| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Professor Kharisheh | + 3069 word(s) | 3069 | 2020-09-25 06:17:26 | | | |
| 2 | Bruce Ren | Meta information modification | 3069 | 2020-09-29 04:07:57 | | | | |
| 3 | Bruce Ren | Meta information modification | 3069 | 2020-10-12 06:22:24 | | |
Video Upload Options
Heightened levels of carbon dioxide (CO2) and other greenhouse gases (GHGs) have prompted research into techniques for their capture and separation, including membrane separation, chemical looping, and cryogenic distillation. Ionic liquids, due to their negligible vapour pressure, thermal stability, and broad electrochemical stability have expanded their application in gas separations.
1. Introduction
Flue gas emissions from the chemical industries have severe impacts on the whole environment and the atmosphere and are considered one of the primary causes of global warming phenomena [1][2][3][4][5][6][7][8]. Consequently, the separation and the capture of these unwanted gases has become necessary. The unwanted gaseous emissions that result from burning fuel and chemicals in chemical engineering power plants include carbon dioxide (CO2), methane (CH4), nitrogen oxides (NOx), sulphur oxides (SOx), and hydrogen sulphide (H2S). Each power plant has the responsibility of mitigating and reducing the amount of these gases to limit their negative impact on the environment.
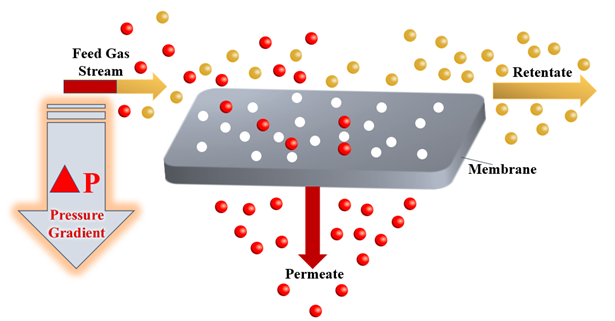
The gas separation process plays a crucial and a fundamental role in the chemical engineering industry as a result of its broad range of applications, ranging from carbon dioxide gas separation from natural gas[9][10][11], recovery of hydrogen from waste gas streams [12], production of nitrogen and oxygen-enriched gases. In the past decades, a large number of different methods for the separation of gases have been developed, including membrane separation[13], chemical looping [14][15][16][17]and cryogenic distillation[18][19][20][21]. The gas separation process using membrane technology depends on the sorption-diffusion mechanism or sieving[22]. Commercial membranes for gas separation use membranes that are nonporous and asymmetric based on a solution-diffusion transport mechanism, and both vapour and gas separation are based on the same mechanism[13]. At the heart of a gas separation process, the driving force for the separation of gases is a pressure gradient. A high-pressure gas mixture moves across a membrane surface that has a selective permeability to one component of the gas mixture. Hence, the permeate produced as a result of this mechanism is enriched with this specific component[23]. Figure 1 below shows a schematic representation of the membrane gas separation process.
Figure 1. Theoretical scheme of the membrane gas separation process.
In the meantime, there is a growing interest in more sustainable gas-separation processes that are mainly based on selective gas absorption or adsorption. Ionic liquids (ILs) provide the best alternative to most of the conventional liquids used in gas separation processes. The unique properties of these ionic liquids distinguish them from all the liquids used in the separation processes [24]. Incorporating ionic liquids into membranes (ILMs) generally increases the membrane selectivity towards specific gases, the permeability of gases through it and the mass transfer. There are three main classifications of ionic liquid membranes (ILMs). These classifications are supported ionic liquid membranes (SILMs), ionic liquid polymeric membranes (ILPMs), and ionic liquid mixed-matrix membranes (ILMMMs) [25]. Each type of ILM has different applications in gas separation processes. The applications of the SILMs, ILPMs, and ionic liquid mixed-matrix membranes (ILMMMs) in the gas separation processes of CO2, CH4, N2, H2 from various gas streams are discussed thoroughly through this paper. Tables 1, 2, and 3 below shows the names and abbreviations of the principal ionic liquid membranes, primary gases, ionic liquids, and polymers mentioned in this study.
2. Ionic Liquid Membranes (ILMs) in gas separation processes
As a result of global warming and the harmful effects of greenhouse gases on the whole environment, the gas separation process plays a fundamental role in the chemical engineering industry as a result of its broad range of applications ranging from carbon dioxide gas separation from natural gas, Recovery of hydrogen from waste gas streams, production of nitrogen and oxygen-enriched gases. During the past decades, a large number of different techniques for the separation of gas have been developed and these methods include membrane separation, chemical looping, and cryogenic distillation. By focusing on the membrane gas separation, many studies and publications were made by several researchers from all over the globe to study the membrane gas separation processes synthesis, implementations, applications, limitations, and development. Figures S7 and S8 give an insight into the interest of researchers about the gas separation processes from the year 2004 to the year 2020.
