
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Jin Li | + 3353 word(s) | 3353 | 2020-09-16 06:30:01 | | | |
| 2 | Catherine Yang | Meta information modification | 3353 | 2020-09-17 06:02:22 | | |
Video Upload Options
Metal nano-particles-modified optical fiber LSPR sensor has high sensitivity and compact structure, which can realize the real-time monitoring of physical parameters, environmental parameters (temperature, humidity), and biochemical molecules (pH value, gas-liquid concentration, protein molecules, viruses). In this paper, both fabrication and application of the metal nano-particles modified optical fiber LSPR sensor probe are reviewed, and its future development is predicted.
1. Introduction
Surface plasmon resonance (SPR) refers to a phenomenon when photons cause the oscillation of the free electron on the metal surface when light is incident on the metal-dielectric interface, and the wave vector component of a light wave along the metal interface matches the wave vector of SPR [1][2]. In the oscillation process, the energy of a light wave is converted into the oscillation energy of the free electron, causing the strong attenuation of light field intensity, where the obvious absorption peak appears at the resonance wavelength position in reflection or transmission spectrum [3]. SPR is divided into long-range SPR (LR-SPR) and localized SPR (LSPR) according to the transferring distance or light field distribution of SPR wave (SPRW) [4][5]]. LR-SPR exists in metal micro/nanostructures, including two-dimensional, three-dimensional structures, and the surface of metal micro/nanowires [6]. This type of SPRW usually propagates along a specific direction and has a certain linear transmission length along the interface. It can be used in all-optical signal modulation, imaging, and biochemical sensors. LSPR exists on the surface of metal nano-particles (NPs) with various morphologies, which can be spherical, ellipsoidal, or even random shape [7][8]. This type of SPRW is limited on the surface of metal NPs, forming an evanescent field around it, so it is very sensitive to the surrounding environment [9][10], mainly being used in the detection and screening of biomolecules and living cells, biochemical reaction process monitoring, biological surface analysis and treatment [11][12][13][14].
In recent years, to explore the optical fiber SPR sensors, the sensing part is modified by the micro/nanostructure of the optical fiber itself, such as using a metal film with nano-thickness or metal nano-array as the sensing layer to replace the coating layer of the optical fiber [15][16]. Or the end face of the optical fiber probe is modified by micro/nanostructure, coated by nano-size noble metal layer or etched by metal nanostructure as the sensing part of the SPR optical fiber sensor [17][18]. These new structures benefit from the progress of metal coating technology and micro/nanostructure processing technology in chemical or physical fields and are the result of interdisciplinary and mutually promoting development [19][20]. In the sensing measurement, the sensing part of the optical fiber will be affected by the object to be measured, so the sensing purpose can be realized by detecting the change of transmitted light or reflected light signal in the optical fiber [21][22]. The research on the high field localization of LSPR generated by metal micro/nanostructures such as single metal NP, NP pairs, and metal NPs arrays to effectively improve the sensitivity, selectivity, spatial resolution, and integrability of SPR sensors and all-optical devices, which has become an important research and application direction in the field of SPR technology in recent years [23][24][25][26][27].
Using “Plasmon,” “Plasmon nanoparticles,” and “Plasmon nanoparticles fiber” as the keywords in Google Scholar, the corresponding number of published papers was compared in Figure 1. Among the reported plasma related articles, 46% were related to NPs. The work on metal NPs modified fiber SPR has been reported since 2000, and almost all the articles (98.6%) have appeared in the last decade.
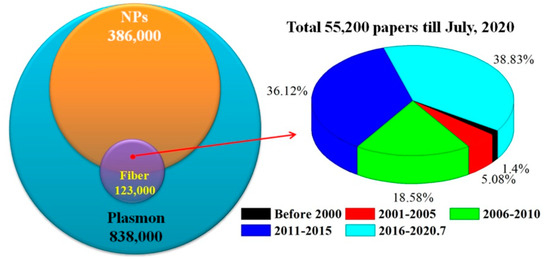
Figure 1. Number comparison of published paper for different key words and interval years.
2. Optical Fiber LSPR Biosensors
High-performance fiber-optic LSPR biosensor probes are designed based on different fiber structures, as well as the modification of metal NPs, antibody, and marker. These biosensors have been used to detect glucose, protein, amino acid, and nucleic acid. The typical structures and characteristics of optical fiber probes are reported in this section in recent years. The role and optimization of metal NPs will be reviewed as well.
2.1. Typical Fiber Structures and Properties
It is well known that the initial SPR was realized by means of the reflection angle modulation of Otto and Krestschmann prism structures to satisfy the SPR coupled resonance condition. Flat surface optical fiber structures, such as rectangular and D-typed fiber, play a similar role [28]. The U-shaped fiber based on fiber microbend for light leakage, the micro/nanofiber with fine diameter fiber to excite evanescent field, the fiber gratings enhanced by multiple reflection interference, the microstructured fibers regulated by periodic array light, and the cascaded fiber with mode decomposition and interference provide the optical action surface to realize the excitation of SPR effect [29].
