
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Yamil Liscano | + 1644 word(s) | 1644 | 2020-09-23 11:38:24 | | | |
| 2 | Vicky Zhou | Meta information modification | 1644 | 2020-09-24 05:49:14 | | | | |
| 3 | Vicky Zhou | -36 word(s) | 1608 | 2020-10-27 09:17:40 | | |
Video Upload Options
Peptides are naturally produced by all organisms and exhibit a wide range of physiological, immunomodulatory, and wound healing functions. Furthermore, they can provide with protection against microorganisms and tumor cells. Their multifaceted performance, high selectivity, and reduced toxicity have positioned them as effective therapeutic agents, representing a positive economic impact for pharmaceutical companies. Currently, efforts have been made to invest in the development of new peptides with antimicrobial and anticancer properties, but the poor stability of these molecules in physiological environments has triggered a bottleneck. Therefore, some tools, such as nanotechnology and in silico approaches can be applied as alternatives to try to overcome these obstacles. In silico studies provide a priori knowledge that can lead to the development of new anticancer peptides with enhanced biological activity and improved stability.
1. Introduction
The necessity to generate new drugs to combat problems of not only cancer drug resistance but also bacterial drug resistance, along with poor selectivity and adverse effects on normal cells has allowed peptides to make their way into the pharmaceutical industry [1][2]. Peptides emerged as one of the alternatives for therapeutic intervention because they mimic the natural metabolic effects within the body with low toxicity and better selectivity [3]. For example, peptides that can mimic hormonal activities are involved in various processes of antimicrobial defense, wound healing, and activation of immune response. To give a clearer example, we could talk about insulin, whose isolation, purification, and subsequent synthetic production have helped generations of people suffering from diabetes; adrenocorticotropic hormone (ACTH); and calcitonin [4].
Other peptides are oxytocin and synthetic vasopressin that paved the way for the synthetic biology market, which is always looking to decrease the use and damage of animals for the extraction and purification of molecules with therapeutic potential [5]. Many of these drugs were located within the “era of chemistry,” and are based on a rational design of compounds in terms of interaction with the target and the design of the ligand and the receptor [5].
In the 21st century, pharmaceutical companies experienced dramatic changes in drug safety regulations, lengthy compound development processes, and financial efforts that influenced investment in research and development [3]. Today, we could say that we are in the era of in silico design because of the tools of bioinformatics and molecular dynamics (simulation) that allow us to obtain information about the genome, transcriptome, and proteomics of organisms, thereby finding patterns and analyzing, modeling, and simulating molecules in systems similar to those presented by nature [5].
However, not everything is perfect with peptides as there are limitations to their use. These limitations include a short plasma half-life, which is due to the presence of peptidases that inactivate the peptides, and the possible immunogenicity that they can arouse in the host [6].
Despite the disadvantages of peptides, to date more than 150 peptides in clinical trials have been approved in the United States, Europe, and Japan [4]. The current market for peptides is approximately USD 19 billion and by the end of 2020, it is expected to reach USD 23 billion [7]. To date, 63 peptides have been approved by the US Food and Drug Administration [7]. Peptides marketed include bacitracin, colistin, daptomycin, enfuvirtide, vancomycin, telavancin, teicoplanin, and dalbavancin [8].
The peptide therapeutics market is increasing year by year, starting with 10 peptides approved in 1980 to about 30 in 2010, with an average of 5 years in clinical studies before their release to the market [4]. This market is estimated at over USD 40 billion per year, equivalent to 10% of the pharmaceutical market [5]. There was a drop in the peptide market between 2010 and 2015 because pharmaceutical companies lost interest in peptides due to their low stability and oral bioavailability, replacing it with interest in small molecules that imitate peptides [4]. However, in recent years, the use of peptides has been on the rise because of advances in formulation, chemical modifications, and peptide delivery technologies [2], such as nanoemulsions, biopolymers (polyethylene glycol), and liposomes [3][4].
The current biopharmaceutical approaches are in search of new peptides from different species as there is an impressive variety, these peptides emerging from evolution mainly through the selective pressure, showing distinctive characteristics, such as selectivity and affinity against bacteria or cancer membranes [3]. One of the future challenges with peptides is to decrease protease degradation in our body through the use of liposomes or nanoparticles, improving bioavailability and peptide lifetime. Other challenges are improving in the solubility of most of the hydrophobic peptides, fast elimination, poor permeability of membranes, and cost of manufacture [8][9].