By looking at figures S7 and S8, it is seen that there is a considerable interest in gas separation processes in all over the world. In the year 2004, a maximum peak (100) was reached. Furthermore, the country that shows the most considerable interest in membrane gas separation is Malaysia (27%). This means that 27% of the total searches made in Google on the gas separation process topic were made by researchers from Malaysia, which is quite a high percentage since there are approximately 2 trillion global Google searches made per year. The second highest region is South Korea (26%), which is a pretty high percentage. By focusing on the membrane gas separation, there are several types and classes of membranes used across the industries. One of the best classes of membranes to be used in the gas separation processes are ionic liquid membranes (ILMs). The incorporation of the ionic liquids into membranes (ILMs) generally increases the membrane selectivity towards specific gases, the permeability of gases through it, and mass transfer. There are three main classifications of the ionic liquid membranes (ILMs). These classifications are supported ionic liquid membranes (SILMs), ionic liquid polymeric membranes (ILPMs), and ionic liquid mixed-matrix membranes (ILMMMs). Each type of ILM has different applications in the gas separation processes.
3.1. Ionic Liquids
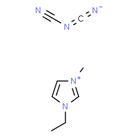
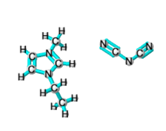
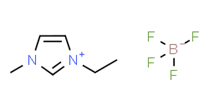
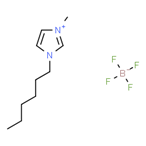
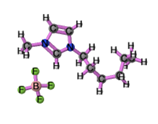
Ionic liquids (ILs) are organic molten salts that have a melting point below 100 Co. Room temperature ionic liquid (RTIL) are ionic liquids that have a melting point less than room temperature (Tmelting < 373 K) [26]. These ionic liquids exhibit many exciting properties that distinguish them from other liquids, including negligible vapour pressures, high thermal stability [27][28][29], inflammability, a liquid range of up to at least 300 Co [30], high solubility for a wide range of inorganic and organic compounds [31]. Moreover, their physicochemical properties can be tailored to satisfy specific chemical tasks by the appropriate selection of anion, cation, and substituents on the cationic constituent. Table 4 below shows the two- and three-dimensional structures of the most used ionic liquids mentioned in this study.
Table 1. Two and three dimensional structures of the most used ionic liquids mentioned in this study.
|
Ionic Liquid |
Two Dimensional (2D) Structure |
Three Dimensional (3D) Structure |
|
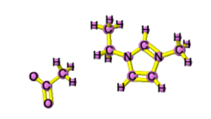
[EMIM][DCA] |
 |
 |
|
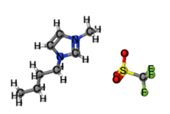
[EMIM][BF4] |
 |
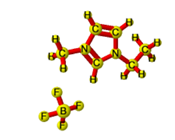 |
|
[EMIM][Ac] |
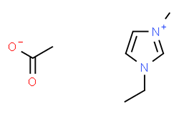 |
 |
|
[EMIM][TFSI] |
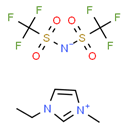 |
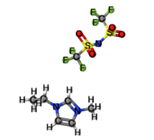 |
[BMIM][TFO] |
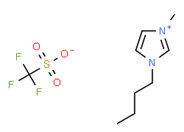 |
 |
|
[BMIM][BF4] |
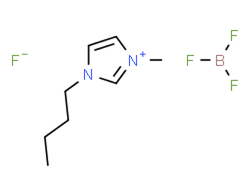 |
 |
|
[BMIM][PF6] |
 |
 |
|
Cyphos 104 |
 |
 |
|
′[HMIM][BF4] |
 |
 |
It is obvious that from Figure S9 that ionic liquids are capturing the special attention of researchers from various countries across the globe. China is ranked the highest country (12%) by searches and interest in ionic liquids, followed by South Korea (9%). Consequently, extensive chemical engineering applications of these innovative fluids have taken place by the end of the last century [32][33][34][35][36][37]. Ionic liquids are made by associating large organic cations with a numerous variety of anions. The most common ionic liquid cations include pyridinium, imidazolium, pyrrolidinium, and theirmono or poly-alkyl derivatives, as well as tetraalkylammonium or phosphonium and trialkylsulfonium. The classical ionic liquids anions include, for the low melting ionic liquids: trifluoromethylsulfate (TfO), bis(trifluoromethylsulfonyl)imide (NTf2), tetra-fluoroborate (BF4) or hexafluorophosphate (PF6) and dicyanamide (N(CN)2). In low stability ionic liquids or ionic liquids that are not liquid at room temperature, the following simple anions are included: iodide, nitrate, bromide, chloride, perchlorate, formate or acetate [38]. Ionic liquids can be produced by other anions, as shown by the selective alkyl-methylimidazolium IL list presented in Table 5 below.
Table 2. Physicochemical properties of selected methylimidazolium ionic liquids sorted by the cation [38].