In order to obtain a stable U-shaped single-mode fiber, flame heating is used to further reduce the bending radius of the fiber. When pre-tension stress is applied to the single-mode fiber and fixed in the capillary [30]. The introduction of large diameter and flexible polymer optical fiber greatly reduces the manufacturing difficulty and cost of SPR optical fiber sensor, which can achieve the low precision measurement with low requirements for optical loss. The U-shaped fiber and etch plane are fabricated from PMMA fiber [31].
Micro/nanofiber provides a stronger optical field, called the evanescent field, to improve the intensity of the optical signal involved in the SPR effect, and enhance the interaction between the optical field and the environment to be measured [32]. In similar fiber structures, the penetration depth of the evanescent field and the optical scattering loss of NPs or structures need to be considered. If the biconical micro/nanofiber is stretched too thin (less than 4 μm) in the waist position, it is easy to be damaged in the cleaning and coating process of functional materials. The tail end of the single cone micro/nanofiber is flat cut by the fiber cutting pen and then coated with functional materials to make the reflective SPR probe [33]. Biconical micro/nanofibers are fabricated from ordinary single-mode fibers by high-temperature melt stretching. Oxy-hydrogen flame, electric heating, and plasma discharge provide a high-temperature environment of thousands of degrees Celsius, which is suitable for processing different types of optical micro/nanofibers [34]. Its lumbar diameter is precisely controlled by heating temperature and stretching speed, while the length of the cone area depends on the stretching speed and heating mode (scanning or single point fixed torch).
Polymer optical fiber was processed into a tapered optical fiber, which is easier to manufacture because of its low melting point and easy decomposition [35]. Tapered micro/nanofibers were obtained by dynamic chemical etching-drawing method [36]. The uncoated end of the single-mode fiber is immersed in hydrofluoric acid and is slowly lifted up to control the taper angle and taper length. The end face of the hollow-core fiber (HCF) is coated with Ag nanospheres and nanorods, and its micropores were easily obtained by capillary action [37]. The LOD of melamine was measured experimentally to be 100 nm, which was far lower than the minimum content of milk powder (1 mg/kg) and other foods (2.5 mg/kg).
For fiber structures with a single resonant peak, such as reflected light of FBG or transmission light of LPG, the ultra-high sensitivity can be obtained when the wavelength of the most resonant peak moves due to the sudden change of light intensity near the peak inflection point [38]. Optical signals can also be reflected to the sensing area through some special optical fiber structures and interact with the environment to be measured. TFBG has been used to effectively control the output direction of the optical signal, and its core mode has the adjustment function of temperature cross sensitive, as shown in Figure 2 [39].
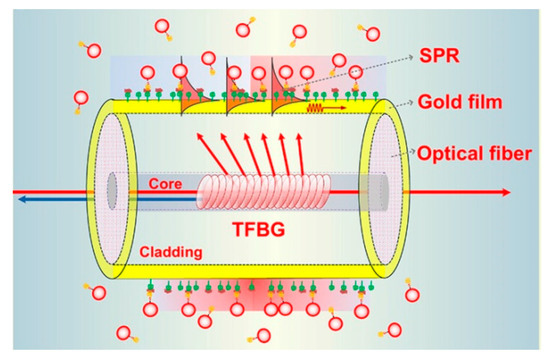
Figure 2. Configuration of Au NPs LSPR fiber sensor based on TFBG [39].
Compared with the LRSPR sensor, the LOD and surface enhancement effects of the LSPR sensor are more obvious. Noble metal NPs provide a larger contact area and achieve efficient information exchange between the metal NPs and the analyte. More importantly, the NPs surface generates a higher concentration of electromagnetic field and the limitations of a single-point amplification effect, which can effectively enhance the detection efficiency of a biological analyte in nanometer size or spacing [40]. In the detection process, the excitation photons meeting the coupling frequency resonate with the surface electrons of the metal NP, thereby generating a stronger and concentrated surface-local evanescent field and realizing the single-molecule nanoprobe having a very low LOD [41].
The cascaded fiber structure guides the high mode light signal onto the fiber surface to improve the sensing performance. The multimode fiber (MMF) and PCF have been verified by experiments, where the LOD of biosensor has been increased to 1 nm, and the influence of environmental temperature was effectively eliminated to limitation less than 7.2 pm/°C [42]. Multiple fiber structures in series or in parallel can form the sensing arrays, which are expected to realize the simultaneous detection of multi-parameters or multi-component samples [43]. The air hole of PCF usually collapses when it is fused with MMF. In order to reduce the cost and improve the repeatability of the sensor probe, the HCF replaces PCF to construct the cascaded fiber structures [44]. The chemical etching method is widely used to fabricate the tapered micro/nanofibers from single-mode fiber, MMF, PCF, etc. [45]. With the decrease of the fiber diameter or the destruction of the cladding structure, the optical signal is leaked to the sensing area. The sensing signal was doubled by constructing multiple sensing regions, but the length of each sensing arm must be strictly controlled to be equal [46]. The different length of the interference region causes the multiple overlapping interference spectra for the multi-mode interference, Mach–Zenhder, Fabry–Perot structures, and other fiber interferometers. This reason results in the demodulation difficulty and failing to enhance the sensing effect.
