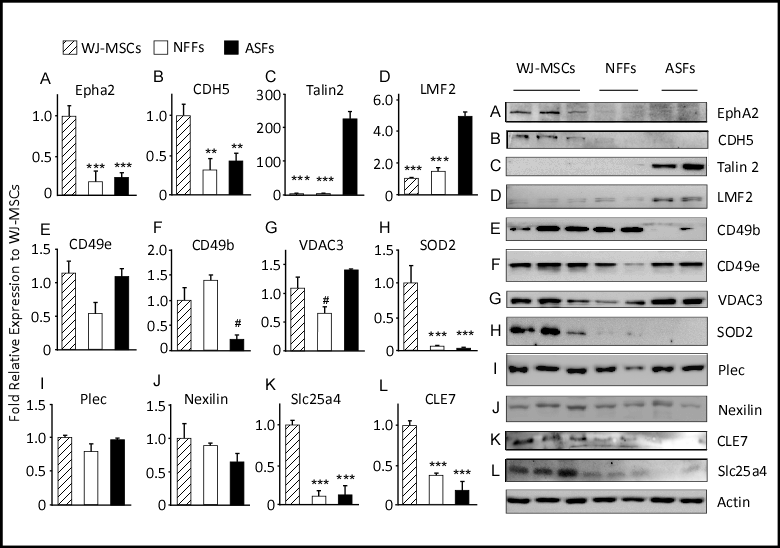
Figure 2. qRT-PCR analysis of 15 selected genes differentially expressed by WJ-MSCs, ASFs, and NFFs. Relative quantification was calculated by comparing gene expression levels in ASFs and NFFs with corresponding WJ-MSCs expression, which was set to 1 (control sample). Gene expression was initially normalized to the geometric mean for the housekeeping genes β-actin, Glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and S18, as references. Data are presented as the means ± standard deviation of six qRT-PCR assays (technical duplicate of three biological samples). * p < 0.05, ** p < 0.01, and *** p < 0.001 are significant to the higher bar. # p < 0.01 is significant to all other bars.
The mRNA expression profiles of the membrane-bound proteins EphA2, SLC25A4, TLN2, LMF2, and CD49b were similar by qRT-PCR analysis and the quantitative MS spectra. EphA2 and SLC25A4 were predominantly expressed by WJ-MSCs. The expression levels of AphA2 in ASFs and NFFs were only 13% and 27% relative to those of WJ-MSCs, respectively. While SLC25A4 transcripts in both fibroblast cell types were <10% relative to those of WJ-MSCs. On the other hand, TLN2 and LMF2 genes were notably expressed by fibroblasts. In both ASFs and NFFs, the expression levels of TLN2 and LMF2 were 43- and 12-fold, and 17- and 15-fold relative to that of WJ-MSCs, respectively (Figure 3). CD49b was expressed mainly by WJ-MSCs, to a lesser extent by NFFs (40% to that of WJ-MSCs), and as low as 15% in ASFs. Alternatively, mRNA levels of VDAC3, SOD2, and plectin-1 (PLEC1) did not reflect the associated protein expression levels determined by MS analysis (Table 3), whereas SOD2 expression levels were 20-fold higher in WJ-MSCs than fibroblasts. The mRNA expression levels of VDAC3 and PLEC1 were equivalent in all cell types (Figure 3).
2.3. Western Blot Analysis
Western blot analysis of selected proteins was performed to validate the quantitative proteomic results obtained by MS and to assess correlations with the mRNA levels determined by qRT-PCR (Figure 4). As compared to the findings of MS and qRT-PCR, the protein levels of CD49b, as determined by Western blot analysis, were approximately 8-fold higher in WJ-MSCs and NFFs relative to ASFs. On the other hand, levels of mitochondrial SOD2 were higher in WJ-MSCs, in agreement with their mRNA levels, but were not detected in ASFs, as predicted by the proteomics approach (Table 3). Western blot analysis using specific antibodies barely detected SOD2 proteins in fibroblasts (Figure 4). Protein levels of PLEC1, Nexilin, TLN2, and CD49e replicated the expression patterns determined by qRT-PCR analysis. However, the expression levels of these proteins in different cell types did not mimic those predicted by the proteomics approach. PLEC1 and Nexilin proteins were comparably expressed by all cell types, whereas CD49e proteins were equivalently detected in WJ-MSCs and ASFs but were 50% lower in NFFs. On the other hand, TLN2 was predominately observed in ASFs and seldomly detected in WH-MSCs or NFFs. Protein levels of LMF2 were 5-fold greater in ASFs relative to WJ-MSCs, but not expressed in NFFs, as observed by both qRT-PCR analysis and the initial MS screening. Similarly, EphA2 and SLC25A4 were predominantly expressed by WJ-MSCs, as predicted by the proteomics approach and validated by qRT-PCR (accounting for <20% in fibroblasts, Figure 4 and Table 3).