Nowadays, the interest in peptides is growing; the evidence of the growth is the rise in the number of publications on the topic of peptides. The most frequent publications on the Science Direct website are related to the topics of plants, mammals, and fish. The least frequent ones are about amphibians and frogs. However, authors, such as Uhlig et al. (2014) [3] point out that these publications are the most frequent in research, specifically on the topic of frog skin.
There are many areas of interest for various researches on peptides worldwide. However, the two aspects that are showing strength are the development of anticancer and antimicrobial peptides because of the resistance and low selectivity of conventional drugs [10][11]. There are two types of conventional anticancer drug resistance, intrinsic and acquired. Intrinsic resistance is related to genetic variations in the somatic cells of patients with tumors. Acquired resistance is due to the expression of energy-dependent transporters that eject anticancer drugs from the cells before they come into contact with the target [12].
In recent years, research has been conducted on a particular type of peptide that fights bacteria and cancer simultaneously. There are two types of anticancer peptides. The first one is active against mammalian, bacterial, and cancer cells. The second one corresponds to those active against bacterial and cancer cells, such as cecropins and magainins [13], and these are referred to as peptides with dual antibacterial–anticancer activity [1][14][15][16]. So, far there have been few studies on the physicochemical properties, structure, and characteristics of these peptides, allowing both bioprospecting and in silico design [1][14][16][17][18]. The study by Felicio et al. (2017) aimed to reveal certain characteristics of these peptides, such as alpha-helix and β-sheets structures, positive net charge, high hydrophobicity, and lengths of up to 30 residues [1].
2. Efforts to Overcome Peptide Limitations
The challenge of overcoming the limitations of peptides has driven the scientific community to search for solutions, such as peptide engineering via amino acid substitution, peptide conjugation, new formulations, and alternative delivery strategies [7]. Peptide engineering is based on the replacement of amino acids with d-amino acids to improve stability and half-life, to which N-acetylation and C-amidation are applied [19].
Peptide conjugation consists of peptide sticking, that is, the addition of polymers, such as poly(lactic-co-glycolic acid), polyvinylpyrrolidone, and polyethylene glycol, to peptides, which contributes to an improvement in bioavailability and an increase in half-life. It can also help prevent immune response and protect against degradation [7][20].
Another conjugation of peptides is with lipids, which is also known as lipidation. It produces amphiphilic peptides with increased bioavailability, improving the half-life, receptor selectivity, potency, and membrane penetration [21]. Daptomycin is one of these lipopeptides, which is produced from natural lipidation and is widely used to treat diseases caused by Gram-positive bacteria. Another example of natural lipidation is surfactin, which exhibits antimicrobial, antiviral, and antitumor activities [19][22]. Some of the peptides on the market that have gone through synthetic lipidation are liraglutide (Victoza®) and insulin detemir (Levemir®) [21].
Cycling, N-methylation, and lactamic bridges have improved the permeability, stability, potency, and solubility of peptides [19]. An alternative use of peptides to improve its potency and selectivity is the synergy of peptides with each other and with other conventional drugs. One example is the study by Kampshof et al. (2019) that shows how peptides, such as melimine and protamine, when combined with cefepime and ciprofloxacin, reduce resistance to fluoroquinolones in Pseudomonas aeruginosa (Figure 1) [23].