The highly integrated optical fiber SPR sensor and planar structure can be fixed by a microfluidic channel [47]. At the same time, it is easier to replace the tested sample with less consumption, and the sample pool is easier to be cleaned. Focused ions beam (FIB) technology has been applied to prepare the Au NPs array at the end face of optical fiber and realized the precise control of the size and morphology of Au NPs [48]. However, high cost and technical difficulty will become a huge obstacle to the commercialization of such devices. In order to improve the surface uniformity of the fiber, the silica NPs were self-assembled on the fiber surface [49]. The sensitivity is affected by the penetration depth of SPRW depending on the fiber structure, the thickness and distribution of metal NPs and other sensitive layers [50].
2.2. Metal NPs Role and Optimization
The concentration of enzyme and the size of NPs should also be considered in the fabrication of fiber-optic biosensors. Too low concentration of enzyme and too small size (<15 nm) of metal NPs may lead to the failure of the enzyme modification [51]. In the fiber optic SPR biosensor probe, both markers, and metal NPs contribute to the high sensitivity and low LOD [52]. Regardless of selectivity, the lack of either of the two factors will greatly affect the sensing performance. The scale and distribution of metal NPs of the functional membrane exerts a significant impact on the SPR effect [53]. The distance/diameter ratio between particles becomes an effective index to reflect the coupling strength of SPRW. The smaller value results in a higher SPR intensity.
Metal NPs can be spherical, rod-shaped, and random in shape, and they will be excited to emit fluorescence with a specific wavelength depending on their morphology and size [54]. Meanwhile, the fluorescence characteristics can also be used to evaluate the morphology and distribution of metal NPs on the fiber surface. With the help of some natural plants, the synthesis of metal NPs also provides a very interesting way for the development of optical fiber SPR sensors [55]. However, the consistency of NPs is poor in some work. The dynamic dissociation adsorption process of Au NPs was used to measure the concentration of heparin [56]. Due to the stronger bond energy between the negatively charged heparin and PDDA modified surface, they replace the Au NPs on the surface of optical fiber and affect the peak wavelength of SPR resonance. In this work, the larger size of Au NPs on the surface of the optical fiber becomes much more unstable and can more sensitively sense the change of the concentration of the substance to be measured, but the structural stability of the response optical fiber probe is poor. Using the metal NPs and nanofilms with different sizes or structures, the multiple SPR resonance peaks can be obtained to realize a simultaneous analysis of different samples [57]. The seed-mediated growth technique was reported to adjust the particle size. The citrate ions were used to reduce Au+ ions to Au0 on the surface of Au NPs seeds during the Au capping process [58]. One can adjust the particle size by changing the growth time [59]. In some work, the introduction of Au NPs does not significantly improve the sensing performance of the fiber probe, which may be due to the fact that the designed structure cannot effectively change the resonance condition of the SPR effect [60]. The structure of the optical fiber, the properties of functional materials, and the morphology or structure of NPs are all factors to be included.
Metal NPs can also be modified on the surface of materials with specific structures before they are modified onto fiber structures. Graphene provides an ideal two-dimensional planar substrate and binding sites for NPs [61]. The three-dimensional functional structure was constructed by stacking multilayer 2D planar graphene [62]. The appropriate number of layers can improve the sensitivity of the sensor, while too many layers will hinder the effective action of the specimen and the optical signal. Compared with the two-dimensional functional film of Au NPs, ZnO nanowires and three-dimensional structure of Au NPs show the better SPR excitation efficiency and sensing performance, which is attributed to the fact that ZnO reduces the optical loss by capturing optical signal and improves the LSPR efficiency through electric field enhancement effect [63].
2-aminoethanethiol (AET) and p-mercaptophenylboronic acid (PMBA) modified Au NPs optical fiber sensor was used to measure blood glucose in urine, showing low detection limit and high selectivity [64]. For the selective detection ability of complex components, Yuan et al. adopted the molecular imprinting technique (MIPT) to construct the SPR structures with specific morphologies and used for the accurate analysis of the species and components of the analytes. On the surface of a metal NPs function layer, the synthetic recognition sites were created by MIPT using conductive polymers or micro/NPs [65]. The ratio of Ag NPs and chitosan doped in the sol determines the information conversion efficiency between the analytes and the light. The photopolymerization time and dip time of the sol will change the thickness of the functional film [66]. Moreover, the concentration of glucose in the sol of metal NPs, the liquid mixing, and the detection methods may damage the tested substance, so pretreatment of the test sample is required [67]. MIPT has been used to construct the characteristic structures in polymers, sols, and micro/NPs to realize selective recognition and concentration analysis of specific molecules [68]. The application of MIPT, SPR, and LSPR technology in optical fiber sensors can refer to the review of Gupta et al. [69].