Figure 3. Expression levels of proteins differentially expressed by WJ-MSCs, ASFs, and NFFs. Representative Western blots of 12 expressed proteins. Normalized proteins are expressed relative to their prospective expression in WJ-MSCs. Data are presented as the means ± standard error of the mean of three independent assays. * p < 0.05, ** p < 0.01, and *** p < 0.001 are significant to the higher bar. # p < 0.01 is significant to all other bars.
2.4. Fluorescence Microscopy
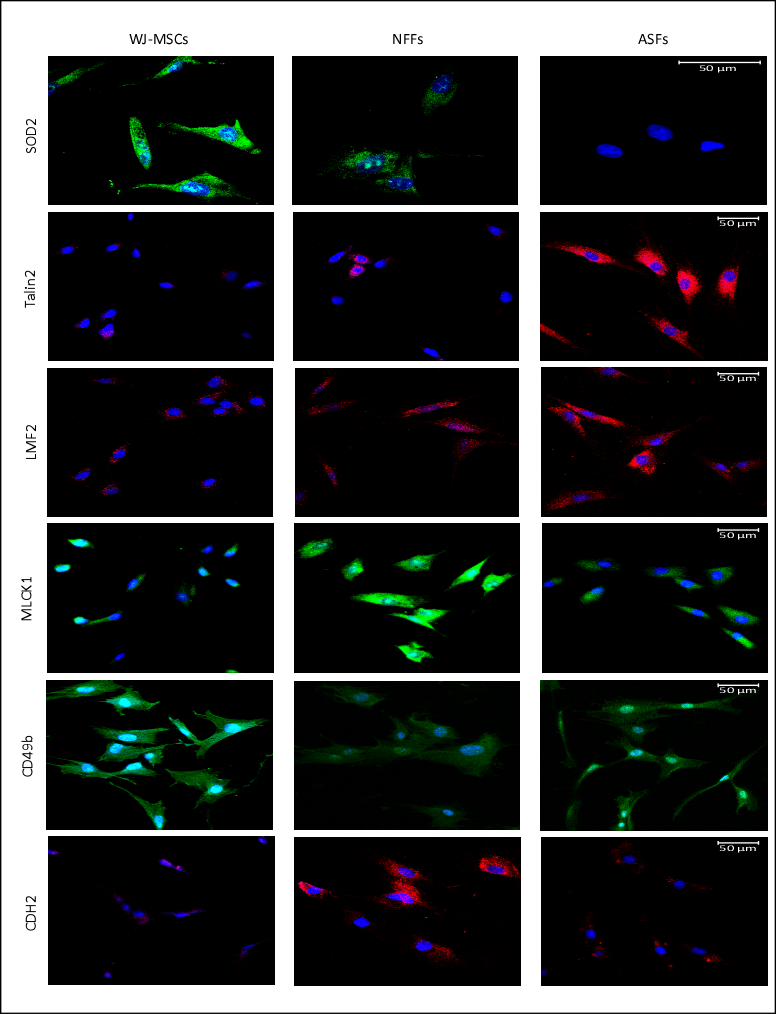
Next, fluorescence microscopy was used to visualize the expression patterns and the cellular localizations of proteins of interest in WJ-MSCs, ASFs, and NFFs (Figure 5). SOD2 was detected predominantly in WJ-MSCs with lower expression seen in NFFs. CD49b was mostly expressed by WJ-MSCs, with some expression detected in ASFs. On the contrary, TLN2 was observed only in ASFs, confirming the results obtained by Western blot analysis. LMF2 was expressed mainly in ASFs, with some minor expression patterns in NFFs, correlating with the pattern detected by Western blot analysis. The myosin light chain kinase MLCK1 was expressed mostly in NFFs, with lower levels in ASFs, similar to the expression profile of N-cadherin (CD325).
Figure 4. Immunofluorescence of selected proteins expressed in WJ-MSCs, NFFs, and ASFs. Representative confocal laser microscopy images of immunofluorescence for primary cells using APEX antibody labeling system for conjugating the indicated primary antibodies as described in the Materials and Methods Section. Shown at 400× magnification. Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI).
3. Discussion
Due to the potential use in regenerative medicine, WJ-MSCs have continued to attract the interest of academic and medical communities in recent years. Despite a multitude of studies, the isolation of WJ-MSCs from contaminating cell populations remains difficult, particularly with fibroblasts. In order to develop more convenient and targeted methods of purification, cell-specific surface markers were identified to precisely isolate WJ-MSCs.