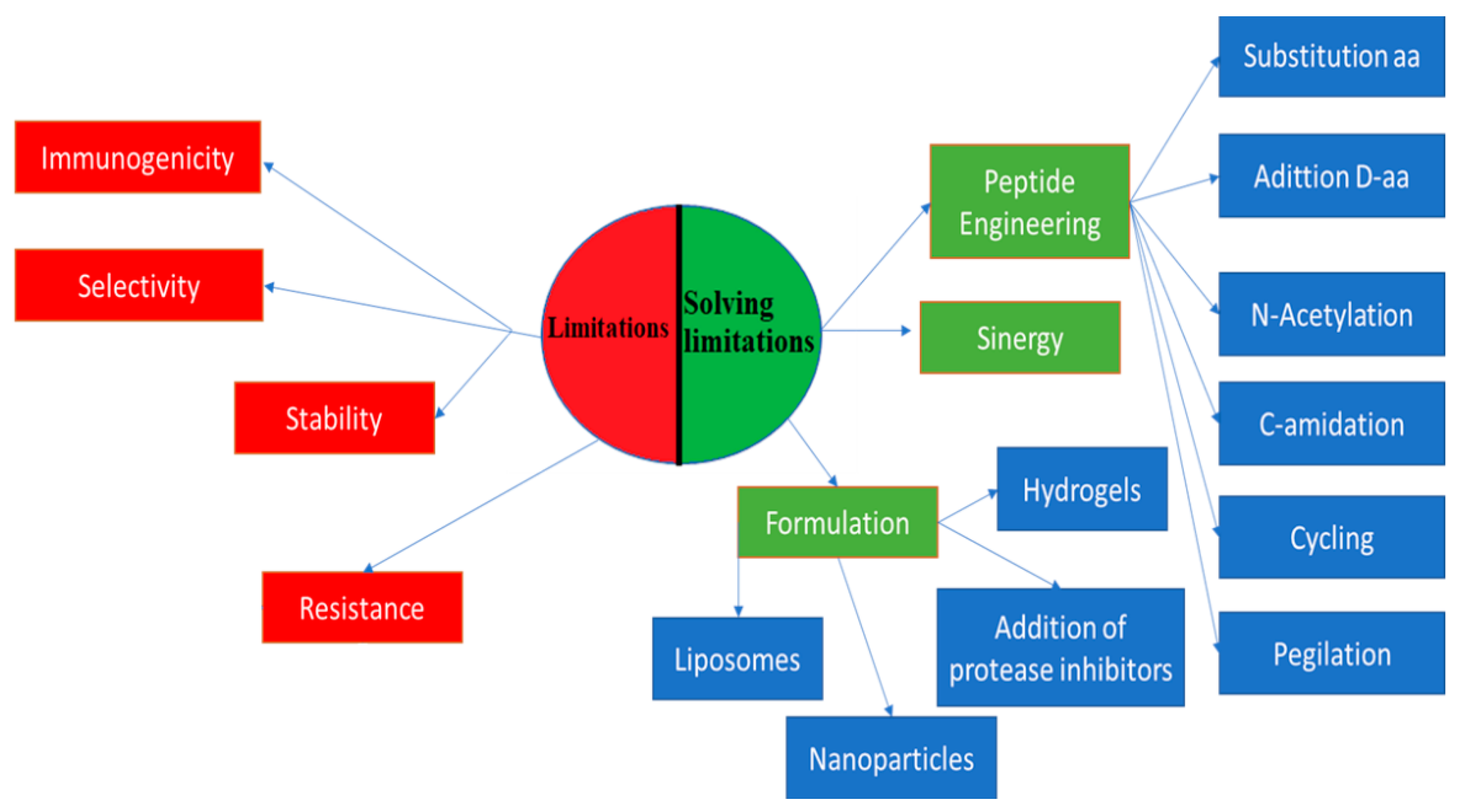
New formulation strategies to protect peptides from degradation include the addition of protease inhibitors. The incorporation of salt, sugar, and heparin in the formulations improves bioavailability, solubility, and stability in vivo [19]. Another type of formulation is hydrogels. They are three-dimensional fiber networks that can retain large amounts of water up to 1000 times their dry weight [25]. They can be made of homopolymers or molecules that can self-assemble into more complex structures [25]. These systems attract attention because of their varied applicability, such as drug delivery and tissue regeneration [26]. Peptide-based hydrogels, such as MAX8 (VKVKVKVK-VDPPT-KVEVKV-NH2) and RAD16 (AcN-RADARADARAD-CONH2), are remarkable because of their biocompatibility, biodegradability, and easy synthesis [7][26]. They also work as drug delivery matrices in a controlled way or as scaffolds to insert stem cells to promote tissue regeneration. Another advantage of these hydrogels is the ease with which they can be administered as they are injectable, thus avoiding surgical interventions [7][26][27].