The combination of LSPR of metal NPs and SPR of nano-film has also become an effective method to improve the performance of SPR optical fiber sensor [70]. It also provides more abundant research content for the structure design of the new optical fiber biochemical sensor. As the function film, PBA Au NPs were used to differentiate the RNA and DNA first, showing excellent selectivity for biomolecules based on the SPR effect of metal NPs [71]. CuS NPs also exhibit the SPR effect and are applied to exploring the LSPR biosensors [72]. In addition, the photothermal effect of metal materials has been revealed and verified by experiments, which has the potential of photothermal diagnosis and treatment. The intensity of the SPR resonance peak will change linearly because the optical power of high-order mode changes with the concentration of the analytes [73]. For the biochemical sensor probe based on the SPR effect and optical interferometer structure, the change of wavelength position or power can be obtained by demodulation. The demodulation method must refer to the changing law of spectral characteristic parameters in the process of sensor calibration.
3. Perspective
The properties for metal NPs based LSPR fiber sensors have been compared with those of plasmonics devices and fiber sensors in Figure 3.
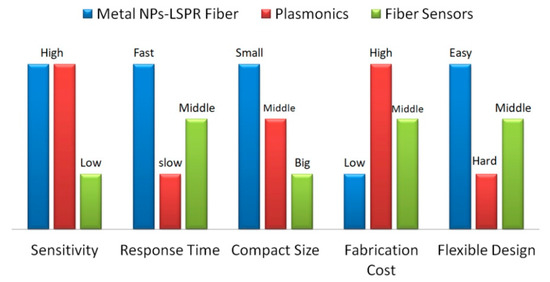
Figure 3. Properties comparison for metal NPs based LSPR fiber sensors, plasmonics devices and fiber sensors.
The sensitivity, response time, compact size, fabrication cost, and flexible design for these techniques have been compared according to the recent works. The LSPR fiber sensors and plasmonics devices were fabricated based on metal nanostructures, having ultra-high sensitivity compared to fiber sensors. The optical fiber supports the high transmission efficiency for the light, resulting in a faster response, which also depends on the size of the devices. LSPR fiber sensors become more compact when the palsmonics structures are prepared on the end or side surface of the optical fiber. Attributing to the focus ion beam or electron beam lithography, the high fabrication cost of plasmonics devices hinders their commercial application way. Either LSPR fiber sensors or common fiber sensors are free-standing and can be integrated with the planar devices.
The reproducible manufacturing technology of probes urgently needs a breakthrough in the future. This must refer to the production and integration methods of plasmonics devices in order to combine them with planar devices through waveguide coupling or sol packaging technology [74][75]. Corresponding sensor arrays and practical sensors are also expected to be developed, similar to the plasmonics chips [76][77]. During the design and fabrication process of metal-NPs-LSPR based optical fiber probes, the size and uniformity of metal NPs, the characteristics and modification methods of auxiliary functional materials, as well as the design and optimization of optical fiber structures should be concerned. Failure to address these issues will result in the low repeatability of such sensors and the limitation of their extensive commercial applications. New nanomaterials and nanofabrication technologies also provide the possibility for the fine design and performance optimization of such probes. As a typical candidate of the biochemical sensors, its response time and recovery effect need special attention. For example, how to improve the interaction efficiency between the LSPR effect and the molecules to be measured? Or how to quickly recover the metal NPs from the contaminated state after the measurement, so as to improve its usage lifetime and reduce the cost.
Inspired by the application of traditional optical fiber sensors, in addition to miniature biochemical sensor probes in environmental pollution, medical assistance, and biomolecular recognition, the application of LSPR sensors can also be extended to structural health monitoring of bridges, dams, and smart cities. However, the current metal NPs-based LSPR sensor is still limited to the development stage of novel biochemical probes in a laboratory environment. This is mainly due to the poor production repeatability, large differences in individual performance, unstable long-term work, and short service life. The preparation process and modification methods of metal NPs, the precise design of metal nanostructures, and the ingenious design of optical fiber structures will be important research directions to promote the development of relevant practical LSPR sensors.
4. Conclusions
The fabrication methods of this kind of fiber probes include the polymer doping-dispersion of metal NPs, nano-photolithography to construct metal nanostructures, dip coating film method, electrostatic layer by layer self-assembly method, and molecular imprinting method. Based on the above technologies, the LSPR effect can also be significantly improved by changing the structure of the metal NPs functional film, laser irradiation technology for NPs size modulation, low dimensional material assisted enhancement, fluorescent material modification, and oriented sensitization of molecular markers. Metal NPs functional films have been modified on U-shaped fiber, D-typed fiber, micro/nanofiber, fiber grating, cascaded fiber structure, and polymer fiber, which are used to develop different types of probes to measure refractive index, heavy metal ions, pH value, gas, nucleic acid, and virus molecules. The simultaneous measurement of different components and concentrations is expected to be realized with the help of the different metal NPs functional films with the special excitation wavelengths and the various fiber structures working on different optical principles.
References
- Homola, J.; Koudela, I.; Yee, S.S. Surface plasmon resonance sensors based on diffraction gratings and prism couplers: Sensitivity comparison. Sens. Actuators B-Chem. 1999, 54, 16–24.
- Barnes, W.L.; Dereux, A.; Ebbesen, T.W. Surface plasmon subwavelength optics. Nature 2003, 424, 824–830.
