
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Marco Valussi | + 2718 word(s) | 2718 | 2020-09-23 03:16:56 | | | |
| 2 | Rita Xu | -625 word(s) | 2093 | 2020-09-24 03:29:24 | | | | |
| 3 | Rita Xu | + 11 word(s) | 2104 | 2020-09-24 10:11:48 | | | | |
| 4 | Michele Antonelli | + 33 word(s) | 2137 | 2020-09-25 12:21:02 | | | | |
| 5 | Michele Antonelli | Meta information modification | 2137 | 2020-09-28 11:31:39 | | |
Video Upload Options
This article provides a biochemical description of forest Biogenic Volatile Organic Compounds (BVOCs), namely any organic substance, except carbon dioxide and monoxide, mostly emitted by plants and having vapor pressure high enough to be vaporized in relevant amounts into the forest air. An estimation of the average contribution of forests to the atmospheric composition is mentioned. Additionally, a brief analysis of functional roles that BVOCs play for plant physiology and forest ecology is reported, including the importance of non-tree-derived BVOCs. Finally, biochemical pathways leading to the natural production of most forest BVOCs are described.
1. Introduction
1.1. Background
The first systematic analysis of volatile organic compounds (VOCs) emitted by plants in the atmosphere above forests was probably performed by climate scientists. In two articles [1][2], Fritz Went and his colleagues hypothesized that the blue haze observed above many forests was caused (at least partially) by VOCs released from plants and tried to estimate the impact that biogenic emissions have on the global atmospheric composition. The first estimate of global terpene emissions by Went, derived from a study on leaf oils in shrubs, was of around 175 millions of tons C/year [1], but more recent evaluations state that worldwide biogenic VOC (BVOC) emissions into the atmosphere could amount to approximately 1 billion of tons C/year [3]. Forests seem to be the greatest BVOC emitters and, in particular, tropical trees appear to be responsible for around 80% of terpenoid emissions and for around 50% of other BVOC emissions, while other tree species seem to contribute to around 10% of total BVOC emissions [3]. Overall, BVOCs seem to form 80% to 90% of the total VOC emissions in the atmosphere each year [4][5].
1.2. Terminology
Some forest VOCs are also defined as “phytoncides” (in ancient Greek: "phyton" = "plant" and "-cide" = "killing"), and this term was invented by the Russian biologist Boris Petrovich Tokin to describe the antimicrobial and insecticidal activity of these substances [6][7]. However, the term “phytoncide” may be misleading because, due its broad definition and etymology, it can indicate either plant-derived volatile substances with antiparasitic properties or any (volatile and nonvolatile) antimicrobial/insecticidal compound released by plants, any volatile VOC, or, in some cases, essential oils obtained from aromatic woods. In order to avoid potential misunderstandings, the term “forest VOCs” is preferable over the less specific “phytoncide”, and BVOCs can be defined as any organic compound, except carbon dioxide and monoxide, mostly emitted by plants and having vapor pressure high enough to be vaporized in relevant amounts. Usually, the definition excludes dimethyl sulfide and methane because it is still debated as to whether they are produced by terrestrial plants [8].
1.3. Natural functions of forest VOCs
More than 1000 different BVOCs are released from plant flowers, vegetative parts, or roots [9]. These substances are largely lipophilic products with molecular masses under 300 Da, and the vast majority are isoprenoids, including hemiterpenes (C5H8) such as isoprene, monoterpenes (C10H16), irregular acyclic homoterpenes (C11H18 or C16H26), and sesquiterpenes (C15H24), both as hydrocarbons and as oxygenated compounds; there is also a number of low-molecular-weight molecules, especially C1 and C2 oxygenated compounds, such as methanol and acetaldehyde, and C6H10 fatty acid derivatives, including lipoxygenase pathway products such as green leaf volatiles (henceforth GLVs), plus benzenoids and phenylpropanoids [5][9][10][11][12][13].