According to the current criteria defined by the International Society for Cellular Therapy, MSCs express the membrane proteins CD105, CD73, and CD90, but not CD45, CD34, CD14, or CD11b, CD79α, or CD19, and HLA-DR [11]. Besides the present study, several previous studies have reported that many of these surface markers are shared with fibroblasts[2][11][42][44][45][46][47]. On the other hand, MSCs express various fibroblast proteins, such as collagen, vimentin, fibroblast surface protein, heat shock protein 47, and α-smooth muscle actin[2][44]. Thus, MSCs are a heterogeneous cell population that lacks a specific surface biomarker, thereby rendering identification and characterization of MSCs rather challenging. In this study, 454 membrane-bound proteins differentially expressed by these cell types were identified. Here, a few highly expressed markers were selected for further analysis, which included EphaA2, CDH5, and the integrin alpha subunits CD49b and CD49e.
EphA2 belongs to the Ephrin receptor subfamily of the protein-tyrosine kinase family. EphA2 and its ligand Ephrin play important roles in cellular migration, survival, and differentiation [48]. In general, Ephrin receptors mediate cell-to-cell binding, leading to contact-dependent bidirectional signaling to neighboring cells[49]. During embryogenesis, Ephrin receptors mediate neuron differentiation, neural-tube formation, and development of the early hindbrain[50]. Ephrin receptors influence a niche of stem cells. In the present study, EphA2 was primarily expressed by WJ-MSCs supporting its role in cell self-renewal and differentiation[51]: the two major characteristics of stem cells that fibroblasts lack. The results of previous gene expression studies indicate that MSCs derived from BM and AT express a wide range of Ephrin receptors, including EphA2[48], whereas MSCs isolated from the umbilical cord blood expresses EphB2[52]. In support of our data, proteomic analysis identified EphA2 as a marker of MSCs derived from the placenta, a cell type that is developmentally related to WJ-MSCs[53] and is believed to secrete prostaglandin E2, an anti-fibrosis and immunomodulator marker[54]. Together, these data indicate that EphA2 is an important surface marker of WJ-MSCs.
CDH5 or VE-cadherin have been previously described to be a surface marker for Adult cardiac progenitor/stem cells but not BM-MSCs[55] or WJ-MSC [56], here we found it to be a good surface marker for WJ-MSCs and to a lesser extent to NFFs. CDH5 is a calcium-dependent cell adhesion protein that ensure integrity of blood and lymph vessels and play an important role in vasculogenesis, angiogenesis, vessel leakage, and leukocyte trafficking[57]. Inhibition of CDH5 expression, by miRNA-6086, blocks human embryonic stem cells’ differentiation into endothelial cells[58]. Together, the elevated levels of CDH5 in WJ-MSCs supports their prospective differentiation into endothelial cells or possible other lineages.
Integrins play an essential role in cellular adhesion and cell surface-mediated signaling. CD49b is integrin alpha 2 subunit, which, in combination with integrin beta 1 subunit, forms a receptor for collagen, collagen C-propeptides, fibronectin, laminin, and E-cadherin[59]. Ligand recognition and binding occur mainly through the alpha subunit of the integrin heterodimer. Once activated, the receptor initiates downstream signaling events engendering changes in cell migration, survival, and growth[60]. Interestingly, integrin activation via intracellular ligands has also been reported. The binding of Talin 2 to the integrin beta subunit leads to a conformational change to its transmembrane domain, leading to integrin activation[61].
Albeit BM-MSCs and fibroblasts express similar levels of CD49b[62]; in the present study, mRNA and protein expression levels of CD49b were 2.5-fold higher in WJ-MSCs than in NFFs. This pattern is corroborated by two large transcriptomic and proteomic studies[63][64], which were generated the Human Protein Atlas (HPA). In the HPA, CD49b is mostly expressed by endothelial cells derived from the umbilical vein and to a lower extent in NFFs. The mRNA and protein expression patterns of CD49e observed in this study also mimicked those from the HPA database: mostly expressed by endothelial cells from the umbilical vein and lower expression in NFFs[59].
While several studies have screened and identified surface markers of MSCs, the identification of MSC-specific surface markers remains challenging. A systematic review of available information noted a great discrepancy in the expression patterns of several surface markers of MSCs in different studies[65]. A possible explanation for this discrepancy is related to the origin or heterogeneity of the MSCs used in different studies. Alternatively, these differences could possibly be related to the different proliferative stages of the cells in culture. In any case, further studies are needed to validate our preliminary findings as well as extrapolate the findings of previous studies to overcome inconsistencies regarding cell surface marker profiles of MSCs with the potential advantage of culture purification of WJ-MSCs via negative or positive selection. A limitation to this study is the comparison of data with only two fibroblast cell lines. Hence, the results must be interpreted with caution.