- Huang, D.W.; Ma, Y.F.; Sung, M.J.; Huang, C.P. Approach the angular sensitivity limit in surface plasmon resonance sensors with low index prism and large resonant angle. Opt. Eng. 2010, 49, 054403.
- Wang, G.Q.; Wang, C.N.; Yang, R.; Liu, W.L.; Sun, S.Q. A Sensitive and Stable Surface Plasmon Resonance Sensor Based on Monolayer Protected Silver Film. Sensors 2017, 17, 2777.
- Morsin, M.; Salleh, M.M.; Umar, A.A.; Sandan, M.Z. Gold Nanoplates for a localized surface plasmon resonance-based boric acid sensor. Sensors 2017, 17, 947.
- Kazuma, E.; Tatsuma, T. Localized surface plasmon resonance sensors based on wavelength-tunable spectral dips. Nanoscale 2014, 6, 2397–2405.
- Shalabney, A.; Abdulhalim, I. Prism dispersion effects in near-guided-wave surface plasmon resonance sensors. Ann. Phys. 2012, 524, 680–686.
- Kang, Y.Q.; Gao, P.; Liu, H.M.; Zhang, J. Large tunable lateral shift from guided wave surface plasmon resonance. Plasmonics 2019, 14, 1289–1293.
- Alharbi, R.; Irannejad, M.; Yavuz, M. A short review on the role of the metal-graphene hybrid nanostructure in promoting the localized surface plasmon resonance sensor performance. Sensors 2019, 19, 862.
- Jeong, H.H.; Erdene, N.; Park, J.H.; Jeong, D.H.; Lee, S.K. Analysis of fiber-optic localized surface plasmon resonance sensor by controlling formation of gold nanoparticles and its bio-application. J. Nanosci. Nanotechnol. 2012, 12, 7815–7821.
- Cennamo, N.; D’Agostino, G.; Dona, A.; Dacarro, G.; Pallavicini, P.; Pesavento, M.; Zeni, L. Localized surface plasmon resonance with five-branched gold nanostars in a plastic optical fiber for bio-chemical sensor implementation. Sensors 2013, 13, 14676–14686.
- Jung, M.; Ji, M.G.; Kim, T.R.; Shim, C.H.; Lee, S.; Woo, D.; Choi, Y.W. Localized surface plasmon resonance in two-dimensional silver nanodot array fabricated using nanoporous alumina mask for chemical sensor platform. Opt. Eng. 2016, 55, 087107.
- Park, J.H.; Byun, J.Y.; Shim, W.B.; Kim, S.U.; Kim, M.G. High-sensitivity detection of ATP using a localized surface plasmon resonance (LSPR) sensor and split aptamers. Biosens. Bioelectron. 2015, 73, 26–31.
- Li, J.Y. Localized surface plasmon resonance sensor based at metallic sphere dimer particle. J. Nanosci. Nanotechnol. 2017, 17, 1443–1446.
- Lee, K.Y.; Lin, K.C.; Tsai, W.H. Side-polished optical fiber sensor coated with a metal/oxide surface-plasmon-resonance sensing film. Optoelectron. Adv. Mat. 2015, 9, 903–906.
- He, Y.J. High-performance localized surface plasmon resonance fiber sensor based on nano-metal-gear array. Sens. Actuators B-Chem. 2014, 193, 778–787.
- Yuan, Y.Q.; Hu, D.; Hua, L.; Li, M. Theoretical investigations for surface plasmon resonance based optical fiber tip sensor. Sens. Actuators B-Chem. 2013, 188, 757–760.
- Ortega-Mendoza, J.G.; Padilla-Vivanco, A.; Toxqui-Quitl, C.; Zaca-Moran, P.; Villegas-Hernandez, D.; Chavez, F. Optical fiber sensor based on localized surface plasmon resonance using silver nanoparticles photodeposited on the optical fiber end. Sensors 2014, 14, 18701–18710.
- Moayyed, H.; Leite, I.T.; Coelho, L.; Santos, J.L.; Viegas, D. Theoretical study of phase-interrogated surface plasmon resonance based on optical fiber sensors with metallic and oxide layers. Plasmonics 2015, 10, 979–987.
- Uh, M.; Kim, J.S.; Park, J.H.; Jeong, D.H.; Lee, H.Y.; Lee, S.M.; Lee, S.K. Fabrication of localized surface plasmon resonance sensor based on optical fiber and micro fluidic channel. J. Nanosci. Nanotechnol. 2017, 17, 1083–1091.
- Chang, C.Y.; Lin, H.T.; Lai, M.S.; Shieh, T.Y.; Peng, C.C.; Shih, M.H.; Tung, Y.C. Flexible localized surface plasmon resonance sensor with metal-insulator-metal nanodisks on PDMS substrate. Sci. Rep. 2018, 8, 11812.
- Teng, C.X.; Zheng, J.; Liang, Q.Y.; Deng, S.J.; Deng, H.C.; Liu, H.Q.; Yuan, L.B. The influence of structural parameters on the surface plasmon resonance sensor based on a side-polished macrobending plastic optical fiber. IEEE Sens. J. 2020, 20, 4245–4250.