Many BVOCs are common to plant species which belong to distant phyla [14]. For example, only some plant species, even of very different taxa (like Bryophyta and Quercus), emit isoprene, while in close taxa, there can be both emitters (Quercus spp.) and non-emitters (Acer spp.) [11], thus suggesting that metabolic pathways related to their production have been highly preserved during the evolutionary history of plants [14].
From the perspective of plant physiology, BVOCs can be divided into constitutive and inducible compounds. All plants can potentially synthesize volatile isoprenoids if triggered by abiotic and biotic stress factors, but only some species can do this constitutively [15].
-
Constitutive forest VOCs are already synthesized in the organism and either stored in specialized structures or constantly emitted without any form of storage [16]. They are emitted at baseline levels and are mainly made of terpenoids, plus shikimate derivatives and polyketides [5].
-
Inducible forest VOCs (herbivore-induced plant volatiles; henceforth HIPVs) are compounds whose synthesis is increased or initiated de-novo after herbivore attacks but also after stimulation by abiotic stressors. They have some metabolic costs but they make the plant phenotypically plastic and herbivore adaptation more unlikely to occur [5].
HIPVs comprise isoprenoids, products of the lipoxygenase (LOX) pathway, such as GLVs, some carotenoid derivatives, indoles and phenolics, and phytohormones such as ethylene, jasmonic acid, and others [17][18]. It is hypothesized that forest VOCs are not only involved in some plant-related physiological functions (biogenic stress response, adaptation to climate changes), but they also have a central role in forest ecosystems (interplant communication, antimicrobial and insecticidal activity against parasites, influence on animals’ feeding behaviors as well as on underwood environmental conditions) [19]. Thus, forest VOCs can have several functions for plant physiology [18] and the most important of them are summarized in Table 1 and Figure 1.
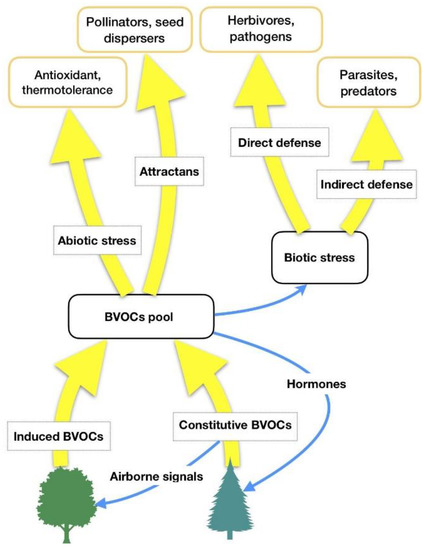
Figure 1. BVOCs’ functions in relation to biotic and abiotic stresses. Adapted from Laothawornkitkul et al. (2009) [8].
Table 1. Functions of constitutive and herbivore-induced forest Volatile Organic Compounds (VOCs).
| Constitutive Forest VOCs | Herbivore-Induced Plant Volatiles (HIPVs) |
|---|---|
| Reduction of abiotic stress. Isoprene and monoterpenes increase general thermal tolerance of photosynthesis, protect photosynthetic apparatus and its activity under high-temperature stress by stabilizing the thylakoid membranes and quenching Reactive Oxygen Species (ROS) | Reduction of abiotic stress. Isoprene and monoterpenes increase general thermal tolerance of photosynthesis, protect photosynthetic apparatus and its activity under high-temperature stress by stabilizing the thylakoid membranes and quenching Reactive Oxygen Species (ROS) |
| Defence against herbivores. Comprises direct mechanisms, mediated by toxic, repellent, anti-nutritive constitutive BVOCs (biogenic Volatile Organic Compounds) or HIPVs, as well as growth and reproductive reducers | Defence against herbivores, mainly indirectly but also directly. HIPVs and volatile compounds that attract, nourish, or otherwise favor another organism that reduces herbivore pressure |
| Inter-plant signalling. HIPVs, especially Green Leaf Volatiles (GLVs), and constitutive BVOCs can travel from a herbivore-damaged part to other plants (both conspecific and heterospecific), activating defence genes and priming a more vigorous response after an attack | Inter- and intra-plant signalling. HIPVs, especially GLVs, and constitutive BVOCs can travel from a herbivore-damaged part to an undamaged one, or to other plants (both conspecific and heterospecific), activating defence genes and priming a more vigorous response after an attack |
| Defence against microbial pathogens | Defence against microbial pathogens |
| Allelopathy. Inhibition of competing species’ seed germination and competition | |
| The attraction of pollinators and seed dispersers |
An ideal chronology of plant emissions could be described as follows [20][14][21]:
-
Prior to herbivore attack: constitutive BVOCs are released and act directly on herbivores by repelling them and/or by inhibiting their feeding; additionally, constitutive BVOCs also inhibit the proliferation of microbial pathogens and repel viral vectors [22].