|
Cation |
Anion |
formula |
m.w g/mol |
m.t. °C |
Visc cP |
Visc cP |
|
MIM |
Cl |
C4H7ClN2 |
118.6 |
74 |
Solid |
Solid |
|
|
NO3 |
C4H7N3O3 |
145.1 |
71 |
Solid |
Solid |
|
MMIM |
Cl |
C5H9ClN2 |
132.6 |
126 |
Solid |
Solid |
|
EMIM |
Cl |
C6H11ClN2 |
146.6 |
89 |
Solid |
Solid |
|
|
SCN |
C7H11N3S |
169.3 |
−6 |
29 |
12 |
|
|
Acetate |
C8H14N2O2 |
170.2 |
-2 |
144 |
39 |
|
|
N(CN)2Br |
C8H11N5 |
177.2 |
-18 |
16 |
9 |
|
|
BF4 |
C6H11BF4N2 |
198 |
13 |
35 |
18 |
|
|
Alaninate |
C9H17N3O2 |
199.2 |
12 |
260 |
62 |
|
|
C(CN)3CH3SO3 |
C10H11N5 |
201.2 |
-9 |
15 |
7 |
|
|
CF3SO3HSO4 |
C7H11F3N2O3S |
260.2 |
-14 |
43 |
20 |
|
|
CH3SO4 |
C7H14N2O4S |
222.3 |
−40 |
79 |
30 |
|
|
C2SO4 |
C8H16N2O4S |
236.3 |
−37 |
98 |
35 |
|
|
C4SO4 |
C10H20N2O4S |
264.3 |
−15 |
181 |
56 |
|
|
C6SO4C8SO4 |
C12H24N2O4S |
292.4 |
-6 |
320 |
86140 |
|
|
I |
C6H11IN2 |
238.1 |
78 |
Solid |
Solid |
|
|
PF6 |
C6H11F6N2P |
256.1 |
60 |
Solid |
Solid |
|
|
AlCl4 |
C6H11AlCl4N2 |
280 |
6.5 |
|
|
|
|
TolSO3 |
C13H18N2O3S |
282.4 |
50 |
Solid |
240 |
|
|
AsF6 |
C6H11AsF6N2 |
300.1 |
53 |
Solid |
Solid |
|
|
NTf2 |
C8H11F6N3O4S2 |
391.3 |
−17 |
33 |
16 |
|
|
N(SO2C2F5)2 |
C10H11F10N3O4S2 |
491.3 |
−1 |
|
|
|
EMMIM |
Br |
C7H13BrN2 |
205.1 |
141 |
Solid |
Solid |
|
|
NTf2 |
C9H13F6N3O4S2 |
405.3 |
25 |
Solid |
|
|
|
N(SO2C2F5)2 |
C11H13F10N3O4S2 |
505.3 |
25 |
Solid |
|
|
PMIM |
Cl |
C7H13ClN2 |
160.6 |
62 |
Solid |
Solid |
|
|
Br |
C7H13BrN2 |
205.1 |
28 |
Solid |
|
|
|
BF4I |
C7H13BF4N2 |
212 |
-17 |
74 |
27 |
|
|
PF6 |
C7H13F6N2P |
270.2 |
38 |
Solid |
|
|
|
NTf2 |
C9H13F6N3O4S2 |
405.3 |
15 |
46 |
19 |
|
BMIM |
Cl |
C8H15ClN2 |
174.5 |
41 |
Solid |
150 |
|
|
SCN |
C9H15N3S |
197.3 |
−6 |
51 |
19 |
|
|
Acetate |
C10H18N2O2 |
198.3 |
−1 |
430 |
67 |
|
|
N(CN)2Br |
C10H15N5 |
205.3 |
-5 |
42 |
19 |
|
|
BF4 |
C8H15BF4N2 |
226 |
−82 |
108 |
36 |
|
|
C(CN)3HSO4 |
C12H15N5 |
229.3 |
-20 |
34 |
12 |
|
|
ClO4 |
C8H15ClN2O4 |
238.7 |
8 |
180 |
57 |
|
|
CH3SO4 |
C9H18N2O4S |
250.3 |
−4 |
94 |
32 |
|
Trifluoroacetate |
||||||
|
|
PF6 |
C8H15F6N2P |
284.2 |
11 |
270 |
74 |
|
|
CF3SO3 |
C9H15F3N2O3S |
288.3 |
14 |
80 |
31 |
|
|
Cyclohexyl sulfamate |
C14H27N3O3S |
317.5 |
72.5 |
Solid |
Solid |
|
|
FeCl4 |
C8H15Cl4FeN2 |
337 |
-12 |
41 870 |
18 |
|
|
NTf2 |
C10H15F6N3O4S2 |
419.4 |
−5 |
42 |
19 |
|
Isobutylmim |
NTf2 |
C10H15F6N3O4S2 |
419.4 |
-16 |
Solid |
Solid |
|
BMIM |
Cl |
C9H17ClN2 |
188.7 |
93 |
Solid |
Solid |
|
|
BF4 |
C9H17BF4N2 |
240.1 |
32 |
Solid |
31 |
|
|
PF6 |
C9H17F6N2P |
298.2 |
38 |
Solid |
|
|
|
NTf2 |
C11H17F6N3O4S2 |
433.4 |
−6 |
350 |
34 |
|
Allylmim |
N(CN)2Cl |
C9H11N5 |
189.2 |
-20 |
20 |
10 |
|
|
CH3SO4 |
C12H16N2O4S |
284.3 |
18 |
4500 |
327 |
|
|
PF6 |
C11H13F6N2P |
318.2 |
130 |
Solid |
Solid |
|
C5mim |
PF6 |
C9H17F6N2P |
298.2 |
16 |
380 |
97 |
|
|
NTf2 |
C11H17F6N3O4S2 |
433.4 |
−9 |
58 |
22 |
|
HMIM |
N(CN)2 |
C12H19N5 |
233.3 |
1 |
50 |
20 |
|
|
BF4I |
C10H19BF4N2 |
254.1 |
-82 |
200 |
58 |
|
|
PF6 |
C10H19F6N2P |
312.2 |
-61 |
480 |
120 |
|
|
NTf2 |
C12H19F6N3O4S2 |
447.4 |
−6 |
70 |
27 |
Aside from their different properties, there are severe limitations that face the usage of ionic liquids [39][40]. The main limitations are the high synthesis price and the high recycling energy requirement. These two limitations could act as a burden on the economic and financial sides of the processes. The use of ionic liquid membrane (ILM) technology can overcome these two drawbacks. The ILM consists of the feed and permeates phases that are separated by a membrane containing IL that allows simultaneous extraction and stripping at each side of ILM. The IL for an ILM can be stabilized by either quasi-solidification to endow material with good mechanical strength or impregnating it inside the pores of the support membrane. The ILM techniques require a fewer amount of IL as a carrier and do not require additional steps for IL recycling. Consequently, ILMs have many advantages, such as the low energy requirements, the ease of fabrication due to the flexible and compact devices, low operating costs, capital and several more merits
Furthermore, due to the special and the outstanding properties of IL, e.g., high viscosity, and negligible vapour pressure, ILMs are considered more stable compared to the traditional supported liquid membranes (SLMs) based on organic solvents. Therefore, ILMs show promising application potential. In the last decade, researchers have reported the use of ILMs in several applications and fields, including the separation of various mixtures, electrochemical devices, and catalytic reactions.