- Sharma, A.K.; Mohr, G.J. On the application of different bimetallic alloy nanoparticle combinations in fiber optic surface plasmon resonance salinity sensor and its performance optimization against thermal effects. J. Nanosci. Nanotechnol. 2010, 10, 3145–3154.
- Tu, M.H.; Sun, T.; Grattan, K.T.V. Optimization of gold-nanoparticle-based optical fibre surface plasmon resonance (SPR)-based sensors. Sens. Actuators B-Chem. 2012, 164, 43–53.
- Bharadwaj, R.; Mukherji, S. Gold nanoparticle coated U-bend fibre optic probe for localized surface plasmon resonance based detection of explosive vapours. Sens. Actuators B-Chem. 2014, 192, 804–811.
- Kumar, S.; Yadav, G.C.; Sharma, G.; Singh, V. Study of surface plasmon resonance sensors based on silver-gold nanostructure alloy film coated tapered optical fibers. Appl. Phys. A 2018, 124, 695.
- Liu, H.; Chen, C.; Zhang, Y.Z.; Bai, B.B.; Tang, S.F. A high-sensitivity methane sensor with localized surface plasmon resonance behavior in an improved hexagonal gold nanoring array. Sensors 2019, 19, 4803.
- Li, W.W.; Sun, C.Y.; Yu, S.L.; Pu, Z.H.; Zhang, P.H.; Xu, K.X.; Song, Z.Q.; Li, D.C. Flattened fiber-optic ATR sensor enhanced by silver nanoparticles for glucose measurement. Biomed. Microdevices 2018, 20, 104.
- Arcas, A.D.; Dutra, F.D.; Allil, R.C.S.B.; Werneck, M.M. Surface plasmon resonance and bending loss-based U-shaped plastic optical giber biosensors. Sensors 2018, 18, 648.
- Chen, K.C.; Li, Y.L.; Wu, C.W.; Chiang, C.C. Glucose sensor using U-Shaped optical fiber probe with gold nanoparticles and glucose oxidase. Sensors 2018, 18, 1217.
- Raj, D.R.; Prasanth, S.; Sudarsanakumar, C. Development of LSPR-based optical fiber dopamine sensor using l-tyrosine-capped silver nanoparticles and its nonlinear optical properties. Plasmonics 2017, 12, 1227–1234.
- Urrutia, A.; Bojan, K.; Marques, L.; Mullaney, K.; Goicoechea, J.; James, S.; Clark, M.; Tatam, R.; Korposh, S. Novel highly sensitive protein sensors based on tapered optical fibres modified with Au-based nanocoatings. J. Sens. 2016, 2016, 8129387.
- Kumar, S.; Kaushik, B.K.; Singh, R.; Chen, N.K.; Yang, Q.S.; Zhang, X.; Wang, W.J.; Zhang, B.Y. LSPR-based cholesterol biosensor using a tapered optical fiber structure. Biomed. Opt. Express 2019, 10, 2150–2160.
- Singh, L.; Zhu, G.; Singh, R.; Zhang, B.Y.; Wang, W.J.; Kaushik, B.K.; Kumar, S. Gold nanoparticles and uricase functionalized tapered fiber sensor for uric acid detection. IEEE Sens. J. 2020, 20, 219–226.
- Dash, S.P.; Patnaik, S.K.; Tripathy, S.K. Investigation of a low cost tapered plastic fiber optic biosensor based on manipulation of colloidal gold nanoparticles. Opt. Commun. 2019, 437, 388–391.
- Cao, J.; Zhao, D.; Mao, Q.H. A highly reproducible and sensitive fiber SERS probe fabricated by direct synthesis of closely packed AgNPs on the silanized fiber taper. Analyst 2017, 142, 596–602.
- Li, L.; Deng, S.X.; Wang, H.; Zhang, R.H.; Zhu, K.; Lu, Y.; Wang, Z.L.; Zong, S.F.; Wang, Z.Y.; Cui, Y.P. A SERS fiber probe fabricated by layer-by-layer assembly of silver sphere nanoparticles and nanorods with a greatly enhanced sensitivity for remote sensing. Nanotechnology 2019, 30, 255503.
- Heidemann, B.R.; Chiamenti, I.; Oliveira, M.M.; Muller, M.; Fabris, J.L. Functionalized long period grating-plasmonic fiber sensor applied to the detection of glyphosate in water. J. Lightwave Technol. 2018, 36, 863–870.
- Lao, J.J.; Han, L.Z.; Wu, Z.; Zhnag, X.J.; Huang, Y.Y.; Tang, Y.; Guo, T. Gold nanoparticle functionalized surface plasmon resonance optical fiber biosensor: In situ detection of thrombin with 1 nM detection Limit. J. Lightwave Technol. 2019, 37, 2748–2755.
- Chen, H.M.; Zhao, L.; Chen, D.Q.; Hu, W.H. Stabilization of gold nanoparticles on glass surface with polydopamine thin film for reliable LSPR sensing. J. Colloid Interface Sci. 2015, 460, 258–263.
- Mayer, K.M.; Hafner, J.H. Localized Surface Plasmon Resonance Sensors. Chem. Rev. 2011, 111, 3828–3857.