-
Herbivore attack: immediate release of constitutive BVOCs upon rupture of storage structures such as glandular trichomes.
-
From a few seconds to a few minutes after the attack: the plant wounding response triggers a cascade of events leading to the synthesis of jasmonic acid, capable of regulating the expression of some genes in the nucleus, thus causing a de novo production of HIPVs (GLVs and terpenoids) that attract natural enemies of herbivore attackers, such as predators and parasitoids. Together with jasmonic acid, salicylic acid, ethylene and other phytohormones, GLVs can also modulate the Systemic Acquired Resistance (SAR) in the same plant and in others, thus priming them for future attacks.
-
Continuous damage, oral secretions, oviposition fluids, infection or HIPVs signalling: the release after 12-24 hours, usually for the next photo-period, of induced phytohormones like jasmonic acid, salicylic acid, ethylene, and de novo synthesis of terpenoids and shikimate derivatives.
1.4. Non-tree derived BVOCs
In temperate forests, the canopy layer of trees acts as the largest emission source of BVOCs [19] and, in general, from a quantitative point of view, plant leaves with their canals, oil glands, and glandular trichomes are the major emitters of these volatile compounds [8][10]. However, all plant organs and tissues produce BVOCs: leaves, flowers, and fruits release them into the atmosphere, whereas roots secrete them into the soil. Moreover, wood, phloem, and bark of trunks can serve as pools of stored BVOCs [19].
Nevertheless, BVOCs are not exclusively tree-derived: soil bacteria, mycorrhizal fungi, and other rhizosphere microbes can also emit BVOCs, but it is not easy to evaluate the exact contribution of these non-plant sources of BVOCs in the soil or their role in the rhizosphere. This is because their emissions cannot be easily separated from those of the roots [19] and because of experimental difficulties [8]. These limits notwithstanding, one study showed that the emission of sesquiterpenes (in particular, (–)-thujopsene) by fungi (Laccaria bicolor) can interact with the ability of Populus trees to develop their root system (in particular, with the proliferation of their lateral roots) [23]. One research article reported that, in the Amazonian rainforest, soil microorganisms can emit large amounts of sesquiterpenes, and these emissions strongly influence atmospheric chemistry near the soil surface and under the canopy, to an extent comparable with canopy emissions themselves [24]. Šimpraga and colleagues reported that there is no similar assessment for temperate and boreal forests, but there are limited data on bacterial VOC profiles (rich in alkenes, alcohols, ketones, and terpenes) and fungal VOC profiles (dominated by alcohols, benzenoids, aldehydes, and ketones) [19]. Apart from soil-derived emission, other, quantitatively less important, BVOC emissions in the forest can originate from understorey vegetation. In an article relative to subarctic forests of Betula pubescens ssp. czerepanovii (mountain birch), the authors found that BVOCs emitted by the understorey vegetation of Rhododendron tomentosum are adsorbed by tree-stands growing above them, thus affecting the volatile emission profile of mountain birches [25].
Overall, as suggested by these limited data, trees are considered as the major emitters of BVOCs in temperate forests and the contribution of non-tree emitters is still difficult to properly characterize in sufficient detail, thus probably having a more indirect effect on the composition of the forest air.