To the best of our knowledge, this is the first study that aimed to identify specific cell markers for WJ-MSCs that are not present in fibroblasts of neonate or adult origin. We confirmed EphA2 as a potential cell surface marker that distinguishes WJ-MSCs from fibroblasts and can be used to prepare pure WJ-MSCs primary cell cultures. CDH5 or VE-Cadherin can be also used as a surface marker, however it would yield a less pure WJ-MSCs population. Whereas, a negative-selection isolation process can be devised using CDH2 to remove neonatal fibroblasts, commonly encountered in WJ-MSCs preparations. Currently, we are aiming to use these surface-specific markers to prepare pure cell cultures from a wide range of MSCs derived from several tissues, including bone marrow, adipose tissue, and dental pulp.
References
- Ali, H.; Al-Yatama, M. K.; Abu-Farha, M.; Behbehani, K.; Al Madhoun, A. Multi-lineage differentiation of human umbilical cord Wharton’s Jelly Mesenchymal Stromal Cells mediates changes in the expression profile of stemness markers. PLoS ONE 2015, 10, e0122465.
- Alt, E.; Yan, Y.; Gehmert, S.; Song, Y. H.; Altman, A.; Gehmert, S.; Vykoukal, D.; Bai, X. Fibroblasts share mesenchymal phenotypes with stem cells, but lack their differentiation and colony-forming potential. Biol. Cell 2011, 103, 197–208.
- Gnecchi, M.; Zhang, Z.; Ni, A.; Dzau, V. J. Paracrine mechanisms in adult stem cell signaling and therapy. Circ. Res. 2008, 103, 1204–1219.
- Friedenstein, A.J.; Piatetzky-Shapiro, I.I.; Petrakova, K.V. Osteogenesis in transplants of bone marrow cells. J. Embryol. Exp. Morphol. 1966, 16, 381–390.
- Zuk, P. A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J. W.; Katz, A. J.; Benhaim, P.; Lorenz, H. P.; Hedrick, M. H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001, 7, 211–228.
- Atari, M.; Barajas, M.; Hernandez-Alfaro, F.; Gil, C.; Fabregat, M.; Ferres Padro, E.; Giner, L.; Casals, N. Isolation of pluripotent stem cells from human third molar dental pulp. Histol. Histopathol. 2011, 26, 1057–1070.
- Berebichez-Fridman, R.; Montero-Olvera, P. R., Sources and Clinical Applications of Mesenchymal Stem Cells: State-of-the-art review. Sultan Qaboos Univ. Med. J. 2018, 18, e264–e277.
- Cheng, H. Y.; Ghetu, N.; Huang, W. C.; Wang, Y. L.; Wallace, C. G.; Wen, C. J.; Chen, H. C.; Shih, L. Y.; Lin, C. F.; Hwang, S. M.; Liao, S. K.; Wei, F. C. Syngeneic adipose-derived stem cells with short-term immunosuppression induce vascularized composite allotransplantation tolerance in rats. Cytotherapy 2014, 16, 369–380.
- Mueller, S. M.; Glowacki, J. Age-related decline in the osteogenic potential of human bone marrow cells cultured in three-dimensional collagen sponges. J. Cell Biochem. 2001, 82, 583–590.
- Choudhery, M. S.; Badowski, M.; Muise, A.; Pierce, J.; Harris, D. T. Donor age negatively impacts adipose tissue-derived mesenchymal stem cell expansion and differentiation. J. Transl. Med. 2014, 12, 8
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F. C.; Krause, D. S.; Deans, R. J.; Keating, A.; Prockop, D. J.; Horwitz, E. M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317.
- Ali, H.; Al-Mulla, F. Defining umbilical cord blood stem cells. Stem Cell Discov. 2012, 2, 15–23.
- Joerger-Messerli, M. S.; Marx, C.; Oppliger, B.; Mueller, M.; Surbek, D. V.; Schoeberlein, A. Mesenchymal Stem Cells from Wharton’s Jelly and Amniotic Fluid. Best Pract. Res. Clin. Obstet. Gynaecol. 2016, 31, 30–44.
- Wang, H. S.; Hung, S. C.; Peng, S. T.; Huang, C. C.; Wei, H. M.; Guo, Y. J.; Fu, Y. S.; Lai, M. C.; Chen, C. C. Mesenchymal stem cells in the Wharton’s jelly of the human umbilical cord. Stem Cells 2004, 22, 1330–1307.