The research progress of gas separation, especially CO2 separation, has been addressed in depth. Martins, et al. [41] conducted a CO2 removal process by using a membrane contactor that is combined with a biocompatible ionic liquid (IL), cholinium lysinate, that has a high absorption capacity (5.9 mol CO2/kg IL). The rate of CO2 removal and IL solution regeneration was examined in their study by changing the feed gas composition, conditions of the ionic liquid flow rate, and the relative humidity. The results obtained from their study have shown that the proposed system is capable of removing CO2 from anaesthetic gas circuits [41]. Shamair, et al. [42] have successfully investigated the performance of a recently synthesized RTIL in SILM, theoretically, and experimentally. In their study, benzimidazolium-1-acetate was used to synthesize SILM over a polyimide support. The synthesized SILM was tested for different gas mixtures (CO2/CH4 and CO2/N2). The prepared SILM results have shown that there is a high selectivity of 37.92 and 40.29 for CO2/CH4 and CO2/N2, respectively. These results strengthen the DFT calculation and indicate that there are up-and-coming applications for SILM in gas separation [42]. Sohaib, et al. [43] have mentioned in their research study that coupled absorption/desorption in combination with ILs, can be considered very useful for continuous post-combustion carbon capture [43]. Zia-ul-Mustafa, et al. [44] focused their research on the effect of imidazolium-based ionic liquids on the PES membrane for CO2/CH4 separation. The polyethersulfone (PES) based gas separation ionic liquid polymeric membranes (ILPMs) were synthesized in their study. Ionic liquids 1-ethyl-3-methylimidazolium tetrafluoroborate [EMIM][BF4] and 1-ethyl-3-methylimidazolium dicyanamide [EMIM][DCA] were added to the doping solution at ten weight % and flat sheet dense membranes were cast by the method of dry phase inversion. The CO2 gas permeability of the synthesized ILPMs was successfully increased by blending the ionic liquids into the polymer matrix as a result of the high affinity and solubility of CO2 in [EMIM][BF4] and [EMIM][DCA] ionic liquids. Membrane PES+IL1 (10%) has shown an approximation of 23.5 folds increase in CO2 gas permeability in comparison to the pure polyethersulfone membrane. Hence, they mentioned that the ILPMs have a remarkable potential for the separation of CO2 from natural gas [44]. Nabais, et al. [45] used poly(ionic liquid)-based engineered mixed matrix membranes for the separation of CO2/H2. The mixed matrix membranes (MMMs) were synthesized by combining IL, a pyrrolidinium-based PIL, and three highly CO2-selective metal-organic frameworks (MOFs). The different MOFs ZIF-8, (MIL-53(Al), and Cu3 (BTC) 2 were used as fillers, that aims to maximize the performance of the membranes towards the purification of syngas. Ideal selectivity and permeability of CO2 were achieved as a result of their usage of different MOFs and loadings (0, 10, 20, and 30 wt %). The authors also mentioned that this has caused mechanical and thermal stabilities of the membranes and has in term increased their performance [45].
3.1.1. Selected Physicochemical Properties of Ionic Liquids
Ionic liquids are very versatile and their anions and cations can be adapted easily to the role that they have to play. Their most valuable and distinguishable physicochemical properties include the following: low melting point (m.p. < 100 Co), high thermal stability, insignificant vapour pressure, a low surface tension [46], high electrical conductivity, a wide electrochemical window [47][48][49], and an adjustable solvent viscosity and/or hydrophobicity and/ or polarity associated with high or low water miscibility [50]. In the separation techniques, the most crucial parameters are the ionic liquid melting point, viscosity, thermal stability, vapour pressure and solvent properties [51].