- Wang, B.T.; Wang, Q. Sensitivity-enhanced optical fiber biosensor based on coupling effect between SPR and LSPR. IEEE Sens. J. 2018, 18, 8303–8310.
- Li, K.W.; Zhou, W.C.; Zeng, S.W. Optical micro/nanofiber-based localized surface plasmon resonance biosensors: Fiber diameter dependence. Sensors 2018, 18, 3295.
- Kumar, S.; Singh, R.; Kaushik, B.K.; Chen, N.K.; Yang, Q.S.; Zhang, X. LSPR-based cholesterol biosensor using hollow core fiber structure. IEEE Sens. J. 2019, 19, 7399–7406.
- Singh, L.; Singh, R.; Zhang, B.Y.; Kaushik, B.K.; Kumar, S. Localized surface plasmon resonance based hetero-core optical fiber sensor structure for the detection of l-cysteine. IEEE Trans. Nanotechnol. 2020, 19, 201–208.
- Zhu, G.; Agrawal, N.; Singh, R.; Kumar, S.; Zhang, B.Y.; Saha, C.; Kumar, C. A novel periodically tapered structure-based gold nanoparticles and graphene oxide-Immobilized optical fiber sensor to detect ascorbic acid. Opt. Laser Technol. 2020, 127, 106156.
- Kim, H.M.; Park, J.H.; Jeong, D.H.; Lee, H.Y.; Lee, S.K. Real-time detection of prostate-specific antigens using a highly reliable fiber-optic localized surface plasmon resonance sensor combined with micro fluidic channel. Sens. Actuators B-Chem. 2018, 273, 891–898.
- Kim, H.M.; Uh, M.; Jeong, D.H.; Lee, H.Y.; Park, J.H.; Lee, S.K. Localized surface plasmon resonance biosensor using nanopatterned gold particles on the surface of an optical fiber. Sens. Actuators B-Chem. 2019, 280, 183–191.
- Liu, L.L.; Marques, L.; Correia, R.; Morgan, S.P.; Lee, S.W.; Tighe, P.; Fairclough, L.; Korposh, S. Highly sensitive label-free antibody detection using a long period fibre grating sensor. Sens. Actuators B-Chem. 2018, 271, 24–32.
- Bharadwaj, R.; Mukherji, S.; Mukherji, S. Probing the localized surface plasmon field of a gold nanoparticle-based fibre optic biosensor. Plasmonics 2016, 11, 753–761.
- Baliyan, A.; Usha, S.P.; Gupta, B.D.; Gupta, R.; Sharma, E.K. Localized surface plasmon resonance-based fiber-optic sensor for the detection of triacylglycerides using silver nanoparticles. J. Biomed. Opt. 2017, 22, 107001.
- Raj, D.R.; Sudarsanakumar, C. Surface plasmon resonance based fiber optic sensor for the detection of cysteine using diosmin capped silver nanoparticles. Sens. Actuators A-Phys. 2017, 253, 41–48.
- Lu, M.D.; Zhu, H.; Bazuin, C.G.; Peng, W.; Masson, J.F. Polymer-templated gold nanoparticles on optical fibers for enhanced-sensitivity localized surface plasmon resonance biosensors. ACS Sens. 2019, 4, 613–622.
- Lee, B.; Park, J.H.; Byun, J.Y.; Kim, J.H.; Kim, M.G. An optical fiber-based LSPR aptasensor for simple and rapid in-situ detection of ochratoxin A. Biosens. Bioelectron. 2018, 102, 504–509.
- Raj, D.R.; Prasanth, S.; Vineeshkumar, T.V.; Sudarsanakumar, C. Surface plasmon resonance based fiber optic dopamine sensor using green synthesized silver nanoparticles. Sens. Actuators B-Chem. 2016, 224, 600–606.
- Yuan, H.Z.; Ji, W.; Chu, S.W.; Liu, Q.; Guang, J.Y.; Sun, G.Y.; Zhang, Y.; Han, X.Y.; Masson, J.F.; Peng, W. Au nanoparticles as label-free competitive reporters for sensitivity enhanced fiber-optic SPR heparin sensor. Biosens. Bioelectron. 2020, 154, 112039.
- Wang, Q.; Wang, X.Z.; Song, H.; Zhao, W.M.; Jing, J.Y. A dual channel self-compensation optical fiber biosensor based on coupling of surface plasmon polariton. Opt. Laser Technol. 2020, 124, 106002.
- Kim, H.M.; Jeong, D.H.; Lee, H.Y.; Park, J.H.; Lee, S.K. Improved stability of gold nanoparticles on the optical fiber and their application to refractive index sensor based on localized surface plasmon resonance. Opt. Laser Technol. 2019, 114, 171–178.
- Loyez, M.; Ribaut, C.; Caucheteur, C.; Wattiez, R. Functionalized gold electroless-plated optical fiber gratings for reliable surface biosensing. Sens. Actuators B-Chem. 2019, 280, 54–61.