- Bagher, Z.; Azami, M.; Ebrahimi-Barough, S.; Mirzadeh, H.; Solouk, A.; Soleimani, M.; Ai, J.; Nourani, M. R.; Joghataei, M. T. Differentiation of Wharton’s Jelly-Derived Mesenchymal Stem Cells into Motor Neuron-Like Cells on Three-Dimensional Collagen-Grafted Nanofibers. Mol. Neurobiol. 2016, 53, 2397–2408.
- Weiss, M. L.; Anderson, C.; Medicetty, S.; Seshareddy, K. B.; Weiss, R. J.; Vanderwerff, I.; Troyer, D.; Mcintosh, K. R. Immune Properties of Human Umbilical Cord Wharton’s Jelly-Derived Cells. Stem Cells 2008, 26, 2865–2874.
- Al Madhoun, A.; Alkandari, S.; Ali, H.; Carrio, N.; Atari, M.; Bitar, M. S.; Al-Mulla, F. Chemically Defined Conditions Mediate an Efficient Induction of Mesodermal Lineage from Human Umbilical Cord- and Bone Marrow- Mesenchymal Stem Cells and Dental Pulp Pluripotent-Like Stem Cells. Cell Reprogram. 2018, 20, 9–16.
- Al Madhoun, A.; Ali, H.; AlKandari, S.; Atizado, V. L.; Akhter, N.; Al-Mulla, F.; Atari, M. Defined three-dimensional culture conditions mediate efficient induction of definitive endoderm lineage from human umbilical cord Wharton’s jelly mesenchymal stem cells. Stem Cell Res. Ther. 2016, 7, 165.
- Liang, J.; Wu, S.; Zhao, H.; Li, S. L.; Liu, Z. X.; Wu, J.; Zhou, L Human umbilical cord mesenchymal stem cells derived from Wharton’s jelly differentiate into cholinergic-like neurons in vitro. Neurosci. Lett. 2013, 532, 59–63.
- Bhandari, D. R.; Seo, K. W.; Sun, B.; Seo, M. S.; Kim, H. S.; Seo, Y. J.; Marcin, J.; Forraz, N.; Roy, H. L.; Larry, D.; Colin, M.; Kang, K. S. The simplest method for in vitro beta-cell production from human adult stem cells. Differentiation 2011, 82, 144–152
- Stern-Straeter, J.; Bonaterra, G. A.; Juritz, S.; Birk, R.; Goessler, U. R.; Bieback, K.; Bugert, P.; Schultz, J.; Hormann, K.; Kinscherf, R.; Faber, A. Evaluation of the effects of different culture media on the myogenic differentiation potential of adipose tissue- or bone marrow-derived human mesenchymal stem cells. Int. J. Mol. Med. 2014, 33, 160–170.
- Balasubramanian, S.; Thej, C.; Venugopal, P.; Priya, N.; Zakaria, Z.; Sundarraj, S.; Majumdar, A. S. Higher propensity of Wharton’s jelly derived mesenchymal stromal cells towards neuronal lineage in comparison to those derived from adipose and bone marrow. Cell Biol. Int. 2013, 37, 507–515.
- Yang, J.; Song, T.; Wu, P.; Chen, Y.; Fan, X.; Chen, H.; Zhang, J.; Huang, C. Differentiation potential of human mesenchymal stem cells derived from adipose tissue and bone marrow to sinus node-like cells. Mol. Med. Rep. 2012, 5, 108–113.
- Meligy, F. Y.; Shigemura, K.; Behnsawy, H. M.; Fujisawa, M.; Kawabata, M.; Shirakawa, T. The efficiency of in vitro isolation and myogenic differentiation of MSCs derived from adipose connective tissue, bone marrow, and skeletal muscle tissue. Vitr. Cell Dev. Biol. Anim. 2012, 48, 203–215.
- Gauthaman, K.; Fong, C. Y.; Suganya, C. A.; Subramanian, A.; Biswas, A.; Choolani, M.; Bongso, A. Extra-embryonic human Wharton’s jelly stem cells do not induce tumorigenesis, unlike human embryonic stem cells. Reprod. Biomed. Online 2012, 24, 235–246.
- Zeddou, M.; Briquet, A.; Relic, B.; Josse, C.; Malaise, M. G.; Gothot, A.; Lechanteur, C.; Beguin, Y. The umbilical cord matrix is a better source of mesenchymal stem cells (MSC) than the umbilical cord blood. Cell Biol. Int. 2010, 34, 693–701.