Nanoparticles are another type of formulation used in inorganic and organic systems. In inorganic systems, nanoparticles are used with metallic ions, such as silver, gold, and zinc oxide. On the other hand, in organic systems, liposomes and polymer nanoparticles are found [28]. Similar to hydrogels, nanoparticles have high biocompatibility and biodegradability, and low cytotoxicity. Homopolymers and copolymers of polylactide or polyglycolic acid are frequently used. They are classified according to their architecture into nanospheres, nanocapsules, conjugated polymers, and polyelectrolyte complexes [28]. Nanoparticles can be used to transport proteins and peptides, whose conjugation generates a synergistic effect that improves the limitations of each one of the materials and achieves uses, such as inhibition of the interactions of pathogenic proteins and high sensitivity in molecular imaging [29][30][31].
References
- Felício, M.R.; Silva, O.N.; Gonçalves, S.; Santos, N.C.; Franco, O.L. Peptides with dual antimicrobial and anticancer activities. Front. Chem. 2017, 5.
- Zompra, A.A.; Galanis, A.; Werbitzky, O.; Albericio, F. Manufacturing peptides as active pharmaceutical ingredients. Future Med. Chem. 2009, 1, 361–377.
- Uhlig, T.; Kyprianou, T.; Martinelli, F.G.; Oppici, C.A.; Heiligers, D.; Hills, D.; Calvo, X.R.; Verhaert, P. The emergence of peptides in the pharmaceutical business: From exploration to exploitation. EuPA Open Proteom. 2014, 4, 58–69.
- Lau, J.L.; Dunn, M.K. Therapeutic peptides: Historical perspectives, current development trends, and future directions. Bioorg. Med. Chem. 2018, 26, 2700–2707.
- Craik, D.J.; Fairlie, D.P.; Liras, S.; Price, D. The future of peptide-based drugs: Peptides in drug development. Chem. Biol. Drug Des. 2013, 81, 136–147.
- Eckert, R. Road to clinical efficacy: Challenges and novel strategies for antimicrobial peptide development. Future Microbiol. 2011, 6, 635–651.
- Koutsopoulos, S. Peptide Applications in Biomedicine, Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2018.
- Correa, W.; Heinbockel, L.; Martinez-de-Tejada, G.; Sánchez, S.; Garidel, P.; Schürholz, T.; Mier, W.; Dupont, A.; Hornef, M.; Gutsmann, T.; et al. Synthetic anti-lipopolysaccharide peptides (SALPs) as effective inhibitors of pathogen-associated molecular patterns (PAMPs). In Antimicrobial Peptides: Basics for Clinical Application; Matsuzaki, K., Ed.; Springer: Singapore, 2019; pp. 111–129.
- Marqus, S.; Pirogova, E.; Piva, T.J. Evaluation of the use of therapeutic peptides for cancer treatment. J. Biomed. Sci. 2017, 24, 21.
- Albericio, F.; Kruger, H.G. Therapeutic peptides. Future Med. Chem. 2012, 4, 1527–1531.
- Mahlapuu, M.; Håkansson, J.; Ringstad, L.; Björn, C. Antimicrobial peptides: An emerging category of therapeutic agents. Front. Cell. Infect. Microbiol. 2016, 6.
- Riedl, S.; Zweytick, D.; Lohner, K. Membrane-active host defense peptides—Challenges and perspectives for the development of novel anticancer drugs. Chem. Phys. Lipids 2011, 164, 766–781.
- Gaspar, D.; Veiga, A.S.; Castanho, M.A.R.B. From antimicrobial to anticancer peptides. A review. Front. Microbiol. 2013, 4.
- Gabernet, G.; Gautschi, D.; Müller, A.T.; Neuhaus, C.S.; Armbrecht, L.; Dittrich, P.S.; Hiss, J.A.; Schneider, G. In silico design and optimization of selective membranolytic anticancer peptides. Sci. Rep. 2012, 9, 11282.
- Hoskin, D.W.; Ramamoorthy, A. Studies on anticancer activities of antimicrobial peptides. Biochim. Biophys. Acta (BBA) Biomembr. 2008, 1778, 357–375.
- Lee, D.; Hahm, K.-S.; Park, Y.; Kim, H.-Y.; Lee, W.; Lim, S.-C.; Seo, Y.-K.; Choi, C.-H. Functional and structural characteristics of anticancer peptide Pep27 analogues. Cancer Cell Int. 2005, 5, 21.