- Singh, L.; Singh, R.; Zhang, B.Y.; Cheng, S.; Kaushik, B.K.; Kumar, S. LSPR based uric acid sensor using graphene oxide and gold nanoparticles functionalized tapered fiber. Opt. Fiber Technol. 2019, 53, 102043.
- Nayak, J.K.; Parhi, P.; Jha, R. Experimental and theoretical studies on localized surface plasmon resonance based fiber optic sensor using graphene oxide coated silver nanoparticles. J. Phys. D Appl. Phys. 2016, 49, 285101.
- Li, C.; Li, Z.; Li, S.L.; Zhang, Y.N.; Sun, B.P.; Yu, Y.H.; Ren, H.Y.; Jiang, S.Z.; Yue, W.W. LSPR optical fiber biosensor based on a 3D composite structure of gold nanoparticles and multilayer graphene films. Opt. Express 2020, 28, 6071–6083.
- Kim, H.M.; Park, J.H.; Lee, S.K. Fiber optic sensor based on ZnO nanowires decorated by Au nanoparticles for improved plasmonic biosensor. Sci. Rep. 2019, 9, 15605.
- Yuan, H.Z.; Ji, W.; Chu, S.W.; Qian, S.Y.; Wang, F.; Masson, J.F.; Han, X.Y.; Peng, W. Fiber-optic surface plasmon resonance glucose sensor enhanced with phenylboronic acid modified Au nanoparticles. Biosens. Bioelectron. 2018, 117, 637–643.
- Shrivastav, A.M.; Usha, S.P.; Gupta, B.D. Highly sensitive and selective erythromycin nanosensor employing fiber optic SPR/ERY imprinted nanostructure: Application in milk and honey. Biosens. Bioelectron. 2017, 90, 516–524.
- Semwal, V.; Shrivastav, A.M.; Gupta, B.D. Surface plasmon resonance based fiber optic trichloroacetic acid sensor utilizing layer of silver nanoparticles and chitosan doped hydrogel. Nanotechnology 2017, 28, 065503.
- Cepeda-Perez, E.; Moreno-Hernandez, C.; Luke, T.L.; Monzon-Hernandez, D.; Plascencia-Villa, G.; de la Rosa, E. Reusable fiber taper sensor based on the metastability of gold nanoparticles. Mater. Res. Express 2019, 6, 026207.
- Sharma, S.; Gupta, B.D. Surface plasmon resonance based highly selective fiber optic dopamine sensor fabricated using molecular imprinted GNP/SnO2 nanocomposite. J. Lightwave Technol. 2018, 36, 5956–5962.
- Gupta, B.D.; Shrivastav, A.M.; Usha, S.P. Surface plasmon resonance-based fiber optic sensors utilizing molecular imprinting. Sensors 2016, 16, 1381.
- Semwal, V.; Gupta, B.D. LSPR- and SPR-based fiber-optic cholesterol sensor using immobilization of cholesterol oxidase over silver nanoparticles coated graphene oxide nanosheets. IEEE Sens. J. 2018, 18, 1039–1046.
- Qian, S.Y.; Lin, M.; Ji, W.; Yuan, H.Z.; Zhang, Y.; Jing, Z.G.; Zhao, J.Z.; Masson, J.F.; Peng, W. Boronic acid functionalized Au nanoparticles for selective microRNA signal amplification in fiber-optic surface plasmon resonance sensing system. ACS Sens. 2018, 3, 929–935.
- Huang, Y.Y.; Chen, P.W.; Liang, H.; Xiao, A.X.; Zeng, S.K.; Guan, B.O. Nucleic acid hybridization on a plasmonic nanointerface of optical microfiber enables ultrahigh-sensitive detection and potential photothermal therapy. Biosens. Bioelectron. 2020, 156, 112147.
- Sharma, P.; Semwal, V.; Gupta, B.D. A highly selective LSPR biosensor for the detection of taurine realized on optical fiber substrate and gold nanoparticles. Opt. Fiber Technol. 2019, 52, 101962.
- Fang, Y.H.; Wen, K.H.; Li, Z.F.; Wu, B.Y.; Chen, L.; Zhou, J.Y.; Zhou, D.Y. Multiple Fano Resonances Based on End-Coupled Semi-Ring Rectangular Resonator. IEEE Photonics J. 2019, 11, 1–8.
- Zhang, L.; Pan, J.; Zhang, Z.; Wu, H.; Yao, N.; Cai, D.W.; Xu, Y.X.; Zhang, J.; Sun, G.F.; Wang, L.Q.; et al. Ultrasensitive skin-like wearable optical sensors based on glass micro/nanofibers. Opto-Electron. Adv. 2020, 3, 190022.
- Li, Z.F.; Wen, K.H.; Fang, Y.H.; Guo, Z.C. Refractive Index Sensing Research on Multi-Fano-Based Plasmonic MDM Resonant System With Water-Based Dielectric. IEEE J. Quantum Electron. 2020, 56, 1–7.
- Chen, Q.; Liang, L.; Zheng, Q.L.; Zhang, Y.X.; Wen, L. On-chip readout plasmonic mid-IR gas sensor. Opto-Electron. Adv. 2020, 3, 190040.