2. Biochemistry of Forest VOCs
Forest VOCs are the product of many different metabolic pathways: the isoprenoids are the most important group of metabolites in terms of diversity and quantities of emissions, but the products of the LOX pathway as well as shikimate derivatives are also highly relevant. Main biosynthetic pathways for BVOCs are graphically displayed in Figure 2.
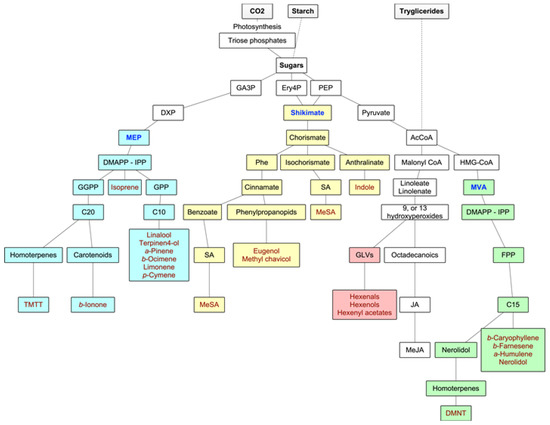
Figure 2. Main biosynthetic pathways for BVOCs. In green and yellow colors, the methylerythritol phosphate (MEP) and mevalonate (MVA) pathways to isoprenoids, respectively, in blue color, the lipoxygenase (LOX) pathway to GLVs, and in purple color, the shikimate pathway to aromatic compounds. Adapted from Maffei et al. (2011) [17].
2.1. Isoprenoids
The volatile isoprenoids include isoprene (C5), monoterpenes (C10), homoterpenes (C11 or C16), and sesquiterpenes (C15), and they all derive from the precursor dimethylallyl diphosphate (DMAPP) and its isomer isopentenyl diphosphate (IPP). These precursors are synthesized by two separate pathways, one active in plastids (the deoxyxylulose-5-phosphate (DXP), also known as the methylerythritol phosphate (MEP) pathway), and the second in the cytosol of the secretory cells themselves (the mevalonate (MVA) pathway) [17]. In fact, IPP and DMAPP seem to originate in the plastids from the MEP pathway, while leucoplasts contain the enzymes for the first steps of monoterpene biosynthesis. In the MVA pathway, DMAPP accepts one IPP (by prenyltransferase—PT), leading to the production of farnesyl diphosphate (FPP) and, consequently, to sesquiterpenoids (although, in some instances, there is a very small production of monoterpenes) and to the homoterpene DMTT. In the MEP pathway, the hemiterpene isoprene is synthesized from DMAPP alone by terpene synthase or cyclase (TPS), and the fusion of DMAPP to one or more IPPs leads to the production of geranyl diphosphate (GPP) (precursor of the monoterpenoids) and geranyl geranyl diphosphate (GGPP), which leads to the synthesis of diterpenes from which the homoterpene TMTT derives. Although the MEP pathway is plastid-located, the enzymes necessary for sesquiterpene synthesis are cytosolic. Hence, MEP-derived IPP or DMAPP need to travel from plastids to the cytosol [17][18]. The biosynthesis of the homoterpenes DMNT and TMTT follows a less direct pathway. DMNT derives from the oxidative degradation of (E)-nerolidol (a C15-alcohol), and TMTT from the oxidative degradation of (E,E)-geranyl linalool (a C20-alcohol) [26].
2.2. Oxylipins
Oxylipins are products of the lipoxygenase (LOX) pathway. They derive from the polyunsaturated C18 fatty acids of the chloroplast membrane (such as 13-hydroperoxylinolenic acid), which, in times of stress or damage, are cleaved by 9-LOX and 13-LOX pathways to give highly reactive products like 6-carbon aldehydes and alcohols (GLVs) and derivatives of jasmonic acid such as methyl jasmonate [9].
2.3. Shikimate Pathway
Another important group of BVOCs consists of compounds containing an aromatic ring, such as indole (derived from reactions of modified amino acids or their precursors) or the derivatives of phenylalanine, either via the phenylpropanoid pathway (eugenol and methyl chavicol) or the benzoate pathway (methyl salicylate) [17] (Figure 2).