3.1.2 Melting Point
Extensive research has been made to understand the low melting points of the ILs and to predict their physicochemical properties by using computational tools. By using a large number of ILS that have been studied, Table 5 above shows the selected physicochemical properties of methylimidazolium ionic liquids only. Low melting points are obtained with salts made of large and asymmetrical ions [49]. Generally speaking, the melting temperature Tm decreases by increasing anisotropy, internal flexibility, and size of the ions. On the other side, the melting point increases by increasing the interactions of the alkyl chains [52]. The change in the melting points of the ionic liquids is linked to the symmetry or cation size, as the chain length changes the melting point changes. Nasirpour, et al. [53] researched the relationship between chain length and the melting point by examining the Ionic liquids melting point versus the length of the alkyl chain for [Cnmim][PF6] and trihexyl-alkylphosphonium hexafluorophosphate [P666n][PF6]). The results of their study have shown that the alkylmethylimidazolium [PF6] ionic liquids have shown a decrease in the melting temperature as the alkyl chain length increase. Shorter alkyl chains with n < 6 have higher melting points since they produce symmetrical Ionic Liquids with better ion cohesion. Also, longer alkyl chains with n > 8 have higher melting points since their cations are more hydrophobic and form Van der Waals forces [53].
3.1.3 Viscosity
Ionic Liquids at room temperature are viscous liquids. By looking at Table 5, at 25 °C the lowest viscosities observed with the methylimidazolium salts are 15 cp for [EMIM][C(CN)3], 16 cp for [EMIM] [N(CN)2], and 20 cP for [allylmim][N(CN)2].[54]. The values of these viscosities are similar to the viscosities at 25 °C of viscous organic solvents such as ethylene glycol (16 cP) [55], dimethyl phthalate (14 cP) [56], or ethanolamine (21 cP) [57]. An increase in temperature dramatically reduces the viscosity, as shown by the 50 °C values listed in Table 5. There is an extensive database in the literature that includes the viscosities of ionic liquids [58].
3.1.4 Vapour Pressure and Thermal Stability
The significant vapour pressure of the ILS is quite challenging to be measured. At high temperatures, a decomposition of the liquids might occur, and at very low temperatures the vapour pressure becomes too low to be measured by typical conventional methods. Several studies focus on finding the vapour pressure of ionic liquids. Ahrenberg, et al. [59] and his research team have focused on determining the vapor pressure of ionic liquids at low temperatures using AC-chip-calorimetry. A highly sensitive method for mass loss determination at temperatures starting from 350 K was successfully developed in their study. They found the vapour pressure from the measured rates of mass loss using the Langmuir equation. The method that they have used has successfully determined the vapour pressure and the vaporization enthalpy of an archetypical ionic liquid 1-ethyl-3-methylimidazolium bis (trifluoromethylsulfonyl) imide ([EMIM][NTf2]) [59]. Maton, et al. [60] have mentioned in their research that the thermal stability of an IL depends on the specific combination of the cation-anion [61]. Also, Xue, et al. [62] have examined the thermal stability of ILs under vacuum by thermogravimetric analysis (TGA). The TGA analysis of their study has shown that many ionic liquids start to decompose thermally at 700 K [61].
References
- Pratibha, G.; Srinivas, I.; Rao, K.V.; Shanker, A.K.; Raju, B.M.K.; Choudhary, D.K.; Srinivas Rao, K.; Srinivasarao, C.; Maheswari, M. Net global warming potential and greenhouse gas intensity of conventional and conservation agriculture system in rainfed semi arid tropics of India. Atmos. Environ. 2016, 145, 239–250, doi:https://doi.org/10.1016/j.atmosenv.2016.09.039.
- Skytt, T.; Nielsen, S.N.; Jonsson, B.-G. Global warming potential and absolute global temperature change potential from carbon dioxide and methane fluxes as indicators of regional sustainability – A case study of Jämtland, Sweden. Ecol. Indic. 2020, 110, 105831, doi:https://doi.org/10.1016/j.ecolind.2019.105831.
- Bartholy, J.; Pongrácz, R. A brief review of health-related issues occurring in urban areas related to global warming of 1.5°C. Curr. Opin. Environ. Sustain. 2018, 30, 123–132, doi:https://doi.org/10.1016/j.cosust.2018.05.014.
- Mika, J.; Forgo, P.; Lakatos, L.; Olah, A.B.; Rapi, S.; Utasi, Z. Impact of 1.5K global warming on urban air pollution and heat island with outlook on human health effects. Curr. Opin. Environ. Sustain. 2018, 30, 151–159, doi:https://doi.org/10.1016/j.cosust.2018.05.013.
- Liu, Y.; Tang, L.; Qiu, X.; Liu, B.; Chang, X.; Liu, L.; Zhang, X.; Cao, W.; Zhu, Y. Impacts of 1.5 and 2.0°C global warming on rice production across China. Agric. For. Meteorol. 2020, 284, 107900, doi:https://doi.org/10.1016/j.agrformet.2020.107900.
- Qi, W.; Liu, J.; Leung, F. A framework to quantify impacts of elevated CO2 concentration, global warming and leaf area changes on seasonal variations of water resources on a river basin scale. J. Hydrol. 2019, 570, 508–522, doi:https://doi.org/10.1016/j.jhydrol.2019.01.015.
- Nordell, B. Thermal pollution causes global warming. Glob. Planet. Chang. 2003, 38, 305–312, doi:https://doi.org/10.1016/S0921-8181(03)00113-9.
- Wijesiri, B.; Liu, A.; Goonetilleke, A. Impact of global warming on urban stormwater quality: From the perspective of an alternative water resource. J. Clean. Prod. 2020, 262, 121330, doi:https://doi.org/10.1016/j.jclepro.2020.121330.