- Fong, C. Y.; Chak, L. L.; Biswas, A.; Tan, J. H.; Gauthaman, K.; Chan, W. K.; Bongso, A. Human Wharton’s jelly stem cells have unique transcriptome profiles compared to human embryonic stem cells and other mesenchymal stem cells. Stem Cell Rev. 2011, 7, 1–16.
- Hsieh, J. Y.; Fu, Y. S.; Chang, S. J.; Tsuang, Y. H.; Wang, H. W. Functional module analysis reveals differential osteogenic and stemness potentials in human mesenchymal stem cells from bone marrow and Wharton’s jelly of umbilical cord. Stem. Cells Dev. 2010, 19, 1895–1910.
- Jiang, W.; Xu, J. Immune modulation by mesenchymal stem cells. Cell Prolif. 2020, 53, e12712.
- Abumaree, M.; Al Jumah, M.; Pace, R. A.; Kalionis, B. Immunosuppressive properties of mesenchymal stem cells. Stem Cell Rev. Rep. 2012, 8, 375–392.
- Melief, S. M.; Geutskens, S. B.; Fibbe, W. E.; Roelofs, H. Multipotent stromal cells skew monocytes towards an anti-inflammatory function: The link with key immunoregulatory molecules. Haematologica 2013, 98, e121–e122.
- Spaggiari, G. M.; Capobianco, A.; Abdelrazik, H.; Becchetti, F.; Mingari, M. C.; Moretta, L. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: Role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood 2008, 111, 1327–1333.
- Obermajer, N.; Popp, F. C.; Soeder, Y.; Haarer, J.; Geissler, E. K.; Schlitt, H. J.; Dahlke, M. H Conversion of Th17 into IL-17A(neg) regulatory T cells: A novel mechanism in prolonged allograft survival promoted by mesenchymal stem cell-supported minimized immunosuppressive therapy. J. Immunol. 2014, 193, 4988–4999.
- Yoo, K. H.; Jang, I. K.; Lee, M. W.; Kim, H. E.; Yang, M. S.; Eom, Y.; Lee, J. E.; Kim, Y. J.; Yang, S. K.; Jung, H. L.; Sung, K. W.; Kim, C. W.; Koo, H. H. Comparison of immunomodulatory properties of mesenchymal stem cells derived from adult human tissues. Cell Immunol. 2009, 259, 150–156.
- Valencia, J.; Blanco, B.; Yanez, R.; Vazquez, M.; Herrero Sanchez, C.; Fernandez-Garcia, M.; Rodriguez Serrano, C.; Pescador, D.; Blanco, J. F.; Hernando-Rodriguez, M.; Sanchez-Guijo, F.; Lamana, M. L.; Segovia, J. C.; Vicente, A.; Del Canizo, C.; Zapata, A. G. Comparative analysis of the immunomodulatory capacities of human bone marrow- and adipose tissue-derived mesenchymal stromal cells from the same donor. Cytotherapy 2016, 18, 1297–1311.
- Melief, S. M.; Zwaginga, J. J.; Fibbe, W. E.; Roelofs, H. Adipose tissue-derived multipotent stromal cells have a higher immunomodulatory capacity than their bone marrow-derived counterparts. Stem Cells Transl. Med. 2013, 2, 455–463.
- Petrenko, Y.; Vackova, I.; Kekulova, K.; Chudickova, M.; Koci, Z.; Turnovcova, K.; Kupcova Skalnikova, H.; Vodicka, P.; Kubinova, S. A Comparative Analysis of Multipotent Mesenchymal Stromal Cells derived from Different Sources, with a Focus on Neuroregenerative Potential. Sci. Rep. 2020, 10, 4290.
- Ribeiro, A.; Laranjeira, P.; Mendes, S.; Velada, I.; Leite, C.; Andrade, P.; Santos, F.; Henriques, A.; Graos, M.; Cardoso, C. M.; Martinho, A.; Pais, M.; da Silva, C. L.; Cabral, J.; Trindade, H.; Paiva, A. Mesenchymal stem cells from umbilical cord matrix, adipose tissue and bone marrow exhibit different capability to suppress peripheral blood B, natural killer and T cells. Stem Cell Res. 2013, 4, 125.
- Kalaszczynska, I.; Ferdyn, K. Wharton’s jelly derived mesenchymal stem cells: Future of regenerative medicine? Recent findings and clinical significance. Biomed. Res. Int. 2015, 2015, 430847.