This encyclopedic entry is based on an article published on https://www.mdpi.com/1660-4601/17/18/6506
References
- F. W. Went; Blue Hazes in the Atmosphere. Nature 1960, 187, 641-643, 10.1038/187641a0.
- Reinhold A. Rasmussen; F. W. Went; VOLATILE ORGANIC MATERIAL OF PLANT ORIGIN IN THE ATMOSPHERE*. Proceedings of the National Academy of Sciences 1965, 53, 215-220, 10.1073/pnas.53.1.215.
- 3. Guenther, A.B.; Jiang, X.; Heald, C.L.; Sakulyanontvittaya, T.; Duhl, T.; Emmons, L.K.; Wang, X; The Model of Emissions of Gases and Aerosols from Nature version 2.1 (MEGAN2.1): an extended and updated framework for modeling biogenic emissions. Geoscientific Model Development Discussions 2012, 5, 1471, 10.5194/gmdd-5-1503-2012.
- Taejoon Kim; Bokyeong Song; Kyoung Sang Cho; Im-Soon Lee; Therapeutic Potential of Volatile Terpenes and Terpenoids from Forests for Inflammatory Diseases. International Journal of Molecular Sciences 2020, 21, 2187, 10.3390/ijms21062187.
- Biology, Controls and Models of Tree Volatile Organic Compound Emissions; Niinemets, Ü., Monson, R.K., Eds.; Springer: Dordrecht, The Netherlands, 2013; ISBN 9789400766051.
- Dyakov, Y.T.; Dzhavakhiya, V.G.; Korpela, T. Comprehensive and Molecular Phytopathology; Elsevier: Amsterdam, The Netherlands, 2007; ISBN 9780444521323.
- Nomura, M. Phytoncide—Its Properties and Applications in Practical Use. In Gas Biology Research in Clinical Practice; Karger Publishers: Basel, Switzerland, 2011; pp. 133–143.
- Jullada Laothawornkitkul; Jane Elizabeth Taylor; N. D. Paul; C. Nicholas Hewitt; Biogenic volatile organic compounds in the Earth system. New Phytologist 2009, 183, 27-51, 10.1111/j.1469-8137.2009.02859.x.
- Abdul Rashid War; Michael Gabriel Paulraj; Tariq Ahmad; Abdul Ahad Buhroo; Barkat Hussain; S. Ignacimuthu; Hari C. Sharma; Mechanisms of plant defense against insect herbivores. Plant Signaling & Behavior 2012, 7, 1306-1320, 10.4161/psb.21663.
- Dušan Materić; Dan Bruhn; Claire Turner; Geraint Morgan; Nigel Mason; V. Gauci; Methods in Plant Foliar Volatile Organic Compounds Research. Applications in Plant Sciences 2015, 3, -, 10.3732/apps.1500044.
- Sonwani, S.; Saxena, P.; Kulshrestha, U. Role of Global Warming and Plant Signaling in BVOC Emissions. In Plant Responses to Air Pollution; Kulshrestha, U., Saxena, P., Eds.; Springer: Singapore, 2016; pp. 45–57, ISBN 9789811012013.
- Francesco Loreto; Jörg-Peter Schnitzler; Jã¶rg-Peter Schnitzler; Abiotic stresses and induced BVOCs. Trends in Plant Science 2010, 15, 154-166, 10.1016/j.tplants.2009.12.006.
- Natalia Dudareva; Eran Pichersky; Jonathan Gershenzon; Biochemistry of Plant Volatiles1. Plant Physiology 2004, 135, 1893-1902, 10.1104/pp.104.049981.
- Gianna Vivaldo; Elisa Masi; Cosimo Taiti; Guido Caldarelli; Stefano Mancuso; The network of plants volatile organic compounds. Scientific Reports 2017, 7, 11050, 10.1038/s41598-017-10975-x.
- Memari, H.R.; Pazouki, L.; Niinemets, Ü. The Biochemistry and Molecular Biology of Volatile Messengers in Trees. In Biology, Controls and Models of Tree Volatile Organic Compound Emissions; Niinemets, Ü., Monson, R.K., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 47–93, ISBN 9789400766068.