- Xu, X.; Song, C.; Miller, B.G.; Scaroni, A.W. Adsorption separation of carbon dioxide from flue gas of natural gas-fired boiler by a novel nanoporous “molecular basket” adsorbent. Fuel Process. Technol. 2005, 86, 1457–1472, doi:https://doi.org/10.1016/j.fuproc.2005.01.002.
- Lee, A.L.; Feldkirchner, H.L.; Stern, S.A.; Houde, A.Y.; Gamez, J.P.; Meyer, H.S. Field tests of membrane modules for the separation of carbon dioxide from low-quality natural gas. Gas. Sep. Purif. 1995, 9, 35–43, doi:https://doi.org/10.1016/0950-4214(95)92175-C.
- Rufford, T.E.; Smart, S.; Watson, G.C.Y.; Graham, B.F.; Boxall, J.; Diniz da Costa, J.C.; May, E.F. The removal of CO2 and N2 from natural gas: A review of conventional and emerging process technologies . J. Pet. Sci. Eng. 2006, 94-95, 123-154, doi:https://doi.org/10.1016/j.petrol.2012.06.016.
- Yáñez, M.; Ortiz, A.; Gorri, D.; Ortiz, I. Comparative performance of commercial polymeric membranes in the recovery of industrial hydrogen waste gas streams. Int. J. Hydrog. Energy 2020, https://doi.org/10.1016/j.ijhydene.2020.04.026.
- Sasikumar, B.; Arthanareeswaran, G.; Ismail, A.F. Recent progress in ionic liquid membranes for gas separation. J. Mol. Liq. 2018, 266, 330–341, doi:https://doi.org/10.1016/j.molliq.2018.06.081.
- Nadgouda, S.G.; Kathe, M.V.; Fan, L.-S. Cold gas efficiency enhancement in a chemical looping combustion system using staged H2 separation approach. Int. J. Hydrog. Energy 2017, 42, 4751–4763, doi:https://doi.org/10.1016/j.ijhydene.2016.12.005.
- Kong, F.; Swift, J.; Zhang, Q.; Fan, L.-S.; Tong, A. Biogas to H2 conversion with CO2 capture using chemical looping technology: Process simulation and comparison to conventional reforming processes. Fuel 2020, 279, 118479, doi:https://doi.org/10.1016/j.fuel.2020.118479.
- Qing, M.; Jin, B.; Ma, J.; Zou, X.; Wang, X.; Zheng, C.; Zhao, H. Thermodynamic and economic performance of oxy-combustion power plants integrating chemical looping air separation. Energy 2020, 206, 118136, doi:https://doi.org/10.1016/j.energy.2020.118136.
- Khan, M.N.; Chiesa, P.; Cloete, S.; Amini, S. Integration of chemical looping combustion for cost-effective CO2 capture from state-of-the-art natural gas combined cycles. Energy Convers. Manag. : X 2020, 7, 100044, doi:https://doi.org/10.1016/j.ecmx.2020.100044.
- Niculescu, A.E.; Bornea, A.; Ana, G.; Zamfirache, M. Development of specific software for hydrogen isotopes separation by cryogenic distillation of ICSI Pilot Plant. Fusion Eng. Des. 2020, 158, 111642, doi:https://doi.org/10.1016/j.fusengdes.2020.111642.
- Brigagão, G.V.; de Medeiros, J.L.; Araújo, O.d.Q.F. A novel cryogenic vapor-recompression air separation unit integrated to oxyfuel combined-cycle gas-to-wire plant with carbon dioxide enhanced oil recovery: Energy and economic assessments. Energy Convers. Manag. 2019, 189, 202–214, doi:https://doi.org/10.1016/j.enconman.2019.03.088.
- Yousef, A.M.; El-Maghlany, W.M.; Eldrainy, Y.A.; Attia, A. New approach for biogas purification using cryogenic separation and distillation process for CO2 capture. Energy 2018, 156, 328–351, doi:https://doi.org/10.1016/j.energy.2018.05.106.
- Ceylan, M.; Jobson, M.; Smith, R. Membrane–cryogenic Distillation Hybrid Processes for Cost-effective Argon Production from Air. In Computer Aided Chemical Engineering, Espuña, A., Graells, M., Puigjaner, L., Eds. Elsevier: 2017; Vol. 40, pp. 1117-1122.
- Fried, J.R. Basic Principles of Membrane Technology By Marcel Mulder (University of Twente, The Netherlands). Kluwer Academic: Dordrecht. 1996. 564 pp. $255.00. ISBN 0-7823-4247-X. J. Am. Chem. Soc. 1997, 119, 8582–8582, doi:10.1021/ja975504k.
- Bernardo, P.; Drioli, E.; Golemme, G. Membrane Gas Separation: A Review/State of the Art. Ind. Eng. Chem. Res. 2009, 48, 4638–4663, doi:10.1021/ie8019032.
- Costa Gomes, M.F.; Husson, P. Ionic Liquids: Promising Media for Gas Separations. In Ionic Liquids: From Knowledge to Application, American Chemical Society: 2009; Vol. 1030, pp. 223-237.
- Zia ul Mustafa, M.; bin Mukhtar, H.; Md Nordin, N.A.H.; Mannan, H.A.; Nasir, R.; Fazil, N. Recent Developments and Applications of Ionic Liquids in Gas Separation Membranes. Chem. Eng. Technol. 2019, 42, 2580–2593, doi:10.1002/ceat.201800519.