- Wang, Q.; Yang, Q.; Wang, Z.; Tong, H.; Ma, L.; Zhang, Y.; Shan, F.; Meng, Y.; Yuan, Z. Comparative analysis of human mesenchymal stem cells from fetal-bone marrow, adipose tissue, and Warton’s jelly as sources of cell immunomodulatory therapy. Hum. Vaccin. Immunother. 2016, 12, 85–96.
- Kendall, R. T.; Feghali-Bostwick, C. A Fibroblasts in fibrosis: novel roles and mediators. Front. Pharmacol. 2014, 5, 123.
- Halfon, S.; Abramov, N.; Grinblat, B.; Ginis, I. Markers distinguishing mesenchymal stem cells from fibroblasts are downregulated with passaging. Stem Cells Dev. 2011, 20, 53–66.
- De Sousa Abreu, R.; Penalva, L. O.; Marcotte, E. M.; Vogel, C. Global signatures of protein and mRNA expression levels. Mol. Biosyst. 2009, 5, 1512–1526.
- Brohem, C. A.; de Carvalho, C. M.; Radoski, C. L.; Santi, F. C.; Baptista, M. C.; Swinka, B. B.; de, A. U. C.; de Araujo, L. R.; Graf, R. M.; Feferman, I. H.; Lorencini, M. Comparison between fibroblasts and mesenchymal stem cells derived from dermal and adipose tissue. Int. J. Cosmet. Sci. 2013, 35, 448–457.
- Denu, R. A.; Nemcek, S.; Bloom, D. D.; Goodrich, A. D.; Kim, J.; Mosher, D. F.; Hematti, P. Fibroblasts and Mesenchymal Stromal/Stem Cells Are Phenotypically Indistinguishable. Acta Haematol. 2016, 136, 85–97.
- Lorenz, K.; Sicker, M.; Schmelzer, E.; Rupf, T.; Salvetter, J.; Schulz-Siegmund, M.; Bader, A. Multilineage differentiation potential of human dermal skin-derived fibroblasts. Exp. Dermatol. 2008, 17, 925–932.
- Sudo, K.; Kanno, M.; Miharada, K.; Ogawa, S.; Hiroyama, T.; Saijo, K.; Nakamura, Y. Mesenchymal progenitors able to differentiate into osteogenic, chondrogenic, and/or adipogenic cells in vitro are present in most primary fibroblast-like cell populations. Stem Cells 2007, 25, 1610–1617.
- Alfaro, D.; Zapata, A. G. Eph/Ephrin-mediated stimulation of human bone marrow mesenchymal stromal cells correlates with changes in cell adherence and increased cell death. Stem Cell Res. 2018, 9, 172.
- Taylor, H.; Campbell, J.; Nobes, C. D. Ephs and ephrins. Curr. Biol. 2017, 27, R90–R95.
- Goldshmit, Y.; McLenachan, S.; Turnley, A. Roles of Eph receptors and ephrins in the normal and damaged adult CNS. Brain Res. Rev. 2006, 52, 327–345.
- Binda, E.; Visioli, A.; Giani, F.; Lamorte, G.; Copetti, M.; Pitter, K. L.; Huse, J. T.; Cajola, L.; Zanetti, N.; DiMeco, F.; De Filippis, L.; Mangiola, A.; Maira, G.; Anile, C.; De Bonis, P.; Reynolds, B. A.; Pasquale, E. B.; Vescovi, A. L. The EphA2 receptor drives self-renewal and tumorigenicity in stem-like tumor-propagating cells from human glioblastomas. Cancer Cell 2012, 22, 765–780.
- Jung, Y. H.; Lee, S. J.; Oh, S. Y.; Lee, H. J.; Ryu, J. M.; Han, H. J. Oleic acid enhances the motility of umbilical cord blood derived mesenchymal stem cells through EphB2-dependent F-actin formation. Biochim. Biophys. Acta 2015, 1853, 1905–1917.
- Shen, S. P.; Liu, W. T.; Lin, Y.; Li, Y. T.; Chang, C. H.; Chang, F. W.; Wang, L. M.; Teng, S. W.; Hsuan, Y. EphA2 is a biomarker of hMSCs derived from human placenta and umbilical cord. Taiwan J. Obs. Gynecol. 2015, 54, 749–756.
- Wen, Y. C.; Du, M. K.; Li, M. W.; Hsuan, Y. C.; Su, Y. C.; Lin, W. EphA2-positive human umbilical cord-derived mesenchymal stem cells exert anti-fibrosis and immunomodulatory activities via secretion of prostaglandin E2. Taiwan J. Obs. Gynecol. 2018, 57, 722–725.