- Peñuelas, J.; Llusià, J. BVOCs: Plant defense against climate warming? Trends Plant Sci. 2003, 8, 105–109, doi:10.1016/S1360-1385(03)00008-6.
- M. E. Maffei; Jürg Gertsch; Giovanni Appendino; Plant volatiles: Production, function and pharmacology. Natural Product Reports 2011, 28, 1359-1380, 10.1039/c1np00021g.
- Mehdi Sharifi-Rad; Antoni Sureda; Gian Carlo Tenore; Maria Daglia; Mehdi Sharifi-Rad; Marco Valussi; Rosa Tundis; Marzieh Sharifi-Rad; Monica R. Loizzo; Adedayo Oluwaseun Ademiluyi; et al.Seyed Abdulmajid AyatollahiMarcello Iriti Biological Activities of Essential Oils: From Plant Chemoecology to Traditional Healing Systems. Molecules 2017, 22, 70, 10.3390/molecules22010070.
- Maja Šimpraga; Rajendra P. Ghimire; Dominique Van Der Straeten; James D. Blande; Anne Kasurinen; Jouni Sorvari; Toini Holopainen; Sandy Adriaenssens; J. K. Holopainen; Minna Kivimäenpää; et al. Unravelling the functions of biogenic volatiles in boreal and temperate forest ecosystems. European Journal of Forest Research 2019, 138, 763-787, 10.1007/s10342-019-01213-2.
- Marcello Iriti; Franco Faoro; Chemical Diversity and Defence Metabolism: How Plants Cope with Pathogens and Ozone Pollution. International Journal of Molecular Sciences 2009, 10, 3371-3399, 10.3390/ijms10083371.
- Niinemets, U.; Monson, R.K.. Biology, Controls and Models of Tree Volatile Organic Compound Emissions; Niinemets, U.; Monson, R.K., Eds.; Springer: London, 2013; pp. 44-49.
- Trowbridge A.M., Stoy P.C.. BVOC-Mediated Plant-Herbivore Interactions, In Biology, Controls and Models of Tree Volatile Organic Compound Emissions. Tree Physiology, vol 5; Niinemets Ü., Monson R., Eds.; Springer: Dordrecht, 2013; pp. 101-119.
- Franck Anicet Ditengou; Anna Muller; Maaria Rosenkranz; Judith Felten; Hanna Lasok; Maja Miloradovic Van Doorn; Valerie Leguã©; Klaus Palme; Jã¶rg-Peter Schnitzler; Andrea Polle; et al. Volatile signalling by sesquiterpenes from ectomycorrhizal fungi reprogrammes root architecture. Nature Communications 2015, 6, 6279, 10.1038/ncomms7279.
- Efstratios Bourtsoukidis; T. Behrendt; Ana María Yañez-Serrano; H. Hellén; Efstathios Diamantopoulos; E. Catão; K. Ashworth; Andrea Pozzer; C. A. Quesada; D. L. Martins; et al.Marta O. SáA. AraújoJoel F. BritoPaulo ArtaxoJürgen KesselmeierJos LelieveldJ. Williams Strong sesquiterpene emissions from Amazonian soils.. Nature Communications 2018, 9, 2226, 10.1038/s41467-018-04658-y.
- Adedayo O. Mofikoya; Kazumi Miura; Rajendra P. Ghimire; James D. Blande; Minna Kivimäenpää; Toini Holopainen; J. K. Holopainen; Understorey Rhododendron tomentosum and Leaf Trichome Density Affect Mountain Birch VOC Emissions in the Subarctic.. Scientific Reports 2018, 8, 13261, 10.1038/s41598-018-31084-3.
- Dorothea Tholl; Reza Sohrabi; Jung-Hyun Huh; Sungbeom Lee; The biochemistry of homoterpenes – Common constituents of floral and herbivore-induced plant volatile bouquets. Phytochemistry 2011, 72, 1635-1646, 10.1016/j.phytochem.2011.01.019.