- Hallett, J.P.; Welton, T. Room-Temperature Ionic Liquids: Solvents for Synthesis and Catalysis. 2. Chem. Rev. 2011, 111, 3508–3576, doi:10.1021/cr1003248.
- Villanueva, M.; Coronas, A.; García, J.; Salgado, J. Thermal Stability of Ionic Liquids for Their Application as New Absorbents. Ind. Eng. Chem. Res. 2013, 52, 15718–15727, doi:10.1021/ie401656e.
- Cao, Y.; Mu, T. Comprehensive Investigation on the Thermal Stability of 66 Ionic Liquids by Thermogravimetric Analysis. Ind. Eng. Chem. Res. 2014, 53, 8651–8664, doi:10.1021/ie5009597.
- Liu, P.; Wang, M.; Cheng, Z.-M. Thermal Stability and Vapor–Liquid Equilibrium for Imidazolium Ionic Liquids as Alternative Reaction Media. J. Chem. Eng. Data 2015, 60, 836–844, doi:10.1021/je5009558.
- Qiao, Y.; Ma, W.; Theyssen, N.; Chen, C.; Hou, Z. Temperature-Responsive Ionic Liquids: Fundamental Behaviors and Catalytic Applications. Chem. Rev. 2017, 117, 6881–6928, doi:10.1021/acs.chemrev.6b00652.
- Zhou, T.; Chen, L.; Ye, Y.; Chen, L.; Qi, Z.; Freund, H.; Sundmacher, K. An Overview of Mutual Solubility of Ionic Liquids and Water Predicted by COSMO-RS. Ind. Eng. Chem. Res. 2012, 51, 6256–6264, doi:10.1021/ie202719z.
- Holbrey, J.D.; Rogers, R.D. Green Industrial Applications of Ionic Liquids: Technology Review. In Ionic Liquids, American Chemical Society: 2002; Vol. 818, pp. 446-458.
- Wishart, J.F. Radiation and Radical Chemistry of Ionic Liquids for Energy Applications. In Ionic Liquids: Current State and Future Directions, American Chemical Society: 2017; Vol. 1250, pp. 251-272.
- Holbrey, J.D.; Turner, M.B.; Rogers, R.D. Selection of Ionic Liquids for Green Chemical Applications. In Ionic Liquids as Green Solvents, American Chemical Society: 2003; Vol. 856, pp. 2-12.
- Roberts, N.J.; Lye, G.J. Application of Room-Temperature Ionic Liquids in Biocatalysis: Opportunities and Challenges. In Ionic Liquids, American Chemical Society: 2002; Vol. 818, pp. 347-359.
- Isosaari, P.; Srivastava, V.; Sillanpää, M. Ionic liquid-based water treatment technologies for organic pollutants: Current status and future prospects of ionic liquid mediated technologies. Sci. Total Environ. 2019, 690, 604–619, doi:https://doi.org/10.1016/j.scitotenv.2019.06.421.
- Marcinkowska, R.; Konieczna, K.; Marcinkowski, Ł.; Namieśnik, J.; Kloskowski, A. Application of ionic liquids in microextraction techniques: Current trends and future perspectives. Trac Trends Anal. Chem. 2019, 119, 115614, doi:https://doi.org/10.1016/j.trac.2019.07.025.
- Berthod, A.; Ruiz-Ángel, M.J.; Carda-Broch, S. Recent advances on ionic liquid uses in separation techniques. J. Chromatogr. A 2018, 1559, 2–16, doi:https://doi.org/10.1016/j.chroma.2017.09.044.
- Stacy, E.W.; Gainaru, C.P.; Gobet, M.; Wojnarowska, Z.; Bocharova, V.; Greenbaum, S.G.; Sokolov, A.P. Fundamental Limitations of Ionic Conductivity in Polymerized Ionic Liquids. Macromolecules 2018, 51, 8637–8645, doi:10.1021/acs.macromol.8b01221.
- Ahmad Adlie, S. IONIC LIQUIDS: PREPARATIONS AND LIMITATIONS. Makara Journal of Science; Vol 14, No 2 (2010): November 2011.
- Martins, C.F.; Neves, L.A.; Chagas, R.; Ferreira, L.M.; Afonso, C.A.M.; Crespo, J.G.; Coelhoso, I.M. CO2 removal from anaesthesia circuits using gas-ionic liquid membrane contactors. Sep. Purif. Technol. 2020, doi:https://doi.org/10.1016/j.seppur.2020.116983.
- Shamair, Z.; Habib, N.; Gilani, M.A.; Khan, A.L. Theoretical and experimental investigation of CO2 separation from CH4 and N2 through supported ionic liquid membranes. Appl. Energy 2020, 268, 115016, doi:https://doi.org/10.1016/j.apenergy.2020.115016.
- Sohaib, Q.; Vadillo, J.M.; Gómez-Coma, L.; Albo, J.; Druon-Bocquet, S.; Irabien, A.; Sanchez-Marcano, J. CO2 capture with room temperature ionic liquids; coupled absorption/desorption and single module absorption in membrane contactor. Chem. Eng. Sci. 2020, 223, 115719, doi:https://doi.org/10.1016/j.ces.2020.115719.