- Toran, J. L.; Lopez, J. A.; Gomes-Alves, P.; Aguilar, S.; Torroja, C.; Trevisan-Herraz, M.; Moscoso, I.; Sebastiao, M. J.; Serra, M.; Brito, C.; Cruz, F. M.; Sepulveda, J. C.; Abad, J. L.; Galan-Arriola, C.; Ibanez, B.; Martinez, F.; Fernandez, M. E.; Fernandez-Aviles, F.; Palacios, I.; L, R. B.; Vazquez, J.; Alves, P. M.; Bernad, A. Definition of a cell surface signature for human cardiac progenitor cells after comprehensive comparative transcriptomic and proteomic characterization. Sci. Rep. 2019, 9, 4647.
- Choi, M.; Lee, H. S.; Naidansaren, P.; Kim, H. K.; O, E.; Cha, J. H.; Ahn, H. Y.; Yang, P. I.; Shin, J. C.; Joe, Y. A. Proangiogenic features of Wharton’s jelly-derived mesenchymal stromal/stem cells and their ability to form functional vessels. Int. J. Biochem. Cell Biol. 2013, 45, 560–570.
- Dejana, E.; Orsenigo, F.; Molendini, C.; Baluk, P.; McDonald, D. M. Discovery and characterization of novel microRNAs during endothelial differentiation of human embryonic stem cells. Stem Cells Dev. 2012, 21, 2049–2057.
- Yoo, J. K.; Kim, J.; Choi, S. J.; Noh, H. M.; Kwon, Y. D.; Yoo, H.; Yi, H. S.; Chung, H. M.; Kim, J. K. Discovery and characterization of novel microRNAs during endothelial differentiation of human embryonic stem cells. Stem Cells Dev. 2012, 21, 2049-2057.
- Adorno-Cruz, V.; Liu, H. Regulation and functions of integrin alpha2 in cell adhesion and disease. Genes Dis. 2019, 6, 16–24.
- Ginsberg, M. H. Integrin activation. BMB Rep. 2014, 47, 655–659.
- Ye, F.; Snider, A. K.; Ginsberg, M. H. Ye, F.; Snider, A.K.; Ginsberg, M.H. Talin and kindlin: The one-two punch in integrin activation. Front Med. 2014, 8, 6–16.
- Cappellesso-Fleury, S.; Puissant-Lubrano, B.; Apoil, P. A.; Titeux, M.; Winterton, P.; Casteilla, L.; Bourin, P.; Blancher, A. Human fibroblasts share immunosuppressive properties with bone marrow mesenchymal stem cells. J. Clin. Immunol. 2010, 30, 607–619.
- Uhlen, M.; Fagerberg, L.; Hallstrom, B. M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, A.; Kampf, C.; Sjostedt, E.; Asplund, A.; Olsson, I.; Edlund, K.; Lundberg, E.; Navani, S.; Szigyarto, C. A.; Odeberg, J.; Djureinovic, D.; Takanen, J. O.; Hober, S.; Alm, T.; Edqvist, P. H.; Berling, H.; Tegel, H.; Mulder, J.; Rockberg, J.; Nilsson, P.; Schwenk, J. M.; Hamsten, M.; von Feilitzen, K.; Forsberg, M.; Persson, L.; Johansson, F.; Zwahlen, M.; von Heijne, G.; Nielsen, J.; Ponten, F. . Proteomics. Tissue-based map of the human proteome. Science 2015, 347, 1260419.
- Thul, P. J.; Akesson, L.; Wiking, M.; Mahdessian, D.; Geladaki, A.; Ait Blal, H.; Alm, T.; Asplund, A.; Bjork, L.; Breckels, L. M.; Backstrom, A.; Danielsson, F.; Fagerberg, L.; Fall, J.; Gatto, L.; Gnann, C.; Hober, S.; Hjelmare, M.; Johansson, F.; Lee, S.; Lindskog, C.; Mulder, J.; Mulvey, C. M.; Nilsson, P.; Oksvold, P.; Rockberg, J.; Schutten, R.; Schwenk, J. M.; Sivertsson, A.; Sjostedt, E.; Skogs, M.; Stadler, C.; Sullivan, D. P.; Tegel, H.; Winsnes, C.; Zhang, C.; Zwahlen, M.; Mardinoglu, A.; Ponten, F.; von Feilitzen, K.; Lilley, K. S.; Uhlen, M.; Lundberg, E. A subcellular map of the human proteome. Science 2017, 356, doi:10.1126/science.aal3321.
- P, M.; S, H.; R, M.; M, G.; W, S. K. Adult mesenchymal stem cells and cell surface characterization—a systematic review of the literature. Open Orthop. J. 2011, 5, 253–260.