- Shoombuatong, W.; Schaduangrat, N.; Nantasenamat, C. Unraveling the bioactivity of anticancer peptides as deduced from machine learning. EXCLI J. 2018, 17, 734–752.
- Ellerby, H.M.; Arap, W.; Ellerby, L.M.; Kain, R.; Andrusiak, R.; Rio, G.D.; Krajewski, S.; Lombardo, C.R.; Rao, R.; Ruoslahti, E.; et al. Anti-cancer activity of targeted pro-apoptotic peptides. Nat. Med. 1999, 5, 1032–1038.
- Agyei, D.; Tan, K.-X.; Pan, S.; Udenigwe, C.C.; Danquah, M.K. Peptides for biopharmaceutical applications. In Peptide Applications in Biomedicine, Biotechnology and Bioengineering; Koutsopoulos, S., Ed.; Woodhead Publishing: Sawston, UK, 2018; pp. 231–251.
- Drayton, M.; Kizhakkedathu, J.N.; Straus, S.K. Towards robust delivery of antimicrobial peptides to combat bacterial resistance. Molecules 2020, 25, 3048.
- Kowalczyk, R.; Harris, P.W.R.; Williams, G.M.; Yang, S.-H.; Brimble, M.A. Peptide lipidation—A synthetic strategy to afford peptide based therapeutics. In Peptides and Peptide-Based Biomaterials and Their Biomedical Applications; Sunna, A., Care, A., Bergquist, P.L., Eds.; Springer International Publishing: Cham, Switzerland, 2017; Volume 1030, pp. 185–227.
- Jacques, P. Surfactin and other lipopeptides from Bacillus spp. In Microbiology Monographs; Springer Science and Business Media: Berlin/Heidelberg, Germany, 2010; Volume 20, pp. 57–91.
- Kampshoff, F.; Willcox, M.D.P.; Dutta, D. A pilot study of the synergy between two antimicrobial peptides and two common antibiotics. Antibiotics 2019, 8, 60.
- Kumar, P.; Kizhakkedathu, J.; Straus, S. Antimicrobial peptides: Diversity, mechanism of action and strategies to improve the activity and biocompatibility in vivo. Biomolecules 2018, 8, 4.
- Jonker, A.M.; Löwik, D.W.P.M.; van Hest, J.C.M. Peptide- and protein-based hydrogels. Chem. Mater. 2012, 24, 759–773.
- Martin, C.; Oyen, E.; Van Wanseele, Y.; Haddou, T.B.; Schmidhammer, H.; Andrade, J.; Waddington, L.; Van Eeckhaut, A.; Van Mele, B.; Gardiner, J.; et al. Injectable peptide-based hydrogel formulations for the extended in vivo release of opioids. Mater. Today Chem. 2017, 3, 49–59.
- Hiew, S.H.; Mohanram, H.; Ning, L.; Guo, J.; Sánchez-Ferrer, A.; Shi, X.; Pervushin, K.; Mu, Y.; Mezzenga, R.; Miserez, A. A short peptide hydrogel with high stiffness induced by 3 10 -helices to β-Sheet Transition in Water. Adv. Sci. 2019, 6, 1901173.
- Montero, N.; Alhajj, M.J.; Sierra, M.; Oñate-Garzon, J.; Yarce, C.J.; Salamanca, C.H. Development of polyelectrolyte complex nanoparticles-PECNs loaded with ampicillin by means of polyelectrolyte complexation and ultra-high pressure homogenization (UHPH). Polymers 2020, 12, 1168.
- Doll, T.A.P.F.; Dey, R.; Burkhard, P. Design and optimization of peptide nanoparticles. J. Nanobiotechnol. 2015, 13, 73.
- Jeong, W.; Bu, J.; Kubiatowicz, L.J.; Chen, S.S.; Kim, Y.; Hong, S. Peptide–nanoparticle conjugates: A next generation of diagnostic and therapeutic platforms? Nano Converg. 2018, 5, 38.
- Pudlarz, A.; Szemraj, J. Nanoparticles as carriers of proteins, peptides and other therapeutic molecules. Open Life Sci. 2018, 13, 285–298.