- Zia-ul-Mustafa, M.; Mukhtar, H.; Nordin, N.; Mannan, H.A. Effect of imidazolium based ionic liquids on PES membrane for CO2/CH4 separation. Mater. Today: Proc. 2019, 16, 1976–1982, doi:https://doi.org/10.1016/j.matpr.2019.06.076.
- Nabais, A.R.; Martins, A.P.S.; Alves, V.D.; Crespo, J.G.; Marrucho, I.M.; Tomé, L.C.; Neves, L.A. Poly(ionic liquid)-based engineered mixed matrix membranes for CO2/H2 separation. Sep. Purif. Technol. 2019, 222, 168–176, doi:https://doi.org/10.1016/j.seppur.2019.04.018.
- Tong, J.; Liu, Q.-S.; Wang, P.-P.; Welz-Biermann, U.; Yang, J.-Z. Surface Tension and Density of Ionic Liquid n-Butylpyridinium Heptachlorodialuminate. J. Chem. Eng. Data 2011, 56, 3722–3724, doi:10.1021/je200471w.
- Wu, F.; Zhu, N.; Bai, Y.; Liu, L.; Zhou, H.; Wu, C. Highly Safe Ionic Liquid Electrolytes for Sodium-Ion Battery: Wide Electrochemical Window and Good Thermal Stability. Acs Appl. Mater. Interfaces 2016, 8, 21381–21386, doi:10.1021/acsami.6b07054.
- O’Mahony, A.M.; Silvester, D.S.; Aldous, L.; Hardacre, C.; Compton, R.G. Effect of Water on the Electrochemical Window and Potential Limits of Room-Temperature Ionic Liquids. J. Chem. Eng. Data 2008, 53, 2884–2891, doi:10.1021/je800678e.
- Nishi, N.; Imakura, S.; Kakiuchi, T. Wide Electrochemical Window at the Interface between Water and a Hydrophobic Room-Temperature Ionic Liquid of Tetrakis[3,5-bis(Trifluoromethyl)phenyl]borate. Anal. Chem. 2006, 78, 2726–2731, doi:10.1021/ac052152o.
- Plechkova, N.V.; Seddon, K.R. Applications of ionic liquids in the chemical industry. Chem. Soc. Rev. 2008, 37, 123–150, doi:10.1039/B006677J.
- Physicochemical Properties of Ionic Liquids. In Ionic Liquids Further UnCOILed, 2014; 10.1002/9781118839706.ch11pp. 275-307.
- García, G.; Atilhan, M.; Aparicio, S. Viscous origin of ionic liquids at the molecular level: A quantum chemical insight. Chem. Phys. Lett. 2014, 610-611, 267-272, doi:https://doi.org/10.1016/j.cplett.2014.07.051.
- Nasirpour, N.; Mohammadpourfard, M.; Zeinali Heris, S. Ionic liquids: Promising compounds for sustainable chemical processes and applications. Chem. Eng. Res. Des. 2020, 160, 264–300, doi:https://doi.org/10.1016/j.cherd.2020.06.006.
- Paduszyński, K.; Domańska, U. Viscosity of Ionic Liquids: An Extensive Database and a New Group Contribution Model Based on a Feed-Forward Artificial Neural Network. J. Chem. Inf. Modeling 2014, 54, 1311–1324, doi:10.1021/ci500206u.
- Jerome, F.S.; Tseng, J.T.; Fan, L.T. Viscosities of aqueous glycol solutions. J. Chem. Eng. Data 1968, 13, 496–496, doi:10.1021/je60039a010.
- De Lorenzi, L.; Fermeglia, M.; Torriano, G. Density, Refractive Index, and Kinematic Viscosity of Diesters and Triesters. J. Chem. Eng. Data 1997, 42, 919–923, doi:10.1021/je970036f.
- DiGuilio, R.M.; Lee, R.J.; Schaeffer, S.T.; Brasher, L.L.; Teja, A.S. Densities and viscosities of the ethanolamines. J. Chem. Eng. Data 1992, 37, 239–242, doi:10.1021/je00006a028.
- Yu, G.; Zhao, D.; Wen, L.; Yang, S.; Chen, X. Viscosity of ionic liquids: Database, observation, and quantitative structure-property relationship analysis. Aiche J. 2012, 58, 2885–2899, doi:10.1002/aic.12786.
- Ahrenberg, M.; Beck, M.; Neise, C.; Keßler, O.; Kragl, U.; Verevkin, S.P.; Schick, C. Vapor pressure of ionic liquids at low temperatures from AC-chip-calorimetry. Phys. Chem. Chem. Phys. 2016, 18, 21381–21390, doi:10.1039/C6CP01948J.
- Maton, C.; De Vos, N.; Stevens, C.V. Ionic liquid thermal stabilities: Decomposition mechanisms and analysis tools. Chem. Soc. Rev. 2013, 42, 5963–5977, doi:10.1039/C3CS60071H.
- Xue, Z.; Qin, L.; Jiang, J.; Mu, T.; Gao, G. Thermal, electrochemical and radiolytic stabilities of ionic liquids. Phys. Chem. Chem. Phys. 2018, 20, 8382–8402, doi:10.1039/C7CP07483B.
- Xue, Z.; Qin, L.; Jiang, J.; Mu, T.; Gao, G. Thermal, electrochemical and radiolytic stabilities of ionic liquids. Phys. Chem. Chem. Phys. 2018, 20, 8382–8402, doi:10.1039/C7CP07483B.